Translate this page into:
Effect of fuel content on nonlinear optical and antibacterial activities of Zn/Cu/Al2O4 nanoparticles prepared by microwave-assisted combustion method
⁎Corresponding authors. kalamravi@gmail.com (B. Ravindran), balasubramani.ravindran@tdtu.edu.vn (B. Ravindran), rameshkumarevr@gmail.com (P. Rameshkumar)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Nonlinear optical nanoparticles of Zn/Cu/Al2O4 were synthesized by microwave-assisted combustion method with different fuel to oxidizer ratio. And, these nanoparticles were characterized by various analytical techniques to examine their suitability in fascinating applications. XRD results enunciate that the nanoparticle crystallizes in cubic spinel structure. The morphology and quantitative chemical compositions of Zn, Cu, Al and O elements were obtained from SEM with EDAX analysis. The prepared compound was confirmed by FTIR spectrum. The optical energy bandgaps are studied from UV–Vis absorption spectra. The luminescence emission peaks are observed at 371 nm (Violet), 425 nm (violet), 471 nm (blue) and 637 nm (red). In the application point of view from the Z-scan technique, the third order nonlinear optical parameters such as nonlinear refractive index (n2), nonlinear absorption coefficient (β), and third-order nonlinear susceptibility (χ3) of title compound are calculated. For biological application, the antibacterial activities were tested against Bacillus cereus, Staphylococcus aureus, Shigella flexneri, and Vibrio cholera bacterial species by measuring the inhibition zone. Existing results point out that the synthesized Zn/Cu/Al2O4 nanoparticles are possible candidates for photonics devices, optical switches, optical limiting and also for pharmaceutical applications.
Keywords
Zn/Cu/Al2O4 nanoparticles
Combustion method
Optical properties
Luminescence
Z-scan
Antibacterial activity
1 Introduction
At the moment, researchers working in the field of nanotechnology are focusing to find promising materials for plenty of applications like telecommunication, optoelectronics, biological and nonlinear optics (Rithesh Raj et al., 2015). Nonlinear optics has a great value and various applications in optical switching, image transmission, data storage and signal processing (Zhang et al., 2008). For the past two decades, number of studies was made on NLO properties of novel materials because of its vital applications (Zhang and Wang, 2017). Recent research on nonlinear optics directs on nanomaterial’s that will be used to fabricate advanced novel optical devices with minimum expense and more efficiency (Yugandhar et al., 2017). Many earlier findings revealed the structural and physical characteristics articulated by ternary metal oxide complex with a spinel structure (Mao et al., 2005). These spinel structure compounds showed high thermal and chemical stability and low temperature sinterability. Also, showed low surface acidity, high quantum yields, better diffusion and hydrophobic behavior gained much more attention in last two decades (Zawadzki, 2006). The spinels are class of binary transition metal oxides of the form AB2O4 with varied structural and potential uses in applications in the field of materials science (Zhang et al., 2011). Among the various spinel structures, zinc aluminate (ZnAl2O4) has multidisciplinary applications like optical materials, electrical, catalyst support, ceramic materials and optical coating or host matrix (Pathak et al., 2014). ZnAl2O4 is a direct wide bandgap semiconductor (3.8 eV), which is used for wide range of applications especially in optoelectronic devices that are operated in the UV region (Wang et al., 2005). Zinc aluminate nanoparticles can be prepared by variety of methods as given by (Cornu et al., 2014). Compared to other methods, one interesting and novel technique is the microwave-assisted combustion method to synthesize nanomaterials. This method is eco-friendly, very high reaction rate, energy saving method, rapid heating and very short reaction time. Investigation about large nonlinear optical properties of these spinel structure nanomaterials is still one of the important works concerning nonlinear optical applications (Panoiu et al., 2018).
Hence, microwave-assisted combustion method was used to synthesize Zn/Cu/Al2O4 nanoparticles with different fuel to oxidizer ratio. These synthesized nanoparticles were characterized by various analytical methods and also tested antibacterial activity. The novelty of this work is to determine the nonlinear properties of Zn/Cu/Al2O4 nanoparticles in order to confirm their suitability in optical limiting device and also to test the antibacterial activity against foodborne pathogens.
2 Experimental procedures
2.1 Materials
Starting metal precursors which are used in this experiment are Zinc nitrate hexahydrate (Zn (NO3)2·6H2O), Copper nitrate hexahydrate (Cu(NO3)2·6H2O), and Aluminum nitrate monohydrate (Al (NO3)3·9H2O) which act as oxidizer and glycine (NH3 COOH CH2) as a fuel.
