Translate this page into:
Effect of different micelles on charging and discharging behavior of phase change material
⁎Corresponding authors. h.alrobei@psau.edu.sa (Hussein Alrobei), rizwanmalik48@yahoo.com (Rizwan Ahmed Malik) rizwan.malik@uettaxila.edu.pk (Rizwan Ahmed Malik)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Phase change material (PCM) offers high-density thermal energy storage, making it attractive for thermal management applications of electronic circuitry and thermal energy storage. However, PCM, like paraffin wax, ideal for such low-temperature applications, has very low thermal conductivity. The present study focused on the improvement of the thermal conductivity of paraffin wax using surfactants. The surfactants used as thermal conductivity enhancers (TCE) are cetyltrimethyl ammonium bromide (CTAB), Dioctyl sodium sulfosuccinate (known as AOT) and sodium dodecyl sulfate (SDS). The surfactant self-aggregation called a micelle, acts as conducting medium inside the paraffin wax, providing better thermal conductivity. The highest heat transfer rate with a peak temperature of 71 °C was observed in the case of AOT micelle paraffin wax. Adding SDS, CTAB, and AOT surfactants increased the highest temperatures by 4%, 8.4%, and 18.33% compared to pure PCM.
Keywords
Phase Changing Material (PCM)
Paraffin wax
Thermal conductivity
Surfactants
Micelles
1 Introduction
As the world progresses with a growing population and economy, energy demand also increases. In the beginning, fossil fuels played a significant role but negatively impacted the environment (Saima et al., 2023, Baghbani et al., 2022). Many researchers have worked in this field to employ renewable energy sources efficiently to fulfill the growing energy demand (Abdelsalam et al., 2020). Renewable energy origins like solar and wind provide massive capabilities of energy production. However, issues like inconsistency and irregularity associated with them are vulnerable to their availability and reliability, particularly during peak times, as a constant supply of energy is required during that phase. For future use, renewable energy can be reserved when it is available. This feature has played an essential role in replacing fossil fuel with renewable energy, thus making a brighter and cleaner future for upcoming generations.
Among different storage methods, thermal energy storage is widely used (Dincer et al., 2002). The excessive energy origins, such as waste heat generation; solar and geothermal energy in industrial and residential applications, gives excellent inspiration for harnessing these energy sources. Thermal energy storage may be achieved in two ways. One of these is sensible heat storage (SHS) which works on the principle of enhancing the temperature of storage material for storing energy. Rocks and water are available materials that have been used for this purpose. Previously for this purpose, rocks were available materials that have been used for decades (Huggins et al., 2015). The second method is latent heat storage (LHS). LHS is a principle of thermal energy storage that relies on the use of phase change materials (PCMs). PCMs are substances with specific melting and freezing points that can store and release a large amount of heat energy during their phase transition, typically from solid to liquid or liquid to solid. This phase change allows the PCM to absorb or release energy while maintaining a nearly constant temperature. As the material goes through a phase shift, the energy is stored as latent heat. LHS has certain advantages over sensible heat storage, such as regulatory features by controlling temperature fluctuations and higher energy density, especially in hot water applications (Mehling et al., 2007). PCMs are categorized as organic and inorganic PCMs. Organic PCMs include substances such as fatty acids and alkanes, with paraffin compounds being a notable example. Organic PCMs are particularly well-suited to a wide range of applications, including electronic device thermal management systems, household hot water systems, and residential air conditioning. This suitability stems from their ability to offer a wide spectrum of phase change temperatures, spanning from 0 to 100 degrees Celsius (Wang et al., 2014). Furthermore, organic PCMs possess several advantages over their inorganic counterparts. These benefits include non-toxicity, chemical stability, and, notably, cost-effectiveness. These appealing attributes have made organic PCMs a focal point of interest for researchers dedicated to advancing thermal storage systems (Wu et al., 2010). PCM, like PT 58 °C paraffin wax is readily available, nontoxic, and relatively