Translate this page into:
Effect of ginger extract on membrane potential changes and AKT activation on a peroxide-induced oxidative stress cell model
⁎Corresponding authors at: Experimental and Computational Biochemistry Research Group, Nutrition and Biochemistry Department, Sciences Faculty, Pontificia Universidad Javeriana, Bogotá, Colombia (L. Morales). Phytochemical Research Group Universidad Javeriana, Department of Chemistry, School of Sciences, Pontificia Universidad Javeriana, Bogotá, Colombia (L.G. Sequeda-Castañeda). lsequeda@javeriana.edu.co (Luis G. Sequeda-Castañeda), ludis.morales@javeriana.edu.co (Ludis Morales)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
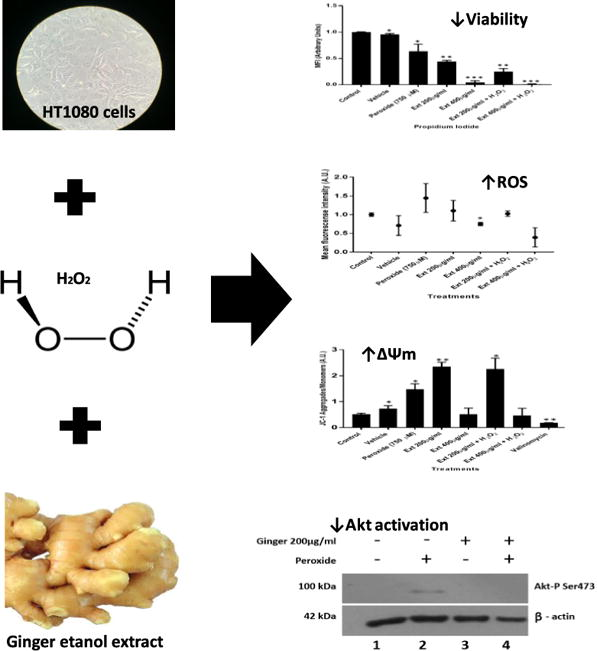
Abstract
Zingiber officinale is a type of Ginger used for the treatment of reactive oxygen species (ROS) associated diseases. Reports describe its use in cellular models, such as pancreatic and intestinal cancer cell lines. However, the biochemical bases of antitumor, anti-inflammatory and antioxidant activities have not yet been fully elucidated. The aim of this study was to evaluate in H2O2-induced oxidative stress the effect of ginger extract (GE) on HT1080 cell viability, ROS production, AKT activation, and mitochondrial membrane potential (ΔΨm). ROS production was measured by DHE probe. Results revealed a significant cell viability decrease with increasing GE concentrations. In addition, GE at 200 μg/ml and 400 μg/ml resulted in decreased ROS production compared with controls. Moreover, GE at 200 μg/ml concentration produced a significant increase in ΔΨm. In contrast, no difference in ΔΨm was observed compared to controls with GE at 400 μg/ml. In untreated HT1080 cells basal AKT activation was not observed. Conversely, treatment with 750 μM H2O2 led to Ser473 phosphorylation. Additionally, treatment with GE at 200 μg/ml decreased AKT activation. Reports in the literature describe GE biological activities; none the less, novel approaches to investigate intracellular changes resulting from GEs are described in this study. In conclusion, this study characterized GE induced intracellular changes, leading to changes in ΔΨm and signaling protein levels, such as AKT, and reduced cell viability.
Keywords
Ginger extract
ROS
Viability
AKT protein
Mitochondrial membrane potential
1 Introduction
Oxidative stress is a metabolic status, which increases reactive oxygen species (ROS) production. Cell injury results in cell viability compromise through apoptosis or necrosis (Fulda et al., 2010). This status affects several cell processes, such as mitochondrial membrane potential (ΔΨm) and protein activation, due to DNA mutations (Rios-Arrabal et al., 2013).
DNA alterations caused by oxidative injury predispose cells to aging, mutagenesis or carcinogenesis (Valko et al., 2007). Oxidative stress has been widely associated with DNA instability, and is one of the main mechanisms involved in cancer (Nourazarian et al., 2014). In neoplasias, macrophages and other immune cells contribute to chronic inflammation. This in turn promotes ROS production, where reduced glutathione, superoxide dismutase, and catalase comprising the anti-oxidant system are unable to control oxygen radical generation. This favors initiation, progression, and tumor invasion in affected tissues (Qian and Pollard, 2010).
It has been shown that signaling pathways responsible for tumor growth and survival, such as PI3K/AKT pathway are affected by mutations (Robbins and Hague, 2015). Activation of PI3K/AKT signaling cascade leads to pro-apoptotic protein expression such as Bcl-2-associated death promoter (BAD) and caspase 9. Oxidative stress can either be reduced through increased consumption of exogenous antioxidants or decreased ROS production by stabilization of mitochondrial energy production (Poljsak, 2011).
As a result of ginger extract (GE) antioxidant activity, recent studies have shown its effectiveness to treat many types of cancers (Chen et al., 2012). These include colon, liver, and breast cancer (Ray et al., 2015; Wee et al., 2015; Zhou et al., 2016a). Reports in the literature describe GE main anticancer component effects including 6- and 10-gingerols, inhibiting cervical cancer (Zhang et al., 2017a; Zhang et al., 2017b). In addition, ginger has the ability to induce apoptosis (Zhou et al., 2016b). Moreover, ginger has antimicrobial (Ewnetu et al., 2014) and anti-inflammatory activities, evidenced by a decreased biological marker like C-reactive protein (CRP) (Arablou et al., 2014). In addition, these extracts have been shown to improve insulin sensitivity and reduce blood sugar levels (Shidfar et al., 2015), among other health benefits.
Given GE anticancer properties, the purpose of this study aimed to evaluate in HT-1080 cells under H2O2-induced oxidative stress the effect of ginger ethanol extract on cell viability, ROS production, AKT protein phosphorylation, and mitochondrial membrane potential (ΔΨm).
