Translate this page into:
Effect of emergence time on some biological aspects of Trichogramma evanescens (Westwood) (Hymenoptera: Trichogrammatidae)
⁎Corresponding authors. elkazafi.taha@agr.kfs.edu.eg (El-Kazafy A. Taha), said19832007@yahoo.com (El-Said M. Elnabawy)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objective
Trichogramma species are minute endoparasitoids of insect eggs that are considered one of the most efficient natural enemies due to their vital role against lepidopteran pests. This experiment was achieved to evaluate the effect of the emergence time of T. evanescens (Westwood) on the emergence rate, % females, % males, sex ratio, longevity, and the emergence of first generation (F1).
Methods
Mass rearing of Sitotroga cerealella and T. evanecsens was performed and the previous parameters were investigated throughout an emergence period extended to 16 hrs.
Results and conclusion
The emergence time has a clear impact on the tested biological parameters. The highest values of % emergence of T. evanecsens was obtained at the 7th hrs of emergence. The female percentage increased gradually to achieve the highest rate (63.58%) at the 3rd hrs, then it declined gradually to record the lowest rate (50.00%) at the 15th hrs and 16th hrs of emergence. A significant decrease in the female numbers was started after 8th hrs. There were significant negative correlations between the emerging time and each of emergence percentages and the % female. There was a negative correlation between the emerging time and longevity of T. evanescens. The longevity was 3.06 days for the parasitoid wasp adults emerged at the 1st hr and enhanced to be 3.19 days at the 3rd hrs. Also, there was a negative correlation between the emerging time and the emerging percentages of F1. Finally, the impact of developmental time on the fitness of female wasps proposes that the high quality of T. evanescens could be enlarged by enhancing the percentage of the individuals emerging early at the 7th hrs of emergence.
Keywords
Biological control
Egg parasitoids
Parasitism
F1 progeny
Sex ratio
1 Introduction
Biological control plays an important role in suppressing various insect pest populations in different crops and forests. The wasps of Trichogramma spp. are considered as one of the most efficient natural enemies widely all over the world, due to easy mass rearing and the ability to decline the population of many insect pests (Li, 1994; Shawer et al., 2021), particularly, lepidopterous insects during the egg stage (Smith, 1996; Shawer et al., 2021). Many factors may influence parasitoid’s fitness, such as availability of food (El-Nabawy et al., 2015) and the female body size (Boivin and Lagacé, 1999). The early adult females were more fecund and larger in body size than the late ones (Doyon and Boivin, 2005). Also, van den Assem et al. (1989) have found that the body size of the adult parasitoid has an impact on its reproductive success. Previous research has shown that the body size of Trichogramma females have an impact on longevity, searching ability, fertility, and the ability to find the hosts. In addition, the body size of Trichogramma males can affect longevity, the numbers of mating, and the ability to find females (Godfray, 1994). Trichogramma species body size seems to be strongly related to their performance (McDougall and Mills, 1997). The emergence percentage of some insects and their developmental averages are very similar for all individual populations when they are reared under the same circumstances (Saunders, 1976; Beck, 1991; Shawer et al., 2021). On the other hand, Shaffer (1983) has indicated that there are some differences in the growth period in many insects of the group reared under similar circumstances. The emergence of T. evanescens, occurs mostly at the starting of the photophase and is happened throughout two days with 95% of the females and 89% of the males emerging during the first day (Doyon and Boivin, 2006). Although Trichogramma evanescens males emerge earlier than females, female growers bigger than males and begins to lay eggs after a very short time of emergence (Charnov et al., 1981; Chassain and Boultreau, 1991). If the parasitoid female wasp has a late of emerging, it consumes the reserved food to continue physical processes, a loss in efficiency is more likely. Individuals who appear later may be penalized by having low-quality mates (Carvalho et al., 1998) and maybe having fewer mating chances (Waage and Ming, 1984). Trichogramma, a short-lived diurnal species, has a unique advantage in that it emerges earlier in the day. The female of T. evanescens lays 56% of the eggs throughout the first day (Boivin and Lagacé, 1999), and the survival in the open field is not going to surpass 1–2 days. Thus, it is very essential to encourage the emergence at early of the day during the possibility of host location.