cost-effective and suitable in low-temperature thermal energy-storage applications. The accessibility and the characteristics to undergo a phase change (from solid to liquid) at a relatively low temperature, making it appropriate for applications where moderate temperature control is required. However, PT 58 °C paraffin wax has very low thermal conductivity and needs improvement to be utilized for energy-storage applications. The primary motivation for improving the thermal conductivity of paraffin wax is to enhance its effectiveness in applications related to electronic circuitry and thermal energy storage. This motivation is driven by the need for efficient thermal management and energy storage solutions in various fields. Electronic devices, such as computer processors and integrated circuits, generate heat during operation. Efficient heat dissipation is crucial to prevent overheating and ensure the reliable and long-lasting performance of these devices. Controlling the temperature of electronic components within safe operating limits is essential to prevent damage and optimize their performance. Improved thermal conductivity in materials like paraffin wax can help transfer heat away from these components more effectively (Cheng, P., et al. 2018), Bar-Cohen, A., et al. 2018). Thermal energy storage plays a critical role in the integration of renewable energy sources like solar and wind power. These sources often produce energy intermittently, and efficient thermal energy storage systems can store excess energy when it's available and release it when needed to ensure a consistent power supply (Sharma, A., et al. (2009), Cabeza, L. F., et al. (2017), Dincer, I., & Rosen, M. A. (2010). Zalba, B., et al. (2003). High-density thermal energy storage using phase change materials (PCMs) like paraffin wax is attractive for its ability to store and release energy with minimal energy loss. In this context, enhancing the thermal conductivity of PCMs is crucial for improving their energy storage and release capabilities.
Researchers study different techniques to boost the thermal conductivity of PCM. The thermal conductivity improvement techniques are extended surfaces (Shatikian et al., 2005; Baby et al., 2012; Sharifi et al., 2011; Ismail et al., 2011; Baby et al., 2013; Akhilesh et al., 2005), suction of the PCM inside the metal foam (Zhao et al., 2010; Baby et al., 2013; Zhao et al., 2011; Chen et al., 2014), using nanoparticles having very high surface area (Kalaiselvam et al., 2012; Motahar et al., 2014; Hosseinizadeh et al., 2012), and geometrical shape optimization of the PCM container.
Different types of nanoparticles derived from metals, ceramics, and carbon fibers are added to the PCM to boost thermal conductivity. Xia et al. (Xia et al., 2010) used expanded graphite (EG) as TCE in the Paraffin. This study reported that increasing the concentration of EG to 10 % improves thermal conductivity 10 times that of pure paraffin. Li et al. examined the phase change materials comprised of nano-graphite (NG) and paraffin. The results revealed a gradual increase in thermal conductivity as the NG content was raised. In the case of the material containing 10 % NG, the thermal conductivity reached 0.9362 W/m K. (Li, 2013). Johansen et al. conducted a study on improving the thermal conductivity of sodium acetate trihydrate by incorporating graphite powder. Their research demonstrated that the addition of 5 wt% graphite powder to a mixture of sodium acetate and water could maintain stable supercooling for a minimum of five months without any indications of spontaneous solidification (Johansen et al., 2015). Fukai et al. investigated carbon fiber/paraffin composites. In this study, it was found that carbon fibers are effective to enhance thermal conductivity (Fukai et al., 2000).
It was demonstrated that Carbon nanotubes (CNTs) at a concentration of 0.01–0.1 wt% increased the thermal conductivity of a water/CNTs composite material by 3.0–3.1 percent (Walvekar et al., 2012). A thermal conductivity improvement ratio of 26 percent was found in a similar discovery (Maré et al., 2015) to increase water's thermal conductivity. A nanocomposite comprising stearic acid (SA) and multi-walled carbon nanotube (MWCNT) is prepared for thermal energy storage purposes. The addition of MWCNT significantly enhances the thermal conductivity of stearic acid but has the side effect of reducing natural convection in the liquid state of stearic acid. When compared to pure stearic acid, the charging rate is reduced by approximately 50 %, while the discharging rate is enhanced by about 91 % when utilizing the SA/5.0 % MWCNT nanocomposite (Li et al., 2013).