2 Materials and methods
2.1 Ginger ethanol extract preparation
Ginger was obtained from a local farmer’s market. 100 g of ginger rhizome was dried at 100 °C for 4 h. To 50 ml of 70% ethanol 10 g of dried powder was added, and mixed gently for one hour. The obtained solution was left at room temperature (RT) for 24 h, before it was stirred again and filtered. Ethanol was chosen as a solvent, since it allows for better extraction of organic compounds (Machado et al., 2015). Ginger extracts were initially evaluated at the following concentrations: 100 µg/ml, 200 µg/ml, 400 µg/ml, 800 µg/ml, 1000 µg/ml, 1200 and 1600 µg/ml.
2.2 Cell culture
HT1080 cell line, derived from a solid tumor human of fibrosarcoma was used in this study. Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% FBS and 1% antibiotic/antimycotic solution [(penicillin/streptomycin/amphotericin B) in a humidified atmosphere at 37 °C and 5% CO2. As a vehicle control 70% ethanol was used in all experiments. All assays were performed in triplicates.
2.3 Cell viability by MTT assay
Cell viability was determined by MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay. 9x103 cells were cultured in 96-well plates containing 100 μl DMEM supplemented with 10% FBS. Effect on cell viability was tested for each hydrogen peroxide concentration. Likewise, for determining GE effect on cell viability all GE concentrations were evaluated. To this end cells were treated with different GE concentrations for 4 h. At the end of the incubation, 20 μl of MTT was added to allow for MTT formazan crystals formation for 4 h at RT. Media was removed and crystals were solubilized in 100 μl of DMSO. Absorbance was recorded at 540 nm. Experiments were performed in triplicates. Data was evaluated as absorbance change with respect to control (Akimoto et al., 2015).
2.4 Cell viability evaluation by propidium iodide assay
4.8x103 cells were cultured in 48-well plates and treated with 100 μl DMEM supplemented with 10% FBS containing two different GE concentrations (200 µg/ml or 400 µg/ml) for 4 h. Media was removed, and cells were trypsinized. After addition of fresh culture media, cells were incubated with 0.01 mg/ml propidium iodide and acquired in a Guava easyCyte Flow cytometer (EMD Millipore, Billerica MA, USA). Experiments were performed in triplicates.
2.5 H2O2 treatment
9 x 103 cells were cultured in 96-well plates with 100 μl DMEM supplemented with 10% FBS. As an oxidative stress inducer H2O2 treatment was used for all experiments to evaluate ROS production. After 12 h of culture cells were treated with the corresponding H2O2 concentration. The following concentrations were assayed: 10 µM, 50 µM, 100 µM, 250 µM, 500 µM, 750 µM and 1,000 µM. For GE effect evaluation 750 µM H2O2 was employed with two different GE concentrations (200 µg/ml or 400 µg/ml) for 4 h. All experiments were performed in triplicates.
2.6 Evaluation of mitochondrial membrane potential
Mitochondrial membrane potential (ΔΨm) was monitored by the JC-1 Mitochondrial Membrane Potential Assay Dye (Sigma, USA). For this assay, 1.2x104 cells were seeded in a 12 well plate and treated as mentioned above. Cells were trypsinized and stained with JC-1. Cells were immediately acquired in a Guava easyCyte flow cytometer. Valinomycin was used as a positive control for mitochondrial membrane potential depolarization. Data was evaluated in triplicates as red/green fluorescence ratio (aggregates/monomers) (Akimoto et al., 2015).
2.7 ROS quantification
Superoxide production was detected by Dihydroethydine (DHE) probe. Briefly, HT1080 cells treated as mentioned above were trypsinized and stained with 1.5 µM DHE for 10 min. Cells were washed once with PBS and acquired in a Guava easyCite flow cytometer. Rotenone was used as a positive control for superoxide production. Results are presented as mean arbitrary units of fluorescence (Anderica-Romero et al., 2016).
2.8 Morphological assessment
Morphological assessment was carried-out through light microscopy to identify events implicated in cell morphological changes, such as cell shrinkage, loss of cell filopodia cell, and detachment (Vijaya Padma et al., 2007).
2.9 Western blotting
Cell extracts were prepared by lysing cells with RIPA buffer (50 mM Tris–HCl, pH 7.4, 150 mM NaCl, 1% NP-40, 0.5% deoxycholate, 0.1% sodium dodecyl sulfate, 2 mM EDTA, protease inhibitor cocktail and phosphatase inhibitor cocktail) on ice for 20 min. Lysates were centrifuged at 5000 × g for 5 min at 4 °C, and supernatants were used for subsequent analyses. Approximately 30 µg of total protein were loaded for each treatment. To assess Akt activation pAkt Ser473 was used at 1:1,000 dilution (Cell Signaling Technology). As a loading control mouse, polyclonal GAPDH at 1: 2500 dilution (Cell Signaling Technology) was used.
2.10 Statistical analysis
Data obtained were analyzed by standard deviation (SD±) and t-student with Graph Pad Prism software (Graph Pad Software, San Diego, CA). T-student test (unpaired variables, two tails) was used for comparing two groups of treatments. A probability value <0.05 was considered statistically significant (Ekstrom and Sørensen, 2015).