Female wasps that are unable to emerge at the starting of the 9th day maybe miss oviposition chances or delay the emergence to the 10th day and probably have lower fitness (Doyon and Boivin, 2005). We may hypothesize that the emergence time maybe have a significant impact on the efficiency of Trichogramma. Thus, this work aimed to compare the efficacy of early and late Trichogramma females' emergence through the following biological parameters: emergence rate, female ratio, longevity, and the emergence of first generation (F1).
2 Materials and methods
2.1 Study site
The study was achieved at the laboratory of Biological Control, Rice Research Center, Agricultural Research Station, Sakha, Kafrelsheikh, Agricultural Research Center, Egypt.
2.2 Mass rearing of Sitotroga cerealella and Trichogramma evanecsens
The mass rearing of angoumois grain moth, Sitotroga cerealella (Olivier) (Lepidoptera: Gelechiidae) was conducted according to the method of Hassan (1995). The mass rearing of T. evanescens was produced as described by Abd EL Hafez (1995) by putting the new eggs of S. cerealella on paper cards (1 × 1 cm) with a thin layer of liquid glue. The paper cards were exposed to adults of T. evanecsens inside glass jars with a few drops of sugar solution as food for the newly emerged adults of T. evanecsens. The glass jars were closed by a piece of cotton cloth. New paper cards were added every day to avoid the super-parasitism of Trichogramma eggs on the old cards.
2.3 Emerging time of T. evanecsens
This experiment was achieved at 25 ± 1 °C and 60 ± 5 RH with photoperiod 16:8 L:D. Paper cards (7 × 12 cm) with new eggs of S. cerealella (<24 hrs old) were exposed to adults of T. evanescens and waited until starting to hatch with newly emerged adults of T. evanecsens. Paper cards were transferred to new glass jars every hour until 16 hrs. Each emergence time was repeated in five replicates. The numbers of the emerged parasitoid, percentage of emergence, female numbers, female percentage, male numbers, and sex ratio of T. evanescens were hourly counted and recorded until 16 hrs of emergence.
The F1 progeny was obtained in the same way of the previous experiment, for each emergence time (from one to 16 hrs) one paper card (1 × 1 cm) with fresh eggs of S. cerealella was inserted into a glass jar to expose them to ten adults of T. evanescens obtained from one emergence time and repeated five times and waited until starting to hatch with newly emerged adults of T. evanecsens.
The emergence rate was estimated using the following equation:
% Emergence = No. emerged wasps × 100.
Total number of eggs.
The longevity (hrs) was evaluated from the emergence time to the time of death. Female and male of T. evanecsens were defined according to the differences in the antenna type. The sex ratio was determined as the number of females: the number of males. The female's percentage was evaluated using the following equation:
% Females = No. females × 100.
Total population.
2.4 Statistical analysis
All data were analyzed using SPSS software (SPSS, 2006). The Shapiro–Wilk normality test was used to test the normality of the data, which indicated the normal distribution of the data. Therefore, the analysis was performed on the original data. One-way analysis of variance (ANOVA) was used to test the differences among emergence times, and Tukey's test was used to find the differences among the treatment means. In addition, the correlations between the emerging time of T. evanecsens and the tested biological parameters were checked statistically according to the Pearson correlation coefficient.
3 Results
Data in Table 1 show that the emergence time had high significant effects (P < 0.01) on the numbers of emerged parasitoids, % parasitoids emergence, female's numbers, sex ratio, numbers of emergence F1, emergence percentage F1, and female longevity of T. evanescens. As shown in Table 2 the emergence ratio progressively increased to reach the highest percentage (8.53%) at 7th hrs of emergence starting, then it decreased gradually until reaching the lowest percentage (4.62%) at the 16th hrs. The emergence rate of T. evanecsens was significantly (P < 0.01) higher at 7th hrs than at 9th, 10th, 11th, 12th, 13th, 14th, 15th, and16th hrs of emergence. Also, the average number of emerged parasitoids increased gradually to reach the maximum value (125.00 parasitoids) at 7th hrs, then a sharp decrease occurred and reached the lowest average number (67.67 parasitoids) at 16th hrs of emergence and it was significantly (P < 0.01) higher at 7th hrs than at 13th, 14th, 15th, and 16th hrs of emergence. The female number started to decrease significantly to be 67.33 parasitoids at 8th hrs. Then, it decreased gradually to perform the lowest number (32.00 and 29.67) at the 15th hrs and 16th hrs of emergence respectively and it was significantly (P < 0.01) greater at 7th hrs than at 1st, 2nd, 8th, 9th, 10th, 11th, 12th, 13th, 14th, 15th, and 16th hrs of emergence. Source of variation = emergence time, degree of freedom = 15, SS = sum of squares, MS = mean squares. Values are the average ± standard error. The means of each column followed by the different letters are significantly different at the 0.01 level. ** indicate P < 0.01.