PCM's thermal conductivity is boosted by the insertion of nanoparticles but at the cost of decreasing the PCM-based system's thermal storage capacity. Two factors cause this compromise in thermal storage capacity. The first factor is the decrease in the quantity of PCM. The second factor is that PCM particles accumulate and attach with the nanoparticles, so not all the molecules participate in the phase change course. It further impairs the PCM's ability to store heat. This second factor is an impetus behind present analysis to assess the impact of surfactant addition on the thermal conductivity of the PCM. The surfactants aggregate and make micelles that enhance thermal conductivity.
So far, few studies have reported the impact of surfactant addition on the charging and discharging behavior of the PCM. The present study experimentally investigates the effect of three different sorts of surfactants on the charging and discharging behavior of paraffin wax. This research aims to design, analyze, and compare the charging and discharging behavior of three different sorts of surfactants incorporated into PT58 oC Paraffin wax.
2 Materials and methods
The heat sink enclosure is made of aluminum. The chamber has a cubical shape. Its size is 50x50x50 mm3. For the present work, PT58 oC Paraffin wax is utilized as PCM, and Sodium dodecyl sulfate (SDS), Cetyltrimethyl ammonium bromide (CTAB), and Dioctyl sodium sulfosuccinate (AOT) were added as a surfactant to enhance the thermal conductivity of the PCM. Thermophysical properties of the PCM are tabulated in Table 1.
Properties
PT 58 °C (Pure Temp.)
Heat Storage Capacity (J/g)
225.0
Melting Point (°C)
58.0
Thermal Conductivity in liquid form (W/m°C)
0.150
Thermal Conductivity in solid form (W/m°C)
0.250
Density in liquid form (g/ml)
0.810
Density in solid form (g/ml)
0.890
Specific heat in liquid form (J/g°C)
2.712
Specific heat in solid form (J/g°C)
2.470
The testing chamber shown in Fig. 1 is fabricated to minimize the effect of surrounding on the testing specimen. Whereas testing chamber is made up of wood covered with glass wool and aluminum foil insulation to avoid any losses of heat. To generate heat inside the chamber two 100-watt bulbs were installed with a fan to circulate the air in the testing chamber and other fan was used as an exhaust fan and these all accessories were controlled via a controller to main, they entered temperature inside the chamber. The schematic diagram of experimental setup is shown in Fig. 2.
Testing Chamber with glass wool and Aluminum foil insulation.
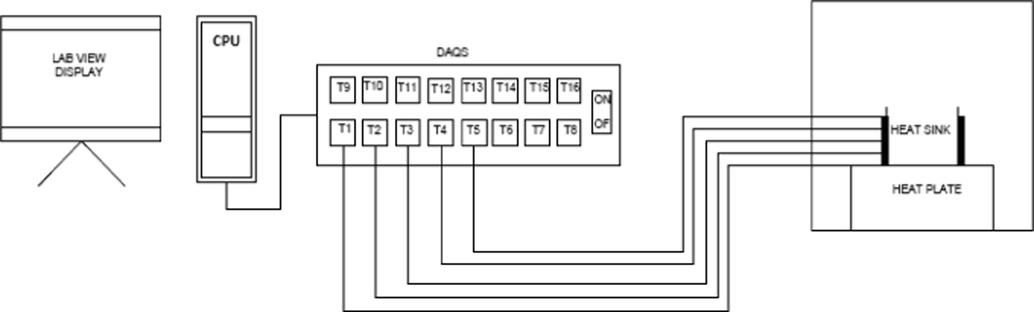
Experimental Setup.