3 - Results
3.1 Effect of hydrogen peroxide concentration on cell viability
To determine the effect of hydrogen peroxide on HT1080 cell viability and ROS production, treatments with increasing hydrogen peroxide concentrations ranging from 10 µM to 1 mM were evaluated. H2O2 at 750 µM had an effect on ROS production without affecting cell viability it was elected as a ROS production inducer for all other experiments (Fig. 1A). For hydrogen peroxide concentrations between 10 µM to 1 mM no effect was observed on cell viability (Fig.1B).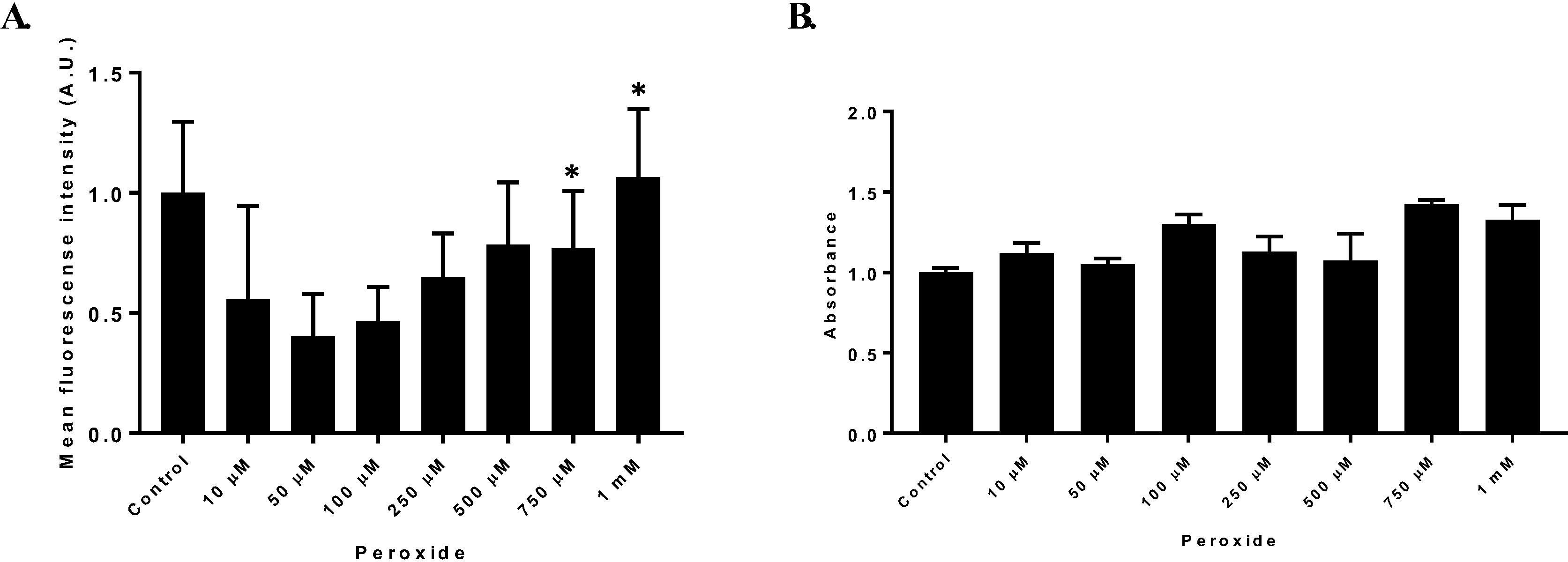
Reactive oxygen species production. (A) HT1080 cells treated with different hydrogen peroxide concentrations to evaluate ROS production through DHE assay. ROS production was established as mean fluorescence intensity arbitrary units (A.U) ± SD (B) Mean fluorescence intensity of HT1080 cell treated with increasing hydrogen peroxide concentrations to evaluate ROS production through DHE probe. n = 3. (*p = 0.05).
3.2 Effect of ginger extracts at different concentrations on cell viability and ROS production
The effect of GE on HT1080 cell viability and ROS production was evaluated with different GE concentrations between 100 µg/ml to 1600 µg/ml. Ginger extract at 1000 µg/ml presented a significantly decrease on cell viability with respect to control (Fig. 2 B). Similar results were observed for 1200 µg/ml and 1600 µg/ml. In contrast, no alterations on cell viability were evidenced with concentrations between 100 µg/ml to 800 µg/ml. For the remaining experiments ginger extract concentrations of 200 and 400 µg/ml were chosen because of their increasing ROS production effects (Fig. 2A).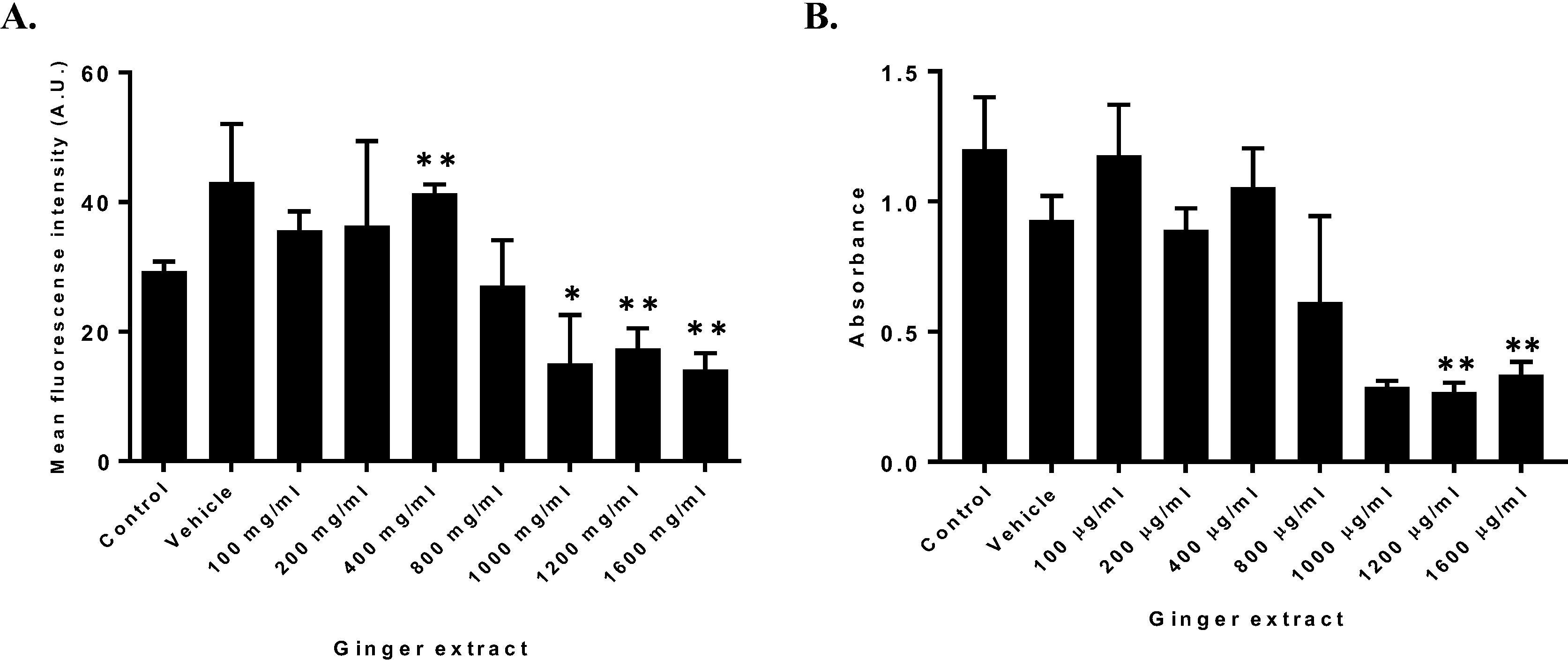