Variables
SS
MS
F value
P value
No. emerged parasitoid
20171.96
1344.79
4.56
<0.0001
% Parasitoids emergence
96.88
6.45
47.40
<0.0001
No. females of T. evanescens
11328.97
755.26
62.93
<0.0001
No. males of T. evanescens
4089.64
272.64
0.92
˃0.0500
Sex ratio
989.89
65.99
7.26
<0.0001
Female longevity
153.71
10.24
23.48
<0.0001
No. emergence F1
4803.64
320.24
23.64
<0.0001
% Emergence F1
783.33
52.22
7.55
<0.0001
Emergence time (hrs)
No. emerging parasitoid
% Emergence
No. females
No. males
Sex ratio (Females: males)
1.00
85.00 ± 2.89abc
5.80 ± 0.21 de
53.00 ± 1.73d
32.00 ± 1.15ab
1.65: 1
2.00
80.00 ± 2.89abc
5.46 ± 0.21 ef
50.00 ± 1.73de
30.00 ± 1.15ab
1.66: 1
3.00
110.00 ± 2.89abc
7.51 ± 0.22b
73.33 ± 1.67ab
36.67 ± 3.33ab
1.99: 1
4.00
110.33 ± 2.60abc
7.53 ± 0.20b
68.00 ± 1.15bc
42.33 ± 1.45ab
1.60: 1
5.00
119.67 ± 3.18ab
8.17 ± 0.24 ab
70.33 ± 2.60abc
49.34 ± 0.67a
1.42: 1
6.00
121.00 ± 5.20ab
8.26 ± 0.37 ab
73.67 ± 3.76ab
47.33 ± 1.45a
1.55: 1
7.00
125.00 ± 2.89a
8.53 ± 0.22 a
77.33 ± 4.33a
47.67 ± 1.45a
1.62: 1
8.00
111.67 ± 3.76abc
7.62 ± 0.26 ab
67.33 ± 0.33bc
44.34 ± 3.48a
1.51: 1
9.00
107.71 ± 7.14abc
7.35 ± 0.31 bc
64.00 ± 0.58c
43.71 ± 4.91a
1.46: 1
10.00
95.00 ± 2.89abc
6.48 ± 0.20cd
52.67 ± 1.45d
42.33 ± 4.33ab
1.24: 1
11.00
87.00 ± 1.73abc
5.94 ± 0.14 de
47.00 ± 1.15de
40.00 ± 2.49ab
1.17: 1
12.00
82.00 ± 3.46abc
5.60 ± 0.25 de
44.00 ± 0.00de
38.00 ± 3.86ab
1.15: 1
13.00
73.00 ± 1.73bc
4.98 ± 0.13 efg
42.67 ± 3.18ef
30.33 ± 1.45ab
1.40: 1
14.00
67.33 ± 0.67c
4.59 ± 0.08fg
34.67 ± 0.33fg
32.66 ± 0.88ab
1.06: 1
15.00
64.00 ± 0.00c
4.37 ± 0.05 gh
32.00 ± 0.00g
32.00 ± 0.00ab
1.00: 1
16.00
67.67 ± 8.67c
4.62 ± 0.04h
29.67 ± 0.33g
38.00 ± 9.00ab
0.78: 1
Significance
**
**
**
**
**
The highest % males were obtained at the latest hrs of emergence, while the lowest % males were obtained at the 3rd hrs of emergence. However, there were no significant differences between male numbers in different emergence times (Table 2). The female percentage was 62.36% at the 1st hr and increased slowly to reach the highest percentage (63.58%) at the 3rd hrs, then moderate decrease occurred until reached the lowest value (50.00%) at 16th hrs (Fig. 1). The female percentage was