Paraffin wax (150 g) was taken into a 250 ml glass beaker. The glass beaker is placed at a hot plate (H4000 H-S, manufactured by Pakistan Council of Scientific and Industrial Research, Pakistan) and the temperature was set to 80 °C. Surfactant amounts (AOT: 13.61 g, CTAB: 10.56 g and SDS: 9.80 g) were added into molten paraffin wax by one gram each time. After addition of particles the liquid paraffin wax was stirred by magnetic stirrer (Fig. 3a) at 1000 rpms and 80 °C for 15 min each time one gram was added. To make sure that particles were well dispersed in the paraffin wax, T 25 digital Ultra Turrex, manufactured by IKA, Germany (Fig. 3b) was used to homogenize the particles at 5000 rpms. In this research, Omega Engineering K-type thermocouples sourced from the USA were employed. These thermocouples have a temperature range, spanning from −200 °C to 1250 °C, and they offer a standard accuracy with a tolerance of either 2.2 °C or 0.75 % of the measured temperature, depending on which value is greater.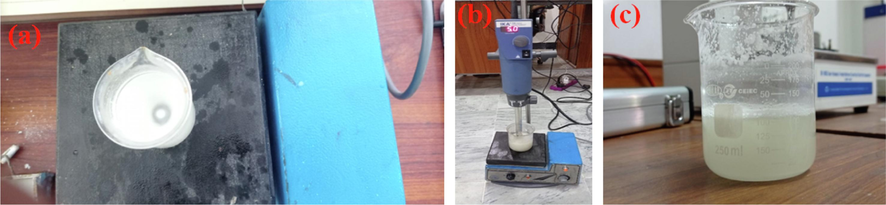
(a) Magnetic Stirring of Paraffin wax and surfactants, (b) Ultra Turrex Homogenizing of surfactants (c) Prepared solution.
3 Results and discussions
To investigate the stability of modified phase-changing materials composite, PT 58 °C paraffin wax was utilized. The pure PT 58 °C underwent a 90-minute charging and 90-minute discharging phase, and the results are presented in Fig. 4.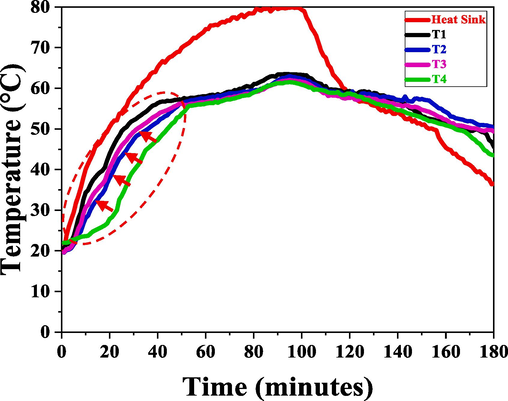
Temperature variation with time at different vertical locations of the Pure-PCM.
Fig. 4 depicts the performance of the heat sink through the charging and discharging stages. T1 thermocouple is positioned at 10 mm from the bottom of the heat sink, T2 is positioned at 20 mm, T3 is positioned at 30 mm, and T4 is positioned at a distance of 40 mm. Throughout the experiment, the thermocouple configuration in the heat sink remains constant. The temperature readings of the pure paraffin wax's phase-changing behavior were acquired from these thermocouples; using these analyses, the graph in Fig. 4 and the data extrapolated from it are displayed in Table 2 respectively.
Pure PCM
Heating
Max. Temperature
Cooling
Paraffin Wax PT 58 °C
Starting Temp.
Phase changing Temp.
Phase changing Temp.
Ending Temp.
T1
20.65 °C
58.09 °C
63.4 °C
59.65 °C
45.54 °C
T2
19.61 °C
57.98 °C
62.8 °C
58.90 °C
50.18 °C
T3
19.57 °C
57.68 °C
62.025 °C
58.11 °C
49.37 °C
T4
20.35 °C
57.85 °C
61.53 °C
58.44 °C
43.54 °C
The information in Table 1 shows that throughout each 90-minute charging and discharging cycle, T1 records a highest peak temperature of 63.4 °C, which is close to the heat sink's base, where the highest temperature of 80 °C was recorded. T1 is usually undergoing a quick phase change since it is at the bottom. This phenomenon moves layer by layer up from the bottom to the top because heat is only delivered at the heat sink's bottom, and this is assumed to be the only direction in which heat flows—from bottom to top. If the phase-changing behavior exhibited in the graph is studied, PCM began to change its state precisely about 58 °C and the top layer T4 reached a maximum temperature of 61.53 °C.