Effect of GE on ROS production and cell viability (A). HT1080 cells treated with increasing GE concentrations to evaluate ROS production through DHE determined as Mean fluorescence intensity (A.U) ± SD. (B) HT1080 cell viability determined by MTT assay of treated cells with increasing extract concentration. n = 3. (*p = 0.05, *p = 0.01).
3.3 Hydrogen peroxide oxidative stress-induced and ginger extract treatments decrease cell viability and increase ROS production
Incubation of HT1080 cells with 750 µM peroxide for 4 h following a treatment with 200 or 400 µg/ml ginger extract, revealed that treatments with only peroxide significantly increased ROS production (Fig. 3A). On the other hand, GE at 400 µg/resulted in decreased ROS production. Regarding cell viability for GE at 400 µg/ml a decreased absorbance value was observed, suggesting decreased cell viability compared to controls (Fig. 3B). However, GE at 200 µg/ml ROS production was significantly higher compared with GE at 400 µg/ml. Significant differences were also observed for cell viability between these two concentrations, where GE at 200 µg/ml had increased cell viability. In co-treatment with hydrogen peroxide, cell viability was reduced in comparison to GE extract only at 200 µg/ml (Fig.3B).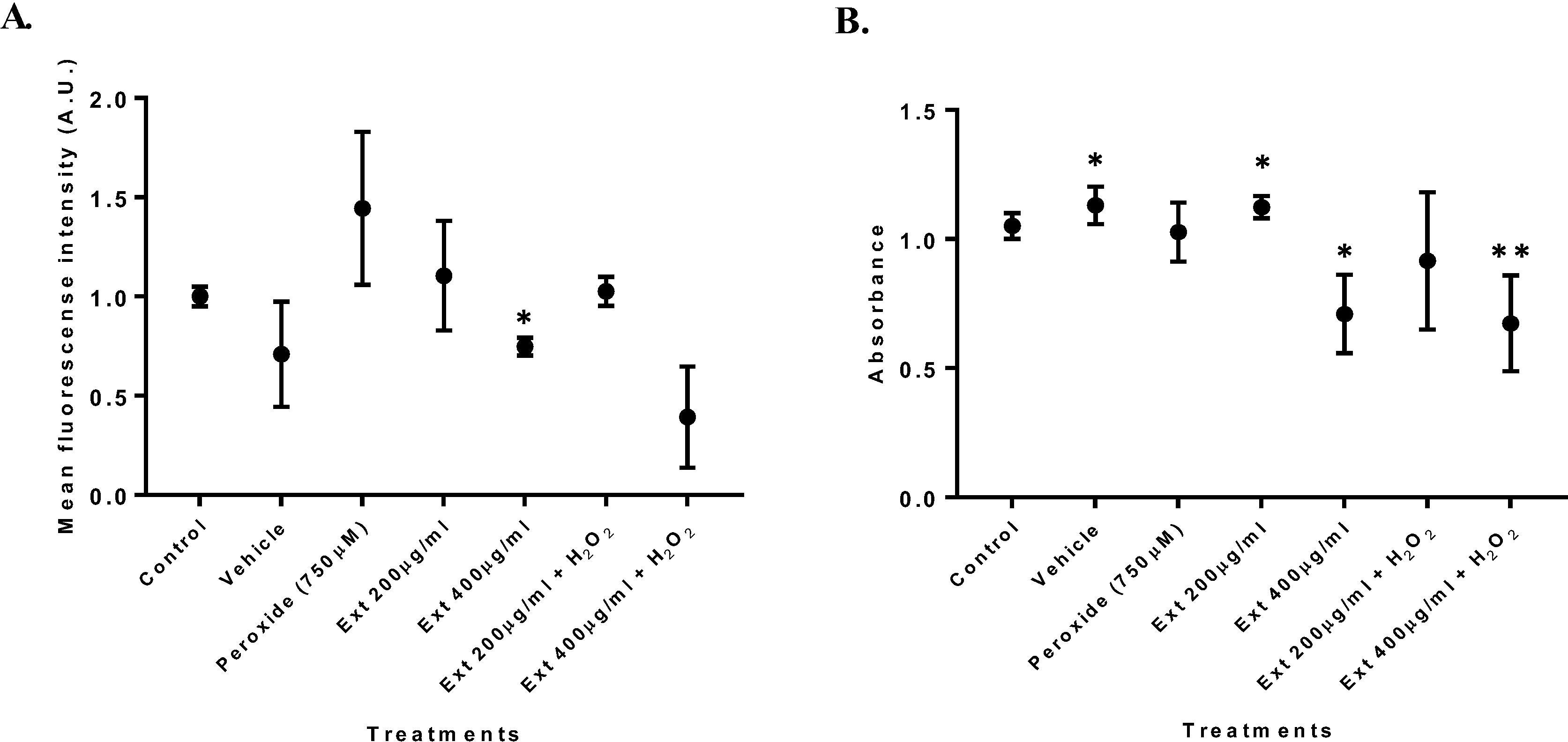
Co-treatment with hydrogen peroxide and GE. (A) ROS production in cells co-treated with hydrogen peroxide and ginger extract for 4 h. (B) MTT assay for HT1080 cells co-treated with hydrogen peroxide and ginger extract for 4 h. n = 3. (*p = 0.05, **p = 0.01).