higher at 7th hrs than at 14th 15th and 16th hrs of emergence. The highest sex ratio of T. evanescens was (1.99: 1) at the 3rd hrs of emergence (Table 2). Also, the statistical analysis indicated that there was a significant negative correlation between the emergence time and numbers of parasitoids, percentage of emergence, female numbers, and sex ratio (Table 3). ** Correlation is significant at the P < 0.01. (2-tailed).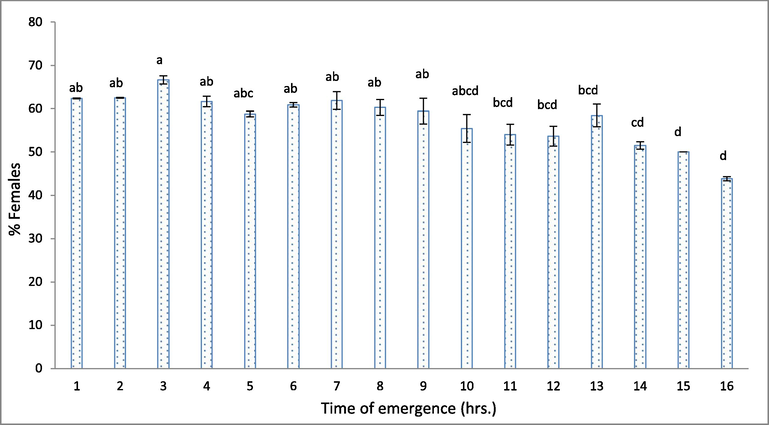
The effect of emergence time on the female percentages. Different letters above the bars mean a significant (P < 0.05) difference.
Parameters
1
2
3
4
5
6
7
8
1. Time of parasitoid emergence
2. No. emerged parasitoid
−0.50**
3. % Parasitoids emergence
−0.60**
0.79**
4. No. females
−0.70**
0.74**
0.97**
5. No. males
−0.08
0.78**
0.27
0.17
6. Sex ratio
−0.82**
0.38**
0.61**
0.76**
−0.14
7. Female longevity
−0.73**
0.67**
0.75**
0.81**
0.24
0.63**
8. No. emergence F1
−0.89**
0.60**
0.76**
0.83**
0.12
0.82**
0.84**
9. % Emergence F1
−0.70**
0.45**
0.56**
0.63**
0.07
0.63**
0.72**
0.76**
The data in Fig. 2 show that the longevity of the T. evanescens adults was 3.06 days for the 1st hr of emergence, then increased to perform the maximum value of 3.19 days at the 3rd hrs, then it decreased progressively by the time to reach 2.08 days at the 14th hrs and then reached the lowest value (1.94 days) at the 15th and 16th hrs of emergence. The longevity of T. evanescens was higher at the 7th hrs of emergence than the 11th, 12th, 13th, 14th, 15th, and 16th hrs of emergence. There was a clear negative correlation between the emerging time and T. evanescens longevity (Table 3). Also, data in Fig. 3 show that the percentages of emerging parasitoids of F1 were almost in the same trend as their parents in Table 2 with a clear negative significant correlation with the emerging time (Table 3). The emergence numbers of progeny F1 were significantly (P < 0.05) higher at 7th hrs than at 10th, 11th, 12th, 13th, 14th, 15th, and 16th hrs of emergence (Fig. 3).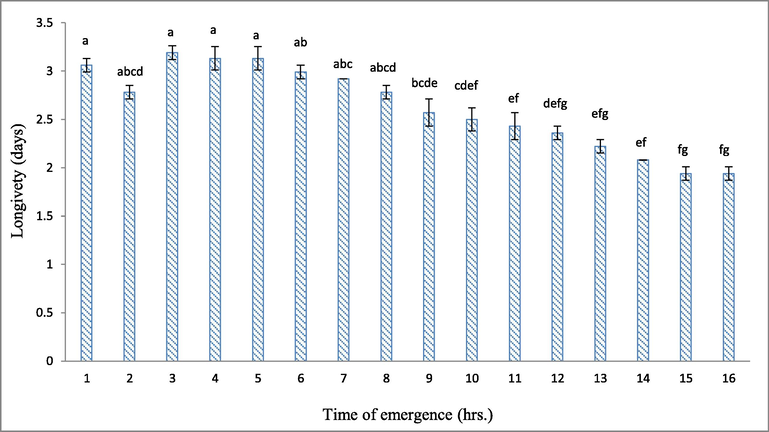
Effect of emergence time on the longevity of Trichogramma evanescens. Different letters above the bars mean a significant (P < 0.05) difference.