2.2 Methods
The Zn0.96Cu0.04Al2O4 nanoparticles were prepared via microwave-assisted combustion method with various fuel to oxidizer ratio such as, stoichiometric (S), fuel lean (L), and rich (R). The constituents in the desired fuel to oxidizer ratio in stoichiometric conditions were suspended in double distilled water (15 mL). The mixture was vigorously stirred at 90 °C for 30 mins until it forms a highly viscous gel, then it was kept in a microwave oven with the output power of 800 Watts. In the early stage, the gel was effervescence and burst into flames with enormous amount of gases which result in yielding of foamy and porous structure. Finally, the yield was collected and calcination was done at 700 °C for 6 h. The same experimental procedure was continued for the remaining fuel to oxidizer ratios like fuel lean and rich.
2.3 Combustion mechanism
Bipropellant chemistry, microwave-assisted combustion synthesis relies on an exothermic redox reaction between glycine and metal nitrate. In this combustion process, metal nitrates like Zn, Cu and Al nitrate function as oxidizers, whereas glycine functions as a reducer. Typically, valency for the elements are Zinc (+2), Copper (+2), Aluminum (+3), Hydrogen (+1), Carbon (+4) and Oxygen (-2). Generally, element nitrogen is assumed to be zero. Hence, equivalent capacity are Zinc nitrate (−10), Copper nitrate (−10), Aluminum nitrate (−15) and glycine (+9). For a stoichiometric ratio, we need 0.96 (−10) + 0.04 (−10) + 2 (−15) + n (+9) = 0, which provides n = 4.44 mol of glycine. Jain et al. (1981) have calculated the nitrate to glycine ratio which is represented by
using the relation,
The coefficients of reducing element for the oxidizer to fuel ratios are for fuel lean, for fuel rich and for stoichiometric. The combustion reaction to prepare Zn0.96Cu0.04Al2O4 reaction is expressed as follows.
1. Fuel lean (
= 0.5, Fuel/Nitrate ratio = 0.74)
It shows that more amount of oxygen released to the surrounding environment.
2. Fuel stoichiometric (
= 1, Fuel/Nitrate ratio = 1.48)
From the reaction, it is very clear that oxidizing fuel reacts with the oxygen from metal nitrate completely and for the complete reaction, no heat exchange is generally required.
3. Fuel rich (
= 1.5, Fuel/Nitrate ratio = 2.22)
It is very clear, to finish the combustion process, further oxygen is needed from the atmosphere.
2.4 Instrumentation
The PXRD analysis was performed to characterize the synthesized nanoparticles using XPERTPRO X-ray powder diffractometer system. The 2θ range analyzed between 10°-80°, the scan speed was set as 0.1 min−1 The morphologies were observed by SEM (Make: CAREL ZEISS, Model: EVO 18). FTIR spectra were analyzed using a Perkin–Elmer spectrophotometer in the range of 400–4000 cm−1 at room temperature. The linear optical absorption and electronic transition of the nanoparticles were investigated using a UV–Visible spectrophotometer (scan range: 200 to 800 nm). The luminescence spectra was recorded in the spectrofluorophotometer (shimadzu/RF 6000).
2.5 Z-Scan measurement
In this experiment, Zn0.96Cu0.04Al2O4 nanoparticles were investigated for third order nonlinear optical properties using CW laser (532 nm, 100 mW) as excitation source. Initially, the prepared Zn0.96Cu0.04Al2O4 nanoparticles were dispersed in ethylene glycol by ultra-sonication with transmittance nearly 65%. The dispersed solution was taken in 1 mm cuvette and was mounted on a translation stage that was slowly shifted into the laser propagation pathway. The corresponding far field transmittance was observed by a photodetector.
2.6 Antibacterial activity
Nanoparticles were subjected for antimicrobial activity studies using disc diffusion method (Vijayalakshmi and Dhanasekaran, 2018) against various bacterial pathogens on Mueller Hinton agar plate. The medium that supports the growth inhibition of bacterial cultures were maintained at 37 °C using an incubator. The sterilized petri dish that was used in the experiment was inhibited at 120 °C for 30 mins. The synthesized nanomaterial was dispersed in deionized water. The dispersed solution was loaded on sterile dishes on Mueller Hinton agar plate seeded with bacteria. The petri plates were incubated for 18 h. After 18 h, the results were observed and the diameter of the zone was measured (mm).
3 Results and discussions
3.1 PXRD analysis
The XRD patterns obtained for the Zn0.96Cu0.04Al2O4 nanoparticles are described in Fig. 1 (a, b, c). It is clear that the diffraction peaks corresponding to the crystallography planes of (2 2 0), (3 1 1), (4 0 0), (3 3 1), (4 2 2), (5 1 1), (4 4 0), (6 2 0), and (5 3 3) are in accordance with JCPDS data (Card No:73-1961). These characters confirmed the presence of cubic spinel structure. However, PXRD pattern did not show any secondary phase diffraction peaks. Debye-Scherrer’s formula was used for the determination of the mean crystallite size (D) as suggested by Pathak et al. (2016).