3.4 Cell viability by propidium iodide assay
A highly significant decrease in cell viability was observed compared with controls, when cells were treated with 400 µg/ml GE (p < 0.001∗∗∗). In contrast, cells treated with GE at 200 µg/ml had a cell viability decrease of only 50% (Fig. 4).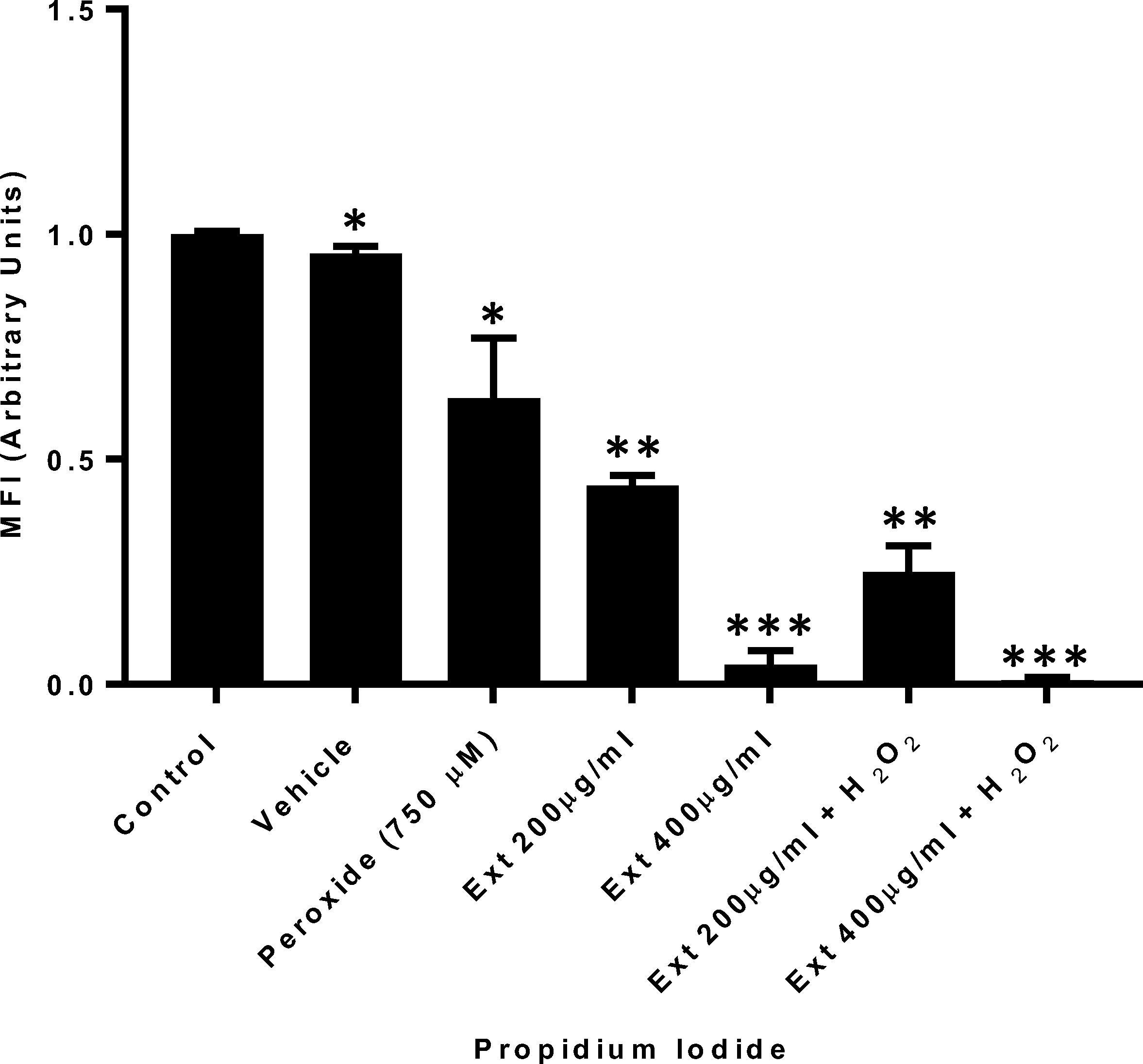
Co-treatment with hydrogen peroxide and GE Cell viability evaluation. Propidium iodide cell viability evaluation of HT1080 cells treated with peroxide concomitantly with ginger extract. n = 3. (*p = 0.05, **p = 0.01, ***p = 0.001).
