Translate this page into:
Effect of Danggui-Shaoyao-San on renal macrophages in STZ-induced DN rats
⁎Corresponding author. baishaoyao@163.com (Xiaobing Li)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objectives: The present study is to explore the influence of DSS on the proliferation and activation of renal macrophages in the STZ-induced diabetic nephropathy rats. Methods: Seventy rats were grouped according to the random digit table means. 8 rats were randomly selected as the control group. The remaining 62 rats were divided into 5 groups and given a high-fat and high-sugar diet for 4 weeks. Diabetic rats were prepared by intraperitoneal injection of 55 mg/kg STZ. Rats in the DSS groups were given 21 g/kg/day, 14 g/kg/day and 7 g/kg/day DSS separately. The rats in the positive control group were given 6 mg/kg/day TP, while the rats of the model group and control group were received the physiological saline by gastric perfusion directly. Blood glucose, 24 h urine protein, urine urea nitrogen and β2 microglobulin of the rats were measured. And the protein levels of ED-1, MCP-1 and TLR4 in rat kidney were detected by Western Blot. ED-1+/TLR4+, ED-1+/PCNA+ and ED-1+/iNOS+ were detected by immunofluorescence. Results: DSS reduced the levels of blood glucose, 24-h urine protein, urine urea nitrogen and β2 microglobulin and repaired kidney tissue damage of the DN rats. In the WB experiment, DSS clearly decreased the protein expression levels of ED-1, MCP-1, and TLR4 in a dose-dependent manner (p < 0.05). In the immunofluorescence experiment, compared with the model group, the level of ED-1+/TLR4+ in the rats of DSS and TP groups and the level of ED-1+/PCNA+ in the rats of DSS-M group were obviously decreased (p < 0.05). Also, the level of ED-1+/PCNA+ and ED-1+/iNOS+ in the rats of DSS-H and TP groups was decreased evidently (p < 0.01). Conclusion: We inferred that DSS has therapeutic effects on the DN model rats, and its mechanism is likely to be connected with inhibition of macrophage proliferation and activation in rat kidney.
Keywords
Diabetic Nephropathy
Rats
Macrophage
Activation
Danggui-Shaoyao-San
- BCA
-
Bovine Carbonic Anhydrase
- CKD
-
Chronic Kidney Diseases
- CON
-
the Control Group
- DAPI
-
4′,6-diamidino-2-phenylindole
- DN
-
Diabetic Nephropathy
- DSS
-
Danggui-Shaoyao-San
- DSS-L
-
the DSS Low-dose Group
- DSS-M
-
the DSS Medium-dose Group
- DSS-H
-
the DSS High-dose Group
- ED-1
-
mouse anti-macrophage monoclonal
- GAPDH
-
Glyceraldehyde-3-phosphate Dehydrogenase
- HE
-
Hematoxylin-Eosin
- HRP
-
Horseradish Peroxidase
- IL-1
-
Interleukin-1
- iNOS
-
inducible Nitric Oxide Synthase
- MCP-1
-
Monocyte Chemotactic Protein-1
- Model
-
the Model Group
- PAS
-
Periodic Acid-Schiff
- PCNA
-
Proliferating Cell Nuclear Antigen
- PMSF
-
phenylmethylsulfonyl Fluoride
- PDGF
-
Platelet-Derived Growth Factor
- PVDF
-
Poly Vinylidene Fluoride
- RIPA
-
Radioimmunoprecipitation assay
- SD
-
Sprague-Dawley
- SDS-PAGE
-
Sodium Dodecyl sulfate–Polyacrylamide Gel Electrophoresis
- STZ
-
Streptozotocin
- SEM
-
Scanning Electron Microscope
- TGF-β
-
Transforming Growth Factor-β1
- TP
-
the Tripterygium Glycosides Group
- TLR4
-
Toll-like Receptor 4
- UUN
-
Urinary Urea Nitrogen
- WB
-
Western Blotting
- β2-MG
-
β2-Microglobulin
Abbreviations
1 Introduction
Diabetic nephropathy (DN) is one of the most common microvascular complications in diabetes. It is a leading cause of disability or death in diabetic patients (Zeng et al., 2019). Diabetic nephropathy has already been the main reason for hospitalization of patients with chronic kidney disease (CKD) in China (Wang et al., 2019). However, we still lack effective treatments to cure diabetic nephropathy patients.