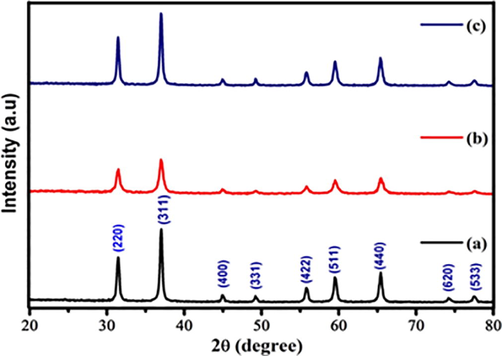
The synthesis Zn0.96Cu0.04Al2O4 nanoparticles for different fuel ratios (a) Fuel lean, (b) Fuel stoichiometric (c) Fuel rich: (1) XRD pattern.
3.1.1 Williamson - Hall analysis
Williamson-Hall (W-H) equation, to calculate the lattice strain is,
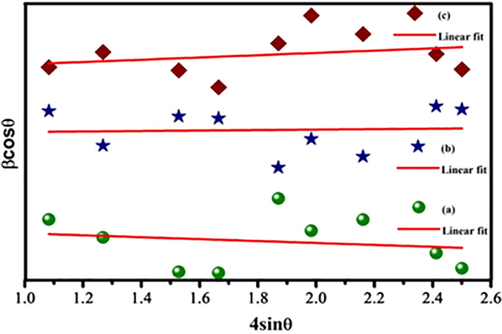
The synthesis Zn0.96Cu0.04Al2O4 nanoparticles for different fuel ratios (a) Fuel lean, (b) Fuel stoichiometric (c) Fuel rich: (2) W-H plots.
3.2 Morphological studies
The morphology of the nanoparticles are analyzed using SEM and are shown in Fig. 3 (a, b, c). The nanoparticles are accompanied by agglomeration and coalescence. In Fig. 3a, it is seen that cubic structure is formed with some nanoparticles deposited on the structure. In Fig. 3b, cubic spinel structure is obtained with some agglomeration. Fig. 3cshows the spongy network structureformed in the nanoparticles. SEM images, reveals slight morphological difference in different size agglomerates covered by smaller particlesdue to selection of fuel.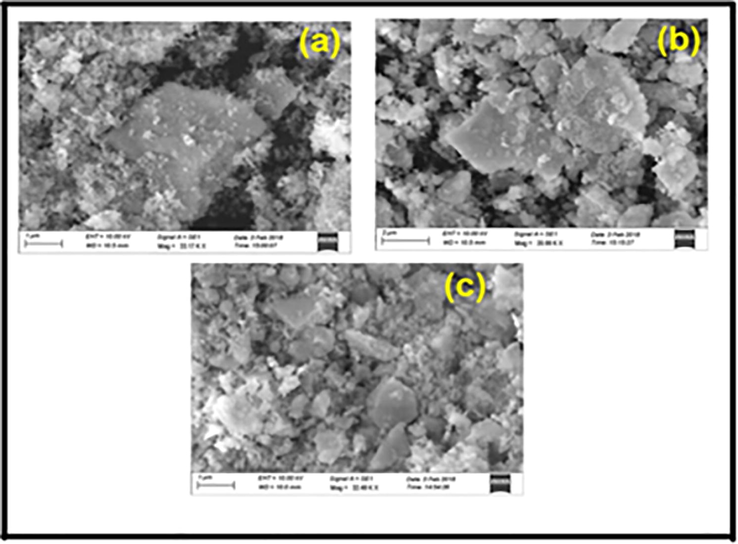
The synthesis Zn0.96Cu0.04Al2O4 nanoparticles for different fuel ratios (a) Fuel lean, (b) Fuel stoichiometric (c) Fuel rich: (3) SEM images.
3.3 Compositional analysis
EDAX was carried out to confirm the elemental composition in the synthesized nanoparticles for fuel (L, S & R) is depicted in the Fig. 4 (a, b, c). The corresponding peaks of the elements O, Al, Zn and Cu were determined for the synthesized nanoparticles. The elements are well distributed and without any characteristic impurity peak indicating the purity of prepared materials. The content of Zn, Cu, Al and O differs with fuel to oxidizer ratio. Moreover, the atomic ratio significantly decreases with increase in the fuel to oxidizer ratio.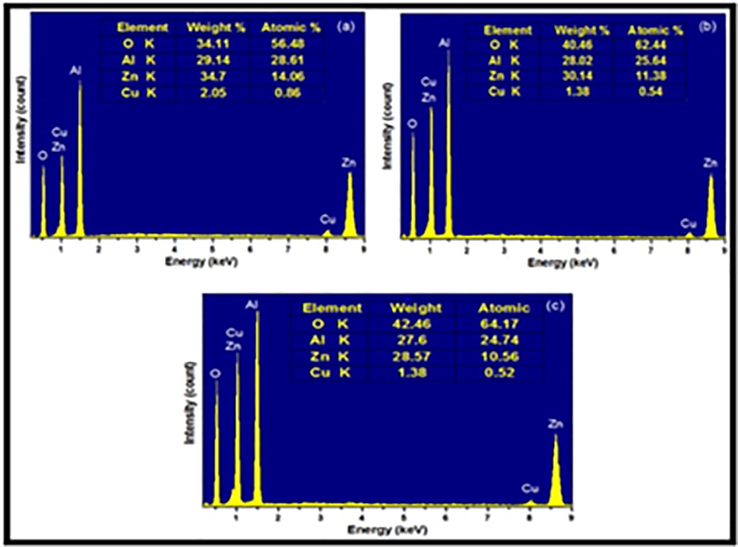
The synthesis Zn0.96Cu0.04Al2O4 nanoparticles for different fuel ratios (a) Fuel lean, (b) Fuel stoichiometric (c) Fuel rich: (4) EDAX spectra.