3.5 Induced hydrogen peroxide oxidative stress and ginger extract treatments are involved in AKT phosphorylation
Incubation of HT1080 cells with 750 µM hydrogen peroxide for 4 h revealed that AKT phosphorylation significantly increased. Additionally, cells did not show major changes when treated only with ginger extract (400 µg/ml). However, cells under induced hydrogen peroxide stress treated with ginger extract at 200 µg/ml, showed a marked decrease in AKT activation (Fig. 5).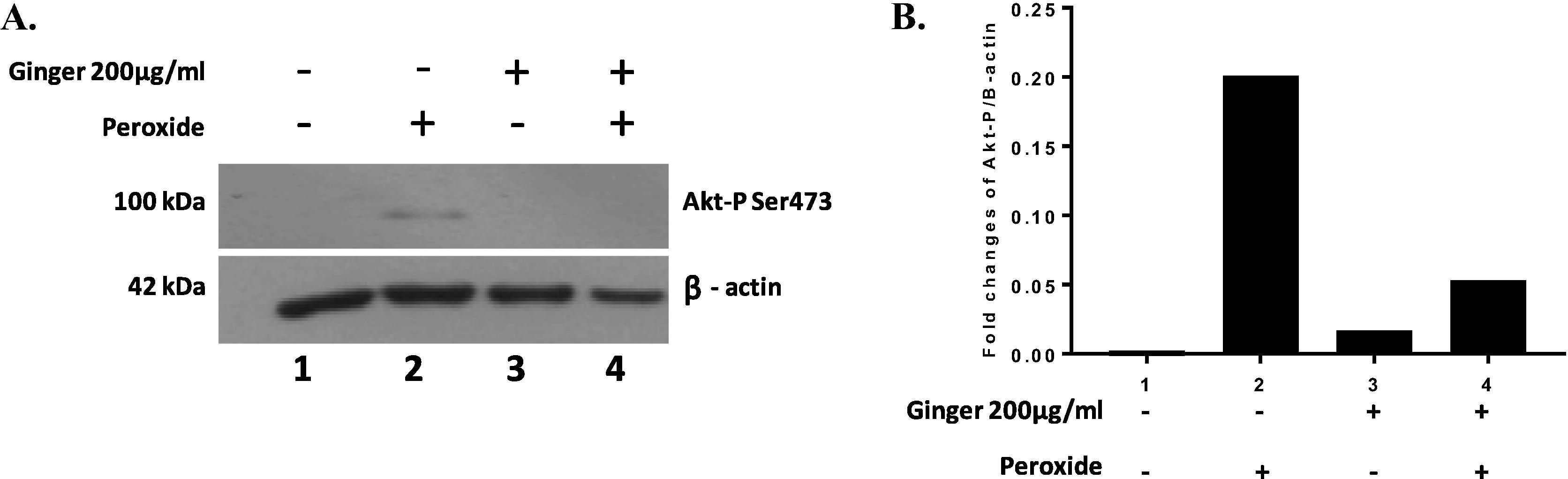
Akt phosphorylation at Ser473 in HT1080 cells treated with hydrogen peroxide and ginger extract, normalized to Beta Actin as a loading control. (A) Representative image of Western blot. (B) Densitometric analysis.
3.6 Morphological assessment
Morphological changes were observed, such as, cell detachment, cell shrinkage, and loss of cell filopodia. On the other hand, untreated cells appear elongated, smoothly attached to the tissue culture plate surface, and some cells were grouped together forming colonies (Fig. 6).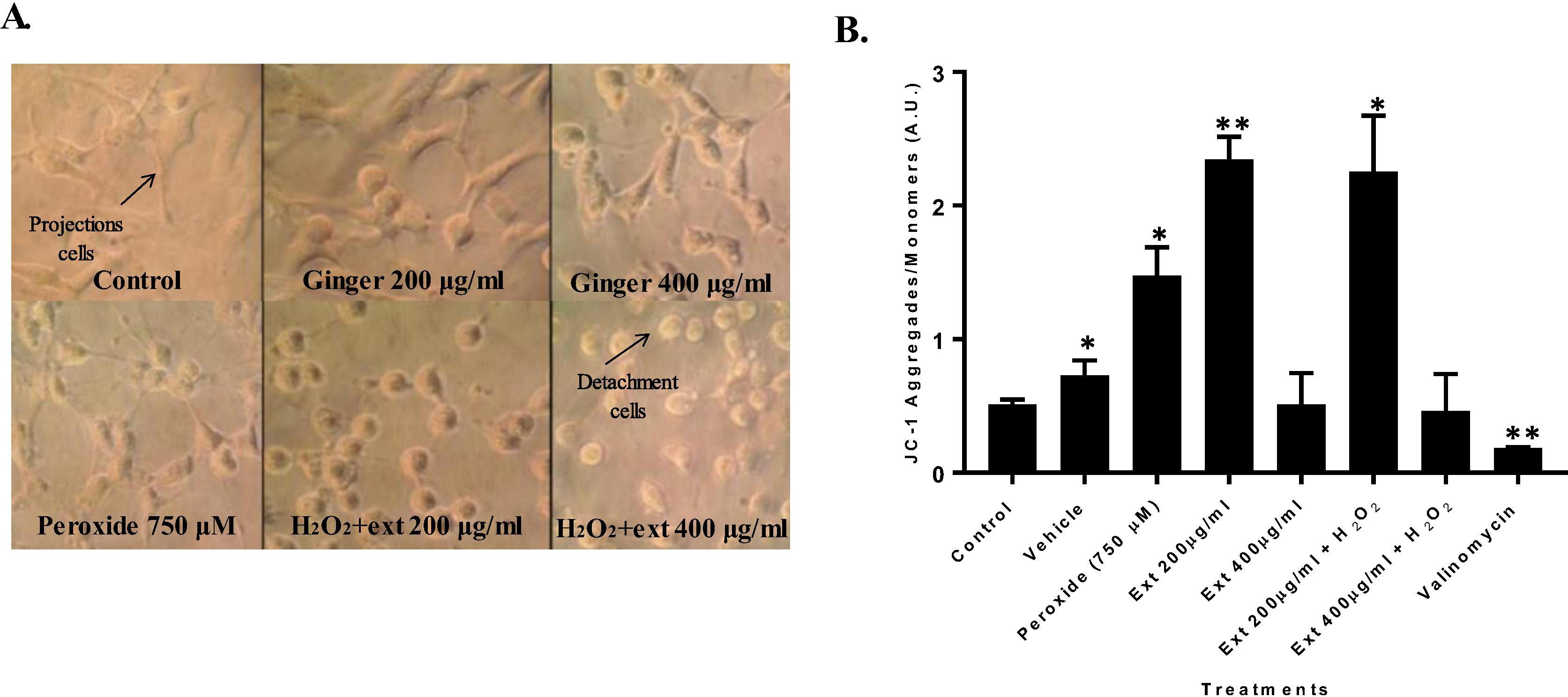
(A). HT1080 cell line morphological changes treated with peroxide and ginger extract. (B) Mean fluorescence intensity of HT1080 cell line treated with peroxide and ginger extract to evaluate mitochondrial membrane potential through flow cytometry with JC-1. (*p = 0.05, **p = 0.01).