Inflammation in the pathogenesis of DN has been widely acknowledged. Hyperglycemia can induce the kidney to produce catalytic factors and pro-inflammatory cytokines, leading to kidney inflammation (Gurley et al., 2018). Studies have shown that these inflammatory factors induce adhesion of macrophages and vascular endothelial cells (Lee et al., 2019). Activated macrophages release nitric oxide, platelet-derived growth factor (PDGF), interleukin-1 (IL-1), transforming growth factor-β1 (TGF-β1) etc. Ultimately, all these factors lead to damage of renal vascular endothelial cells and proliferation of fibroblasts, glomerulus hypertrophy and renal interstitial fibrosis (Rousselle et al., 2017).
Danggui-Shaoyao-San (DSS) is drawn from the Synopsis of Golden Chamber, which is Zhang Zhongjing’s famous prescription for treating liver and spleen disorders. It composes of Angelica sinensis, Paeonia lactiflora, Ligusticum Chuanxiong, Poria cocos, Atractylodes macrocephala and Alisma orientale. It can regulate the liver and spleen, promote blood circulation, dredge collaterals and keep the flow of qi and alleviate water retention. We found that “pressure-regulating lipid-lowering capsules” which was made by DSS can decrease blood pressure and blood lipids (Li X.B. et al., 2013). Previous researches have proved that DSS can reduce TGF-β1 in kidney tissue of rats with early diabetic kidney injury (Zhao et al., 2011). TGF-β1 is a well-studied fibrogenic cytokine, which is the main cause of renal fibrosis (Zeng et al., 2019). In the inflammatory response, macrophages can regulate TGF-β1 production (Juban et al., 2018). Macrophage infiltration is the key to DN inflammatory response, and inflammation is closely related to renal fibrosis in diabetic nephropathy (Goldfine and Shoelson 2017). Therefore, in our research, we investigated the influence of DSS on macrophage proliferation and activation in the kidneys of diabetic nephropathy rats.
2 Materials and methods
2.1 Animals
Male Sprague-Dawley rats, were purchased from the Animal Experimental Center of Zhengzhou University. The rats were kept in a temperature-controlled room with a 12-h light/dark cycle. Animals were provided with free access water and a standard diet.
2.2 Animal models and treatment protocols
The rats who had adapted for 1-week were divided into two groups. The rats in the high-sugar and high-fat groups (n = 62) were fed with high-sugar and high-fat diet for 4 weeks, and the control group (CON, n = 8) were fed with routine diet. The rats of high-sugar and high-fat groups were given intraperitoneally injecting of 55 mg/kg streptozotocin (STZ). Three days after the injection of STZ, the rats with blood glucose levels ≥16.7 mmol/L were enrolled in the research.
Diabetic rats were divided arbitrarily into five groups: the diabetic model group (Model), the DSS low-dose group (DSS-L), the DSS medium-dose group (DSS-M), the DSS high-dose group (DSS-H), and the Tripterygium Glycosides group (TP). The rats of the control group and the model group were gavage with normal saline which is equivalent volume. The rats of other groups were gavaged with DSS-L 7 g/kg, DSS-M 14 g/kg, DSS-H 21 g/kg or TP 6 mg/kg once a day respectively for 8 weeks. The blood glucose, urinary urea nitrogen (UUN), β2-macroglobulin (β2-MG) and 24 h urinary protein levels were detected.