3.4 FTIR analysis
The chemical bonding and functional groups of synthesized nanoparticles were identified by FTIR spectrum. The infrared spectra of the prepared sample are depicted in Fig. 5 (a, b, c). The absorption band at 3443 cm−1 and 1633 cm−1 could be assigned to O–H stretching vibration due to water (H2O) adsorption (Theophil Anand et al., 2019). A band observed at 2924 cm−1 could assign to C–H stretching vibration. Also observed a band at the wave number 2340 cm−1 could attributed to stretching vibration modes of CO2 molecule in the air. The peak at 1712 cm−1 is due to the C = O carbonyl group. In this present work, metal–oxygen stretching frequencies appear as three main peaks between 400 and 800 cm−1. The bands observed at 667 cm−1, 558 cm-1and 501 cm−1 are mainly attributed to intrinsic metal stretching vibration of the
siteand stretching
site (Ahmed et al., 2011). These bands in the formation of crystallite phase spinel structure which is in accordance with the XRD results.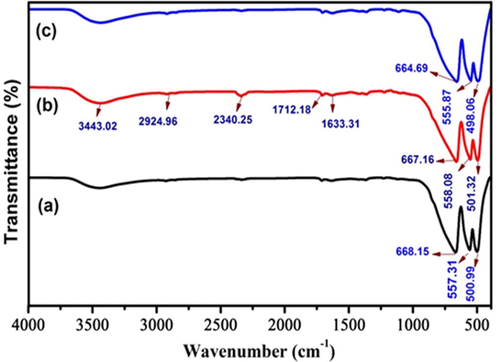
The synthesis Zn0.96Cu0.04Al2O4 nanoparticles for different fuel ratios (a) Fuel lean, (b) Fuel stoichiometric (c) Fuel rich: (5) FTIR spectra.
3.5 Optical studies
The optical absorption spectra are shown in Fig. 6a for the prepared nanoparticles have maximum absorption edge around 331 nm (L), 335 nm (S) and 337 nm (R). There are small variations in the absorption edges and also intensity of edges increase with increase in F/O ratios of glycine. The optical energy bandgap were calculated by the Tauc’s equation (Bhaumik et al., 2014),
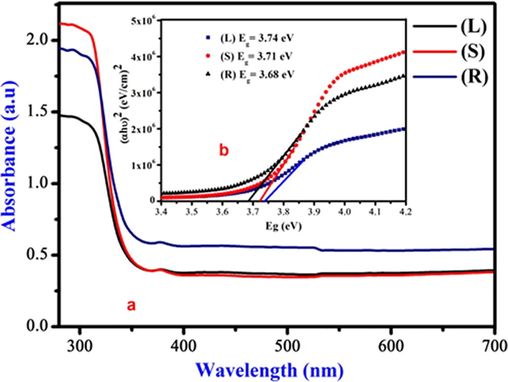
The synthesis Zn0.96Cu0.04Al2O4 nanoparticles for different fuel ratios (a) Fuel lean, (b) Fuel stoichiometric (c) Fuel rich: (6a) UV–Vis absorption spectra (6b) Optical bandgap.