Fig. 5 shows the effects of charging and discharging SDS-based micellated paraffin. The research demonstrates that paraffin wax with concentrations larger than CMC had a superior rate of heat transfer than the micelles produced by CMC. The peak temperature for the surfactant CMC concentration was measured at 64.08 °C, while the phase transition temperature was 57.7 °C. For larger concentrations, temperatures were measured at 64.72 °C for the peak and 57.13 °C for the phase, respectively.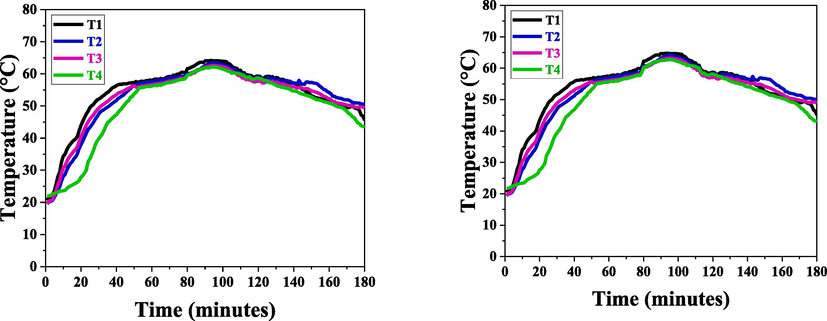
(A) Charging and discharging of SDS based Paraffin at CMC, (B) Charging and discharging of SDS based Paraffin above CMC.
For CTAB-based micellated paraffin wax, the same charging and discharging experiment was carried out once more and results were depicted in Fig. 6. The peak temperature was 55.5 °C and the findings for the phase transition were 56.5 °C and 65.8 °C, respectively.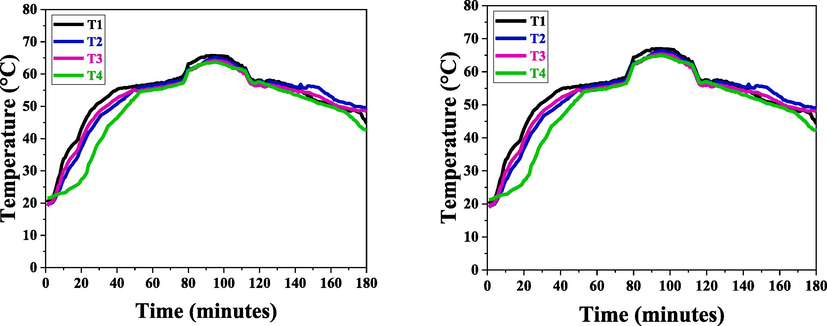
(A) Charging and discharging of CTAB based PCM at CMC, (B) Charging and discharging of CTAB based PCM above CMC.
The phase transition and peak temperatures for AOT-based micellated paraffin wax were 54.6 °C and 68.98 °C for the concentration at CMC, and more than CMC values were 54 °C and 71 °C, as depicted in Fig. 7.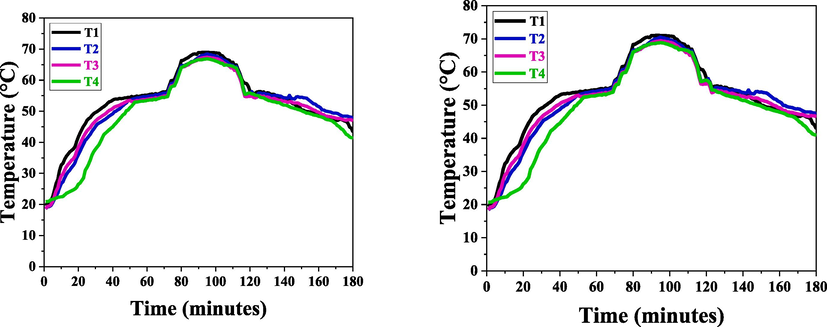
(A) Charging and discharging of AOT based Paraffin wax above CMC, (B) Charging and discharging of AOT based Paraffin wax below CMC.