3.7 Effect of ginger extract on mitochondrial membrane potential (ΔΨm)
A significant mitochondrial membrane potential hyperpolarization was observed for GE at 200 µg/ml (p < 0.002). Additionally, co-treatment of GE at 200 µg/ml with hydrogen peroxide also had significant mitochondrial membrane potential hyperpolarization compared with control. However, GE at 400 µg/ml or GE at this concentration with hydrogen peroxide treatment did not display differences compared to controls. Valinomycin treatment was used as depolarization positive control (Fig. 6B).
4 Discussion
This study revealed GE ability to reduce HT1080 cell viability. This effect has also been observed in other cell models (Akimoto et al., 2015). In colon cancer (HT29 cells), GE and combined with gelam honey inhibited tumor cells growth by inducing early apoptosis. In addition, expression of PI3K/AKT pathway was modulated. Moreover, inflammation was suppressed via NFκB pathway (Tahir et al., 2015). In Hep-2 cell GE reduced cell viability; this result was confirmed by protein and nucleic acid estimation, where increasing GE doses were related to DNA, RNA and protein reduction (Vijaya Padma et al., 2007). It is important to note, other studies with primary cultures have demonstrated GE oxidative stress-free antioxidant capacities (Akimoto et al., 2015).
The mechanism induced by ginger is not fully elucidated yet. On the one hand, it is believed ROS production is involved in decreasing of antioxidant compounds (reduced glutathione). On the other hand, it has been related to induce apoptosis through signaling pathways. Namely GE can induce caspase 3 activation, mTOR inhibition, and p53 protein activation. All of them related to induction of apoptotic processes (Zhang et al., 2012). It is known that 6-Shogaol -one of ginger’s constituents- is involved in apoptosis (Pan et al., 2008). Additionally, GE apoptosis capability through released caspases has not only been demonstrated in eukaryote cell models, but also in protozoan models (Arbabi et al., 2016), suggesting a conserved process. Since our results demonstrated GE capacity to reduce ROS, taking this evidence in consideration, GE or its compounds could represent novel chemoprotective alternative for pathologies associated with ROS production.
Another mechanism related to cell death is through necrosis. It has also been characterized by increased ROS production, affecting lipids in cell membranes and mitochondrial membrane potential (ΔΨm) describe by Arbabi et al. (2016). However, reports have described ginger ethanol extract necrosis induction to be rather insignificant (less than 5%), pointing to apoptosis as the principal process responsible for cell death (Arbabi et al., 2016).
In regard to Akt protein, its activation was observed under hydrogen peroxide induction. It is known that hydrogen peroxide can activate protein kinases such as Erk1/2, c-Jun and Akt, in a Ca2+ and PI3K (Crossthwaite et al., 2002). In this study, it was observed GE decreased H2O2 induced-AKT phosphorylation. It has been described ginger decreases AKT and ERK gene expression, which are overexpressed in tumor cells (Tahir et al., 2015). Ginger extract capacity to inhibit activation has been elucidated in other cell models, such as human gliobastoma cells (Ramachandran et al., 2015). Other tumor cells models, have shown the mechanism associated with apoptosis induction is through modulation of PI3K/AKT/mTOR genes. These genes produce progression of the autophagic processes mediated by mTOR protein dephosphorylation (Wee et al., 2015). mTOR is one of the signaling pathways frequently associated with various cancers, and it is a downstream target of PI3K/Akt (Khan et al., 2013). Mutations of genes, such as PIK3CA and PTEN of PI3KAkt-mTOR signaling pathway lead to cell proliferation and survival of colorectal cancer cells (Gulhati et al., 2009; Wu et al., 2013). Ginger is an inexpensive spice, worldwide readily available with multiple biological properties, thus it represents an important subject in cancer biology. Therefore, this study paves the way to understand ginger extract components effect at the molecular and cellular level in cancer cell lines. However, it would be desirable to perform these assays in primary cultures as well. Therefore, further studies by the Biochemical and Phytochemical groups at the Pontificia Universidad Javeriana will continue to shed light on these mechanims.
5 Conclusion
In general, data obtained in this study indicates GE or one of its components, induce intracellular changes, including mitochondrial membrane potential changes ΔΨm and signaling protein activation such as Akt, which could be implicated in early event promotion associated with apoptotic processes.
Responses evidenced in the presence of induced stress, suggest GE has a role in PI3K/AKT signaling pathway activation. Thus, this study showed a new approach to characterize intracellular changes elicited by GE. These mechanisms should be further explored for novel chemoprotective alternatives in pathologies associated with excessive ROS production.
Acknowledgements
This work was funded by Pontificia Universidad Javeriana-Bogotá (Project 7628).
Conflict of interest
There are no conflicts of interest.
References
- Anticancer effect of ginger extract against pancreatic cancer cells mainly through reactive oxygen species-mediated autotic cell death. PLoS ONE. 2015;10:e0126605.
- [Google Scholar]
- The MLN4924 inhibitor exerts a neuroprotective effect against oxidative stress injury via Nrf2 protein accumulation. Redox Biol.. 2016;8:341-347.