3.6 Luminescence analysis
Luminescence spectra analysis was performed and the results were shown in Fig. 7. The entire sample exhibiting emission peaks at 371, 425, 471 and 637 nm are found in the luminescence spectra under the excitation wavelength of λexc = 330 nm. In this study, violet emission peaks were observed at 371 and 425 nm and this characteristic emission peaks were previously determined by Geetha Devi and Sakthi Velu (2016). The blue emission peaks were observed near 471 nm and this kind of blue emission peak was previously reported by Vijayaprasath et al. (2015). A red emission peak was observed at 637 nm mainly due to surface state and deep trap emission. The luminescence emission intensity is observed to significantly decrease with high increase in fuel to oxidizer ratio and there is also a slight peak shift as depicted in the spectrum. The luminescence emission peak shift is due to the variation position of the defect levels within the bandgap. These results indicate that, it has a potential application in monochromatic laser and other optoelectronic devices (Karthik et al., 2017).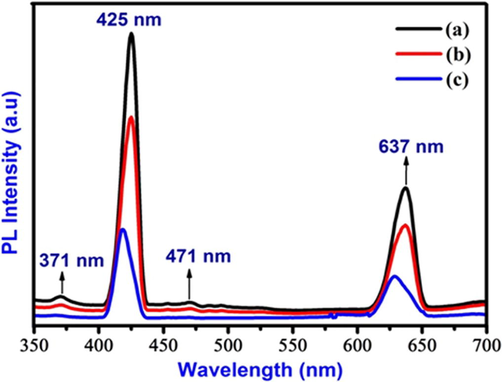
The synthesis Zn0.96Cu0.04Al2O4 nanoparticles for different fuel ratios (a) Fuel lean, (b) Fuel stoichiometric (c) Fuel rich: (7) Emission spectrum.
3.7 Z-Scan analysis
This technique has been developed by Sheik-bahae et al. (1989) to determine both the nonlinear absorption coefficient and nonlinear refractive index for the prepared nanoparticles. The recorded closed aperture and open aperture Z-Scan curves are described in Fig. 8a and b respectively. In this study, open aperture Z-Scan curves were determined. This observation revealed that the sample exhibit the self-defocusing nonlinearity and reverse saturable absorption (RSA). The nonlinear optical parameters are calculated using standard relations (Yugandhar et al., 2017). The third order nonlinear optical susceptibility
can be estimated using,
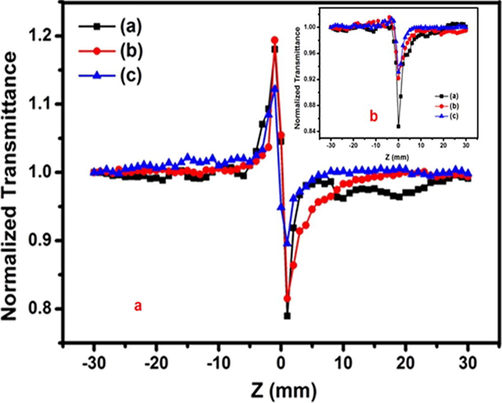
The synthesis Zn0.96Cu0.04Al2O4 nanoparticles for different fuel ratios (a) Fuel lean, (b) Fuel stoichiometric (c) Fuel rich: (8a) Closed aperture (8b) Closed aperture Z-scan analysis.
Hence,
is the permittivity of vacuum (8.854 × 10−12F/m),
is the linear refractive index of the nanomaterial and c is the velocity of the light in vacuum (3
108 m/s). Then, the third order nonlinear optical susceptibility
of the prepared nanoparticles can be calculated from
The analyzed parameters of third-order nonlinear optical nonlinearity are described in Table 1. The open aperture Z-Scan curves of this study clearly illustrate decrease in transmission with significant increase in the input intensity. This finding indicates the availability of RSA and has been previously reported by Henari (2001). The obtained results confirm that Zn0.96Cu0.04Al2O4 nanoparticles are suitable material for optical limiting device applications.
Parameters
Fuel Lean
Fuel Stoichiometric
Fuel Rich
Linear absorption coefficient (α)
85.441
81.756
75.999
Linear refractive index
1.3602
1.3526
1.3412
Nonlinear refractive index
2.4615
2.1503
1.4224
Nonlinear absorption coefficient (β)
cm/W
1.2949
0.6632
0.5803
Real part
esu
1.1557
0.9984
0.6493
Imaginary part of
esu
2.5752
1.3042
1.1221
Third order nonlinear susceptibility
esu
1.1840
1.0068
0.6589
3.8 Antibacterial activity
The synthesized nanoparticles were evaluated against foodborne bacterial pathogens such as, Staphylococcus aureus, Bacillus cereus, Shigella flexneri, Vibrio cholerae bacteria. Nanoparticles showed activity against these bacterial pathogens (Fig. 9). Photograph of antibacterial activity of Zn0.96Cu0.04Al2O4 nanoparticles is shown in Fig. 10. By the formation of zone of inhibition, the results prove that the samples have good antibacterial activity. The important mechanism of metal oxide in antimicrobial property is the disruption of cell wall by various mechanisms, including generation of reactive oxygen species (ROS) which causes cell disruption (Karthik et al., 2019a).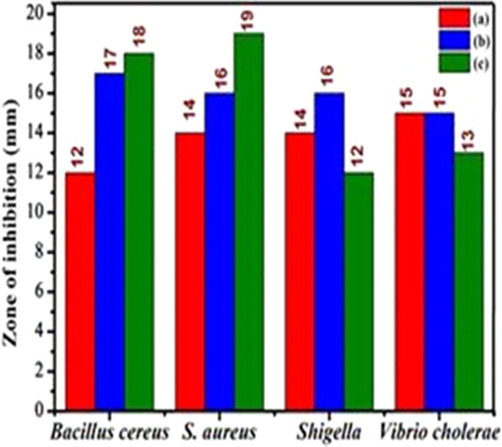
The antibacterial activity of synthesized nanoparticles for different fuel ratios (a) Fuel lean, (b) Fuel stoichiometric (c) Fuel rich: (9) zone of inhibition.