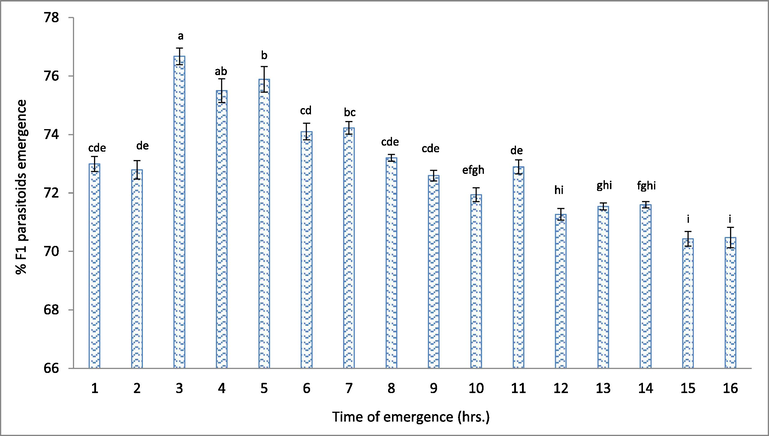
Effect of emergence time on the emergence percentage of F1. Different letters above the bars mean a significant (P < 0.05) difference.
4 Discussion
The statistical analysis of our experimental data shows that the emergence rate, female ratio, female longevity, and progeny emergence of F1 of T. evanescens were varied significantly depending on the emergence time. This is maybe because of many reasons make the late females with less fitness such as late female consume reserved food, the occurrence of super parasitism, and the female's body size.
The emergence numbers decreased with the late female emergence after the 8th hrs of emergence. It may be due to females feeding on some of their natural resources during the pupal stage to keep in existence and wait for emergence. Also, Yadav et al. (2001) have reported that super parasitism is often occurred in Trichogramma wasps under lab conditions, which may also be the reason for the expansion in the developmental period in late female parasitoids. The egg of S. cerealella often supports a single adult parasitoid per one egg for its development. However, some egg parasitoids are able to lay more than one egg in a single host egg (Klomp and Teerink, 1978). Corrigan et al. (1995) have reported that larval conflict leads to the loss of some parasitoids and survival of others. Moreover, super parasitism affects parasitoid development (Ahmad et al., 2002) and it is a reason that leads to prolonged emergence period in numerous Trichogramma species (Parra et al., 1988).
In hymenopteran parasitoids, sex ratios of offspring are well-known to differ and male wasps are produced from unfertilized eggs and female wasps from fertilized eggs (Flanders, 1965). Flanders (1965) and Suzuki et al. (1984) have indicated that after hymenopteran parasitoids mating, females store spermatic fluid in the spermatheca and can control the sex ratio of their offspring by manipulating the entry of sperm to the egg throughout oviposition. Boivin and Lagacé (1999) have shown that the gradual rising in sex ratio was noticed as a result of sperm depletion of the female. Oogenesis in hymenopteran parasitoid wasps begins throughout the prepupal stage (Volkoff and Daumal, 1994) and continues after the emergence of the adult (Mills and Kuhlmann, 2000). Pak and Oatman (1982) and Bai et al. (1992) have considered that Trichogramma wasps are proovigenic species (the hymenopteran parasitoids species that reach the adult with a whole compliment of eggs that they deposit in a short period and no new additional eggs created throughout the parasitoid's life). Also, Trichogramma species have been described as moderate synovigenic species (almost parasitoids) of hymenopteran wasps which continue to release eggs during the adult stage and the eggs' production depends on the feeding of the female adult more than the metabolites of the immature stages (Jervis et al., 2001).