- [Google Scholar]
- The effect of ginger consumption on glycemic status, lipid profile and some inflammatory markers in patients with type 2 diabetes mellitus. Int. J. Food Sci. Nutr.. 2014;65:515-520.
- [Google Scholar]
- Ginger (Zingiber officinale) induces apoptosis in Trichomonas vaginalis in vitro. Int. J. Reprod. Biomed. (Yazd). 2016;14:691-698.
- [Google Scholar]
- Hydrogen peroxide-mediated phosphorylation of ERK1/2, Akt/PKB and JNK in cortical neurones: dependence on Ca(2+) and PI3-kinase. J. Neurochem.. 2002;80:24-35.
- [Google Scholar]
- Chen, H., Lv, L., Soroka, D., Warin, R.F., Parks, T.A., Hu, Y., Zhu, Y., Chen, X., Sang, S., 2012. Metabolism of [6]-shogaol in mice and in cancer cells. Drug metabolism and disposition: the biological fate of chemicals 40, 742-753.
- Introduction to Statistical Data Analysis for the Life Sciences. Boca Raton, FL: CRC Press, Taylor & Francis Group; 2015.
- Ewnetu, Y., Lemma, W., Birhane, N., 2014. Synergetic antimicrobial effects of mixtures of ethiopian honeys and ginger powder extracts on standard and resistant clinical bacteria isolates. Evidence-based complementary and alternative medicine: eCAM 2014, 562804.
- Cellular stress responses: cell survival and cell death. Int. J. Cell Biol.. 2010;2010:23.
- [Google Scholar]
- Targeted inhibition of mammalian target of rapamycin signaling inhibits tumorigenesis of colorectal cancer. Clin. Cancer Res. Official J. Am. Assoc. Cancer Res.. 2009;15:7207-7216.
- [Google Scholar]
- Targeting the PI3K-AKT-mTOR signaling network in cancer. Chin. J. Cancer. 2013;32:253-265.
- [Google Scholar]
- Determination of parameters for the supercritical extraction of antioxidant compounds from green propolis using carbon dioxide and ethanol as co-solvent. PLoS ONE. 2015;10:e0134489.
- [Google Scholar]
- Roles of oxidative stress in the development and progression of breast cancer. Asian Pac. J. Cancer Prev.: APJCP. 2014;15:4745-4751.
- [Google Scholar]
- 6-Shogaol induces apoptosis in human colorectal carcinoma cells via ROS production, caspase activation, and GADD 153 expression. Mol. Nutr. Food Res.. 2008;52:527-537.
- [Google Scholar]
- Strategies for reducing or preventing the generation of oxidative stress. Oxid. Med. Cell. Longevity. 2011;2011
- [Google Scholar]
- Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39-51.
- [Google Scholar]
- Inhibition of AKT signaling by supercritical CO2 extract of mango ginger (Curcuma amada Roxb.) in human glioblastoma cells. J. Complementary Integr. Med.. 2015;12:307-315.
- [Google Scholar]
- 6-Shogaol inhibits breast cancer cells and stem cell-like spheroids by modulation of notch signaling pathway and induction of autophagic cell death. PLoS ONE. 2015;10:e0137614.
- [Google Scholar]
- The PI3K/Akt pathway in tumors of endocrine tissues. Front. Endocrinol.. 2015;6:188.
- [Google Scholar]
- The effect of ginger (Zingiber officinale) on glycemic markers in patients with type 2 diabetes. J. Complementary Integr. Med.. 2015;12:165-170.
- [Google Scholar]
- Combined ginger extract & Gelam honey modulate Ras/ERK and PI3K/AKT pathway genes in colon cancer HT29 cells. Nutr. J.. 2015;14:31.
- [Google Scholar]
- Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol.. 2007;39:44-84.
- [Google Scholar]
- Vijaya Padma, V., Arul Diana Christie, S., Ramkuma, K.M., 2007. Induction of apoptosis by ginger in HEp-2 cell line is mediated by reactive oxygen species. Basic Clin. Pharmacol. Toxicol. 100, 302-307.
- Mechanism of chemoprevention against colon cancer cells using combined gelam honey and ginger extract via mtor and wnt/beta-catenin pathways. Asian Pac. J. Cancer Prev.: APJCP. 2015;16:6549-6556.
- [Google Scholar]
- Dysregulation and crosstalk of cellular signaling pathways in colon carcinogenesis. Crit. Rev. Oncol./Hematol.. 2013;86:251-277.
- [Google Scholar]
- 10-Gingerol, a Phytochemical Derivative from “Tongling White Ginger”, inhibits cervical cancer: insights into the molecular mechanism and inhibitory targets. J. Agric. Food Chem.. 2017;65:2089-2099.
- [Google Scholar]
- Zhang, F., Zhang, J.G., Qu, J., Zhang, Q., Prasad, C., Wei, Z.J., 2017b. Assessment of anti-cancerous potential of 6-gingerol (Tongling White Ginger) and its synergy with drugs on human cervical adenocarcinoma cells. Food and chemical toxicology: an international journal published for the British Industrial Biological Research Association.
- Zhang, S., Liu, Q., Liu, Y., Qiao, H., Liu, Y., 2012. Zerumbone, a Southeast Asian Ginger Sesquiterpene, Induced Apoptosis of Pancreatic Carcinoma Cells through p53 Signaling Pathway. Evidence-based complementary and alternative medicine: eCAM 2012, 936030.
- Dietary natural products for prevention and treatment of liver cancer. Nutrients. 2016;8:156.
- [Google Scholar]
- Zhou, Y., Zheng, J., Li, Y., Xu, D.P., Li, S., Chen, Y.M., Li, H.B., 2016. Natural Polyphenols for Prevention and Treatment of Cancer. Nutrients 8.