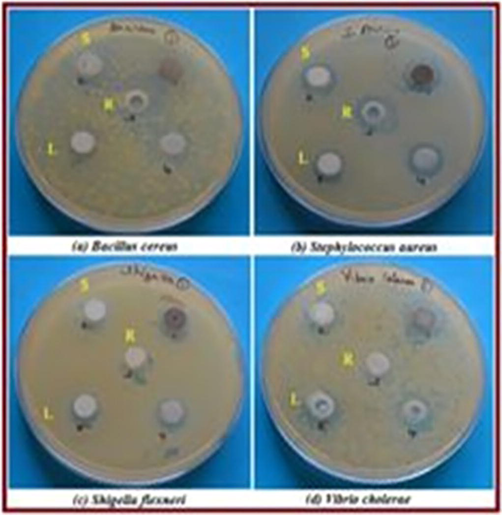
The antibacterial activity of synthesized nanoparticles for different fuel ratios (a) Fuel lean, (b) Fuel stoichiometric (c) Fuel rich: (10) Antibacterial activity plate photos and.
Fig. 11 illustrates the antibacterial mechanism of Zn/Cu/Al2O4 nanoparticles. The process involved attraction of positive charged heavy metal ions (Zn2+, Cu2+ and Al3+) with negative charged cell wall and penetrate into the cell wall. Certain bacteria produce ROS production in host organism, including human and may cause cancer (Sadiq et al., 2009). Zn/Cu/Al2O4 nanoparticles have the capacity to inhibit the production of ROS in bacteria and protect mammalian cell damage (Parham et al., 2016). Hence, the synthesized nanoparticles are efficient material against the foodborne pathogens which are suitable for medical devices and paramedical applications (Karthik et al., 2019). The prepared Zn/Cu/Al2O4 nanoparticles have potential antibacterial activity than other metal oxides (Table 2).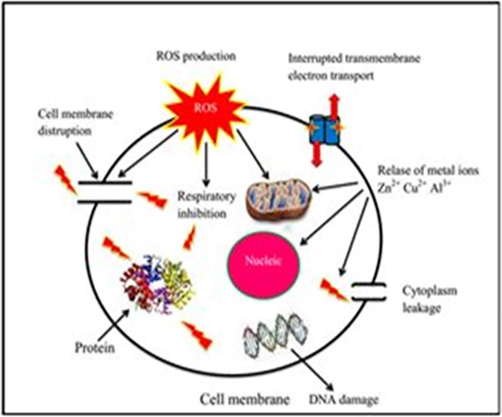
The antibacterial activity of synthesized nanoparticles for different fuel ratios (a) Fuel lean, (b) Fuel stoichiometric (c) Fuel rich: (11) antibacterial mechanism.
Bacteria species
Materials
Zone of inhibition (mm) ± standard deviation
References
Staphylococcus aureus
NiO
10
(Karthik et al., 2018)
Al2O3
12
(Manyasree et al., 2018)
MgO
12 ± 0.10
(EL-Moslamy et al., 2018)
CuO
9 ± 0.8
(Yugandhar et al., 2017)
ZnO
11 ± 0.23
(Zare et al., 2019)
Ni-ZnO
12
(Brintha and Ajitha, 2016)
Al-ZnO
12
(Brintha and Ajitha, 2016)
NiFe2O4
6.4
(Allafchian et al., 2016)
NiFe2O4@Ag
12.5
(Allafchian et al., 2016)
Zn/Cu/Al2O4
19
Present work
Bacillus cereus
MgO
15 ± 0.03
(EL-Moslamy et al., 2018)
Ni-ZnO
8
(Brintha and Ajitha, 2016)
Al-ZnO
12
(Brintha and Ajitha, 2016)
NiFe2O4
6.4
(Allafchian et al., 2016)
NiFe2O4@Ag
11.5
(Allafchian et al., 2016)
Zn/Cu/Al2O4
18
Present work
Vibrio cholera
ZrO2
12
(Karthik et al., 2019c)
CdO
12
(Karthik et al., 2017)
CdO-MgO
12
(Karthik et al., 2019a)
Zn/Cu/Al2O4
15
Present work
Shigellaflexneri
Ta2O5
10 ± 0.2
(Nagaraju et al., 2019)
MgO
10 ± 2.3
(EL-Moslamy et al., 2018)
TiO2
12
(Karthik et al., 2019d)
Zn/Cu/Al2O4
16
Present work
4 Conclusion
In our study, Zn0.96Cu0.04Al2O4 eco-friendly microwave-assisted combustion method was used for the synthesis of nanoparticles. These were characterized by various analytical techniques and antibacterial activities were assayed. The synthesized nanoparticles showed cubic spinel structure. The morphological and elemental composition was confirmed by SEM and EDAX. The optical bandgap is found to be 3.74 eV (L), 3.71 eV (S) and 3.68 eV (R) with absorption wavelength at 331 (L), 335 (S) and 337 nm (R) respectively. The luminescence nature of the nanoparticles was studied by luminescence spectra which can be effectively used in monochromatic laser and also in optoelectronic devices. The third order NLO properties results enunciate that the sample can be used in nonlinear optical limiting device applications. The sample inhibits the growth of various foodborne pathogens and exhibits potential activity against both Gram-positive and Gram-negative bacteria. The above experimental result reveals that, Zn0.96Cu0.04Al2O4 nanoparticles provide potential applications in photonic devices and antibacterial activity which restricts the selected bacterial growth confirms the suitability in pharmaceutical applications.