The late females were not able to hatch at the start of the photoperiod on the 9th day, so those females had to spend extra time to become adults. This increased maturation time as pupae or subimago may have to use some of their energy stores, resulting in decreased body size and fertility. Early females of parasitoids are bigger and much more fecund than later ones, and able to produce more offspring (Doyon and Boivin, 2005). Earlier females that emerged on the ninth day after oviposition had higher efficiency than later females on the tenth day because every egg released helps to the reproductive success of females. Consequently, early females had a great impact on the female's body size and their egg production. Whereas the impact of emerging time on emergence rate was practically significant, the difference in the body size among early and late females accounted for the majority of the effect. Early males can sexually mature before the female's emergence and the early males could have a good production of spermatozoids throughout the time between emergence and mating (Olsson and Madsen, 1996). Also, early males have the time to enhance their fitness (Wedell, 1992) by increasing their ability to mate with greater receptivity females. Those might be the reasons for the high female ratio at the 3rd hrs of emergence.
The negative effect of emergence time of Trichogramma on the emergence numbers, females' percentage, longevity, and the emergence number of F1suggests that the quality of the parasitoids produced in mass production might be increased by enhancing the rate of the parasitoid population emerging early at 7th and 8th hrs of emergence.
5 Conclusion
In conclusion, the emergence time of T. evanescens has a clear effect on the tested biological parameters. The highest emergence ratio was obtained at 7th hrs and the female numbers started to decrease significantly at 8th hrs. Also, there was an obvious negative correlation between the emerging time and T. evanescens longevity and the emerging percentages of F1. Finally, the negative effect of longer developmental time on the fecundity of female wasps suggests that the high quality of the individuals of T. evanescens produced in mass production could be enhanced by increasing the rate of the early emerging individuals.
Acknowledgment
The authors extend their appreciation to Taif University for funding the current work by Taif University Researchers Supporting Project number (TURSP-2020/119), Taif University, Taif, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- A comparison of thermal requirements and some biological aspects of Trichogramma evanescens (Westwood) and Trichogrammatoidea bactrae (Najgaja) reared from eggs of the pink and spiny bollworm. Ann. Agric. Sci. Ain Shams Univ. Cairo. 1995;4:901-912.
- [Google Scholar]
- Superparasitism by Trichogramma poliae in the eggs of Clostera cupreata (Lepidoptera: Notodontidae) and its effect on offspring. J. Trop. Forest Sci.. 2002;14:61-70.
- [Google Scholar]
- The effect of host size on quality attributes of the egg parasitoid, Trichogramma pretiosum. Entomol. Exp. Appl.. 1992;64:37-48.
- [Google Scholar]
- Thermoperiodism. In: Lee R.E., Denlinger D.L., eds. Insects at Low Temperature. Boston, MA: Springer US; 1991. p. :199-228.
- [Google Scholar]
- Effet de la taille sur la fitness de Trichogramma evanescens (Hymenoptera: Trichogrammatidae) Ann. Soc. Entomol. France. 1999;35:371-378.
- [Google Scholar]
- Protandry and females size-fecundity variation in the tropical butterfly Brassolis sophorae. Oecologia. 1998;116:98-102.
- [Google Scholar]
- Genetic variability in quantitative traits of host exploitation in Trichogramma (Hymenoptera: Trichogrammatidae) Genetica. 1991;83(3):195-202.
- [Google Scholar]
- Effects of parasitoid to host ratio and time of day of parasitism on development and emergence of Trichogramma minutum (Hymenoptera: Trichogrammatidae) parasitizing eggs of Ephestia kuehniella (Lepidoptera: Pyralidae) Ann. Entomol. Soc. Am.. 1995;88:773-780.
- [Google Scholar]
- The effect of development time on the fitness of female Trichogramma evanescens. J. Insect Sci.. 2005;5(4)
- [Google Scholar]
- Impact of protandry on mating capacity of males in Trichogramma evanescens Westwood. Biocontrol. 2006;51:703-713.