The temporal variations of the heat sink temperature are suppressed by addition of the PCM. The PCM stores thermal energy and lowers the peak temperatures of the PCM rectangular enclosure. The melting behavior of PCM is non-linear in nature. Initially at the very start of the melting, conduction is the leading mode of heat transmission from the bottom of the PCM enclosure. The heat transfer through the thin PCM layer and the solid phase is via the conduction mode of heat transmission. The lower thermal conductivity of the PCM is responsible for the initial higher variation of the temperatures along the vertical direction, from bottom to the top of the PCM. Now as appreciable amount of PCM is melted which offers thermal resistance between the heat source (base) and the solid PCM. So, the conduction mode of heat transferal is suppressed with the growth of melted PCM layer between the heat source and the solid PCM. The charging rate is now supported by enhancement of the convection mode of heat transfer. This convection mode of heat transfer is dependent on the temperature dependent density difference of the PCM. So as most of the PCM has been melted and uniform temperatures field is developed across the PCM, the convection heat transfer gets weakened. Now both the conduction and convection mode of heat transfer are suppressed, causing a very slow charging rate depicted in the results of temporal variation of temperatures. During charging and discharging of PCM, the maximum temperature achieved increased with addition of the surfactants. The increase in the temperature represents higher heat transfer across the PCM that is because of the improvement in thermal conductivity of the PCM. The concertation of surfactants more than their CMC value offered better results as compared to the concentration equal to the CMC. Addition of SDS, CTAB and AOT surfactants increased the highest temperatures by 4, 8.4 and 18.33 % as matched to the pure PCM.
4 Conclusion
An experimental investigation is performed to explore the impact of incorporation of surfactants in PCM on the thermal conductivity of PCM. The surfactants named cetyltrimethyl ammonium bromide (CTAB), dioctyl sodium sulfosuccinate (known as AOT) and sodium dodecyl sulphate are added to the paraffin wax. It was observed that micellated paraffin wax samples showed improved heat transfer rate as compared to pure paraffin wax. These micelles may act as conducting medium between the paraffin layers. To conclude, during charging and discharging of PCM, the maximum temperature achieved increased with addition of the surfactants. The higher temperatures represent an enhancement in thermal conductivity of PCM. Addition of SDS, CTAB and AOT surfactants increased the highest temperatures by 4, 8.4 and 18.33 % as compared to unmodified PCM.
Acknowledgment
The authors extend their appreciation to Prince Sattam bin Abdulaziz University for funding this research work through the project number (PSAU/2021/01/18816).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Hybrid thermal energy storage with phase change materials for solar domestic hot water applications: Direct versus indirect heat exchange systems. Renew. Energ.. 2020;147:77-88.
- [Google Scholar]
- Method to improve geometry for heat transfer enhancement in PCM composite heat sinks. Int. J. Heat Mass Transfer. 2005;48:2759-2770.
- [Google Scholar]
- Experimental investigations on phase change material based finned heat sinks for electronic equipment cooling. Int. J. Heat Mass Transfer. 2012;55:1642-1649.
- [Google Scholar]
- Thermal optimization of PCM based pin fin heat sinks: an experimental study. Appl. Therm. Eng.. 2013;54:65-77.
- [Google Scholar]
- Experimental investigations on thermal performance enhancement and effect of orientation on porous matrix filled PCM based heat sink. Int. Commun. Heat Mass Transfer. 2013;46:27-30.
- [Google Scholar]
- 2022, Numerical simulation effect of PCM storage on flat storage on flat plate solar heater in different kinds of weather conditions. Int. J. Energy Environ.. 2022;13:135-152.
- [Google Scholar]
- Advances in thermal management of electronic systems. Heat Transfer Engineering. 2018;39(10):803-810.
- [Google Scholar]
- A review on high-temperature phase change materials for solar thermal energy storage. Renewable and Sustainable Energy Reviews. 2017;76:1287-1299.
- [Google Scholar]
- Experimental and numerical study on melting of phase change materials in metal foams at pore scale. Int. J. Heat Mass Transfer. 2014;72:646-655.