Acknowledgements
One of the authors A. Lakshmanan wish to thank the UGC-RGNF [F1-17.1/2016-17/RGNF-2015-17-SC-TAM-21802] and P. Surendran is grateful to UGC-NFHE [F1-17.1/2015-16/NFST-2015-17-ST-TAM-1335] New Delhi, India, for the financial support. G. Ramalingam acknowledges financial support from RUSA 2.0, Grant/Award Number: RUSA 2.0 grant No.F.24‐51/2014‐U, Policy (TN Multi-Gen) & DST-SERB/2016/198 for instrumentation facility used at Alagappa University, Karaikudi. The authors extend their appreciation to The Researchers supporting project number (RSP-2019/108) King Saud University, Riyadh, Saudi Arabia. This work was supported by the Korea Institute of Energy Technology Evaluation and Planning(KETEP) and the Ministry of Trade, Industry & Energy(MOTIE) of the Republic of Korea (No. 20194110300040).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Preparation and characterizations of polyaniline (PANI)/ZnO nanocomposites film using solution casting method. Thin Solid Films.. 2011;519:8375-8378.
- [Google Scholar]
- Preparation, characterization, and antibacterial activity of NiFe2O4/PAMA/Ag–TiO2 nanocomposite. J. Magn. Magn. Mater.. 2016;404:14-20.
- [Google Scholar]
- Lattice strain estimation for CoAl2O4 nano particles using Williamson-Hall analysis. J. Alloys Compd.. 2016;676:606-612.
- [Google Scholar]
- Significant enhancement of optical absorption through nano-structuring of copper based oxide semiconductors: Possible future materials for solar energy applications. Phys. Chem. Chem. Phys.. 2014;16:11054-11066.
- [Google Scholar]
- Synthesis, Structural and Antibacterial Activity of Aluminium and Nickel Doped ZnO Nanoparticles by Sol-gel Method. Asian J. Chem. Sci.. 2016;1:1-9.
- [Google Scholar]
- Luminescence switch of Mn-Doped ZnAl2O4powder with temperature. J. Mater. Chem. C.. 2014;2:9512-9522.
- [Google Scholar]
- Bioprocessing strategies for cost-effective large-scale biogenic synthesis of nano-MgO from endophytic Streptomyces coelicolor strain E72 as an anti-multidrug-resistant pathogens agent. Sci. Rep.. 2018;8:3820.
- [Google Scholar]
- Structural, optical and photoluminescence properties of copper and iron doped nanoparticles prepared by co-precipitation method. J. Mater. Sci. Mater. Electron.. 2016;27:10833-10840.
- [Google Scholar]
- Optical switching in organometallic phthalocyanine. J. Opt. A: Pure Appl. Opt.. 2001;3:188-190.
- [Google Scholar]
- A new approach to thermochemical calculations of condensed fuel-oxidizer mixtures. Combust. Flame. 1981;40:71-79.
- [Google Scholar]
- Ultrasonic-assisted CdO–MgO nanocomposite for multifunctional applications. Mater. Technol.. 2019;34:403-414.
- [Google Scholar]
- Fabrication of MgO nanostructures and its efficient photocatalytic, antibacterial and anticancer performance. J. Photochem. Photobiol. B Biol.. 2019;190:8-20.
- [Google Scholar]
- Microwave-Assisted ZrO2 Nanoparticles and Its Photocatalytic and Antibacterial Studies. J. Clust. Sci.. 2019;30:311-318.
- [Google Scholar]
- Multifunctional Applications of Microwave-Assisted Biogenic TiO2 Nanoparticles. J. Clust. Sci.. 2019;30:965-972.