- [Google Scholar]
- Attractiveness of spiders and insect predators and parasitoids to flowering plants. Egypt. J. Biol. Pest Cont.. 2015;25:245-250.
- [Google Scholar]
- On the sexuality and sex ratios of hymenopterous populations. Am. Nat.. 1965;99(909):489-494.
- [Google Scholar]
- Parasitoids: Behavioral and Evolutionary Ecology. Princeton, New Jersey: Princeton University Press; 1994.
- Hassan, S.A., 1995. Improved method for the production of the Angoumois grain moth Sitotroga cerealella Oliv. Trichogramma and other egg parasitoids conference. Cairo, Egypt, Ed IUNRA, Paris, pp 157–160.
- Life history strategies in parasitoid wasps: a comparative analysis of ovigeny. J. Anim. Ecol.. 2001;70:442-458.
- [Google Scholar]
- Li, L.Y., 1994. Worldwide use of Trichogramma for biological control on different crops: a survey in “biological control with egg parasitoids” (E. Wajnberg, and S. A.Hassan, Eds.), pp. 37–53. CABInternational, Wallingford, U.K.
- Elimination of supernumerary larvae of the gregarious egg-parasitoid Trichogramma embryophagum (Hymenoptera: Trichogrammatidae) in eggs of the host Ephestia kuehniella (Lepidoptera: Pyralidae) Entomophaga. 1978;23:153-159.
- [Google Scholar]
- Dispersal of Trichogramma platneri (Hymenoptera: Trichogrammatidae) from point-source releases in an apple orchard in California. J. Appl. Entomol.. 1997;121:205-209.
- [Google Scholar]
- The relationship between egg load and fecundity among Trichogramma parasitoids. Ecol. Entomol.. 2000;25:315-324.
- [Google Scholar]
- Costs of mating with infertile males selects for late emergence in female sand lizards (Lacerta agilis) Copeia. 1996;2:462-464.
- [Google Scholar]
- Parra, J.R.P., Zucchi, R.A., Silveira Neto, S., 1988. Perspectives of biological control using Trichogramma and/or Trichogrammatoidea in the state of Sào Paulo (Brazil). In: Trichogramma and other egg parasites 43, 527–540. INRA.
- Insect Clocks. New York: Pergamont; 1976.
- Prediction of variation in developmental period of insects and mites reared at constant temperatures. Environ. Entomol.. 1983;12:1012-1019.
- [Google Scholar]
- The impact of cold storage durations on Trichogramma evanescens (Westwood) (Hymenoptera: Trichogrammatidae) during their pupal stage. Saudi J. Biol. Sci.. 2021;28(12):7202-7206.
- [Google Scholar]
- Biological control with Trichogramma: advances, success and potential of their use. Ann. Rev. Entomol.. 1996;41:375-406.
- [Google Scholar]
- SPSS, 2006. SPSS15.0 for Windows. SPSS Inc. Chicago, IL.
- Sex allocation and the effects of superparasitism on secondary sex ratios in the gregarious parasitoid, Trichogramma chilonis (Hymenoptera: Trichogrammatidae) Anim. Behav.. 1984;32:478-484.
- [Google Scholar]
- Ovarian cycle in immature and adult stages of Trichogramma cacoeciae and T. brassicae (Hymenoptera: Trichogrammatidae) Entomophaga. 1994;39:303-312.
- [Google Scholar]
- The reproductive strategy of a parasitic wasp I. Optimal progeny and sex allocation in Trichogramma evanescens. J. Anim. Ecol.. 1984;53:401-415.
- [Google Scholar]
- Protandry and mate assessment in the wartbiter Decticus verrucivorus (Orthoptera: Tettigoniidae) Behav. Ecol. Sociobiol.. 1992;31:301-308.
- [Google Scholar]
- Effect of host egg density on parasitism and adult emergence in Trichogramma chilonis Ishii (Hymenoptera: Trichogrammatidae) in two systems. J. Biol. Control. 2001;15:11-14.
- [Google Scholar]