- [Google Scholar]
- Thermal management of electronic devices using phase change materials: A review. Applied Thermal Engineering. 2018;139:354-372.
- [Google Scholar]
- Thermal energy storage: systems and applications. John Wiley & Sons; 2002.
- Thermal energy storage systems and applications (2nd ed.). John Wiley & Sons; 2010.
- Thermal conductivity enhancement of energy storage media using carbon fibers. Energy Convers Manag. 2000;41:1543-1556.
- [Google Scholar]
- Numerical investigations of unconstrained melting of nano-enhanced phase change material (NEPCM) inside a spherical container. Int. J. Therm. Sci.. 2012;51:77-83.
- [Google Scholar]
- Energy storage: fundamentals, materials and applications. Springer; 2015.
- Fins and turbulence promoters for heat transfer enhancement in latent heat storage systems. Exp. Therm. Fluid Sci.. 2011;35:1010-1018.
- [Google Scholar]
- Thermal conductivity enhancement of sodium acetate trihydrate by adding graphite powder and the effect on stability of supercooling. Energy Procedia. 2015;70:249-256.
- [Google Scholar]
- Analytical and experimental investigations of nanoparticles embedded phase change materials for cooling application in modern buildings. Renewable Energy.. 2012;39:375-387.
- [Google Scholar]
- A nano–graphite/paraffin phase change material with high thermal conductivity. Appl. Energy. 2013;106:25-30.
- [Google Scholar]
- Enhancement of heat transfer for thermal energy storage application using stearic acid nanocomposite with multi–walled carbon nanotubes. Energy.. 2013;55:752-761.
- [Google Scholar]
- Unexpected sharp peak in thermal conductivity of carbon nanotubes water–based nanofluids. Int. Commun. Heat Mass Transf.. 2015;66:80-83.
- [Google Scholar]
- Phase change materials and their basic properties in thermal energy storage for sustainable energy consumption. Springer 2007:257-277.
- [Google Scholar]
- A novel phase change material containing mesoporous silica nanoparticles for thermal storage: a study on thermal conductivity and viscosity. Int. Commun. Heat Mass Transfer. 2014;56:114-120.
- [Google Scholar]
- Experimental and numerical analysis for the size, charging and discharging characteristics of a phase changing material as a thermal energy storage. J. Energy Storage. 2023;58:106228
- [Google Scholar]
- Enhancement of PCM melting in enclosures with horizontally-finned internal surfaces. Int. J. Heat Mass Transfer. 2011;54:4182-4192.
- [Google Scholar]
- Review on thermal energy storage with phase change materials and applications. Renewable and Sustainable Energy Reviews. 2009;13(2):318-345.
- [Google Scholar]
- Numerical investigation of a PCM-based heat sink with internal fins. Int. J. Heat Mass Transfer. 2005;48:3689-3706.
- [Google Scholar]
- Thermal conductivity of carbon nanotube nanofluid—experimental and theoretical study. Heat Transf. – Asian Res.. 2012;41(2):145-163.
- [Google Scholar]
- Improved thermal properties of paraffin wax by the addition of TiO2 nanoparticles. Appl. Therm. Eng.. 2014;73:1541-1547.
- [Google Scholar]
- Preparation and melting/freezing characteristics of Cu/paraffin nanofluid as phase-change material (PCM) Energy & Fuels. 2010;24:1894-1898.
- [Google Scholar]
- Preparation and Thermal Characterization of Expanded Graphite/Paraffin Composite Phase Change Material. Carbon.. 2010;48:2538-2548.
- [Google Scholar]
- Review on thermal energy storage with phase change: materials, heat transfer analysis and applications. Applied Thermal Engineering. 2003;23(3):251-283.
- [Google Scholar]
- Heat transfer enhancement of high temperature thermal energy storage using metal foams and expanded graphite. Sol. Energy Mater. Sol. Cells. 2011;95:636-643.
- [Google Scholar]
- Heat transfer enhancement for thermal energy storage using metal foams embedded within phase change materials (PCMs) Sol. Energy. 2010;84:1402-1412.
- [Google Scholar]