- [Google Scholar]
- Nanostructured CdO-NiO composite for multifunctional applications. J. Phys. Chem. Solids. 2018;112:106-118.
- [Google Scholar]
- Andrographis paniculata extract mediated green synthesis of CdO nanoparticles and its electrochemical and antibacterial studies. J. Mater. Sci. Mater. Electron.. 2017;28:7991-8001.
- [Google Scholar]
- Synthesis, Characterization and Antibacterial Activity of Aluminium Oxide Nanoparticles. Int. J. Pharm. Pharm. Sci.. 2018;10:32-35.
- [Google Scholar]
- Synthesis of classes of ternary metal oxide nanostructures. Chem. Commun.. 2005;46:5721-5735.
- [Google Scholar]
- Mobeen, A., Maria Magdalane, C., Jasmine Shahina, S.K., Lakshmi, D., Sundaram, R., Ramalingam, G., Raja, A., Madhavan, J., Letsholathebe, D., Bashir, A.K.H., Maaza, M., Kaviyarasu, K., 2019. Investigation on antibacterial and photocatalytic degradation of rhodamine-B dye under visible light irradiation by titanium molybdate nanoparticles prepared via microwave method. Surfaces and Interfaces 100381.
- Ultrasound-assisted Ta2O5nanoparticles and their photocatalytic and biological applications. Microchem. J.. 2019;147:749-754.
- [CrossRef] [Google Scholar]
- Antimicrobial treatment of different metal oxide nanoparticles: a critical review. J. Chinese Chem. Soc.. 2016;63:385-393.
- [Google Scholar]
- Photoluminescence and EPR studies on Fe3+ doped ZnAl2O4: an evidence for local site swapping of Fe3+ and formation of inverse and normal phase. Dalt. Trans.. 2014;43:9313-9323.
- [Google Scholar]
- Effect of fuel content on luminescence and antibacterial properties of zinc oxide nanocrystalline powders synthesized by the combustion method. RSC Adv.. 2016;6:97770-97782.
- [Google Scholar]
- Surface plasmon resonance based fiber optic dopamine sensor using green synthesized silver nanoparticles. Sensors Actuators, B Chem.. 2015;224:600-606.
- [Google Scholar]
- Antimicrobial sensitivity of Escherichia coli to alumina nanoparticles. Nanomedicine Nanotechnology. Biol. Med.. 2009;5:282-286.
- [Google Scholar]
- Green synthesis of ZnO nanoparticle using Prunus dulcis (Almond Gum) for antimicrobial and supercapacitor applications. Surfaces and Interfaces.. 2019;17:100376
- [Google Scholar]
- Growth and characterization study of γ-glycine crystal grown using different mole concentrations of zinc sulphate as structure-directing agents. J. Cryst. Growth. 2018;498:372-376.
- [CrossRef] [Google Scholar]
- Enhancement of ferromagnetic property in rare earth neodymium doped ZnO nanoparticles. Ceram. Int.. 2015;41:10607-10615.
- [Google Scholar]
- Synthesis and photoluminescence characteristics of Dy3+-doped ZnAl2O4 nanocrystals via a combustion process. J. Alloys Compd.. 2005;394:255-258.
- [Google Scholar]
- Structural, magnetic, elastic, dielectric and electrical properties of hot-press sintered Co1−xZnxFe2O4 (x = 0.0, 0.5) spinel ferrite nanoparticles. J. Magn. Magn. Mater.. 2018;447:48-57.
- [Google Scholar]
- Bioinspired green synthesis of copper oxide nanoparticles from Syzygiumalternifolium (Wt.) Walp: characterization and evaluation of its synergistic antimicrobial and anticancer activity. Appl. Nanosci.. 2017;7:417-427.
- [Google Scholar]
- Novel green biomimetic approach for synthesis of ZnO-Ag nanocomposite; antimicrobial activity against food-borne pathogen, biocompatibility and solar photocatalysis. Sci. Rep.. 2019;9:8303.
- [Google Scholar]
- Synthesis of nanosized and microporous zinc aluminate spinel by microwave assisted hydrothermal method (microwave-hydrothermal synthesis of ZnAl2O4) Solid State Sci.. 2006;8:14-18.
- [Google Scholar]
- Modulation of third-order nonlinear optical properties by backbone modification of polymeric pillared-layer heterometallic clusters. Adv. Mater.. 2008;20:1870-1875.
- [Google Scholar]
- First-principles study of the structural, mechanical and electronic properties of ZnX2O4 (X=Al, Cr and Ga) Chin. Phys. B.. 2011;20:047102
- [Google Scholar]
- Nonlinear optical properties of metal nanoparticles: A review. RSC Adv.. 2017;7:45129-45144.
- [Google Scholar]