2.3 Drugs and reagents
DSS is composed of Angelica sinensis, Paeonia lactiflora, Ligusticum Chuanxiong, Poria cocos, Atractylodes macrocephala, and Alisma orientale. The ratio of the six materials is 1:4:2:1:1:2. All herbs were purchased from the Henan Zhang Zhongjing Pharmacy Company. The immunohistochemistry kit was obtained from Maxim (Fuzhou, China). lgG-DyLight 594, lgG-DyLight 488 and DAPI Staining solution were purchased from Santa Cruz (CA, USA). Anti-TLR4 antibody, Anti-MCP-1 antibody, Anti-PCNA antibody, Anti-iNOS antibody and Anti-ED-1 were obtained from Abcam (Cambridge, UK). Urinary microalbumin ELISA and β2 microglobulin ELISA kit were purchased from Shanghai Enzyme Biotechnology Co, Ltd.
2.4 Experiment instrument
Zeiss LSM laser confocal microscope (Zeiss Company, German); Blood glucose meter (Sannuo Company, Changsha, China); Chemray 240 automated biochemical analyzer (Shenzhen Redu Lifeience and Technology, Shenzhen, China).
2.5 The detection method of blood glucose, 24-h urine protein, β2 microglobulin and urinary urea nitrogen
The blood glucose was measured by a conventional blood glucose meter, the β2 microglobulin was determined by the ELISA kit, the 24-h urine protein of the rats was measured by the rat urine protein kit and the urinary urea nitrogen of the rats was measured by Chemray 240 automated biochemical.
2.6 Histopathological analysis
After the rats were sacrificed, the kidneys were cut into half. The Half of the kidneys was fixed with 10% formalin for 48 h, then paraffin-embedded and sectioned routinely, and the specimens were stained with hematoxylin-eosin (HE) and Periodic Acid-Schiff (PAS). Finally, renal pathological changes in each group were observed by light microscopy (magnification × 400).
2.7 Westen Blotting
Homogenization of kidney tissue samples in RIPA buffer supplemented with PMSF using a homogenizer, and overall protein was quantified by the BCA method. The protein specimens were separated by 10% SDS-PAGE and electrophoretically transferred to PVDF membrane (0.45 μm). After washing with Tris, membranes were incubated overnight at 4 °C with polyclonal antibodies. All antibodies were used at a dilution of 1:1000. The next day, the incubation with appropriate HRP-conjugated secondary antibodies was performed, keeping at room temperature for 1 hour. After the last wash, the specific reaction was visualized by using a chemiluminescent reagent, and image acquisition was performed using a Tanon 6600 illuminating imaging workstation.
2.8 Immunofluorescence double labeling test
Double Immunofluorescence staining for Toll-like receptor4 positive (TLR4+)/macrophage (ED-1+), proliferating cell nuclear antibodies positive (PCNA+)/macrophage (ED-1+) and inducible nitric oxide synthase positive (iNOS+)/macrophage (ED-1+) was performed. Firstly, the tissue sections were incubated with 0.4% Triton X-100 for 10 min at room temperature. Then the sections were placed in a 500ml solution of 0.1 M sodium citrate buffer (pH6.0) and microwave-treated for 10 min to complete their antigen retrieval. Subsequently, the tissue sections were incubated with 5% normal goat serum for 10min. After discarding the serum, an anti-PCNA/ED-1 antibody, an anti-iNOS/ED-1 antibody and an anti-TLR4/ED-1 antibody at a concentration of 1:100 were added and reacted at 4 °C overnight. Immediately, lgG-DyLight 594-labeled secondary antibody (1:500) and IgG-DyLight 488-labeled secondary antibody (1:500) were added and incubated for 1–2 h in the dark environment at 37 °C.
2.9 Statistical analysis
All values that are expressed as means ± SEM were analyzed by one-way analysis of variance (ANOVA). A value of p < 0.05 was considered to have statistical significance.
3 Results
3.1 Biochemical parameters of experimental animals
In contrast to the control rats, blood glucose was increased notably in the model rats (P < 0.01). Compared with the model group, the blood glucose level was decreased in DSS-M group and DSS-H group (Fig. 1A).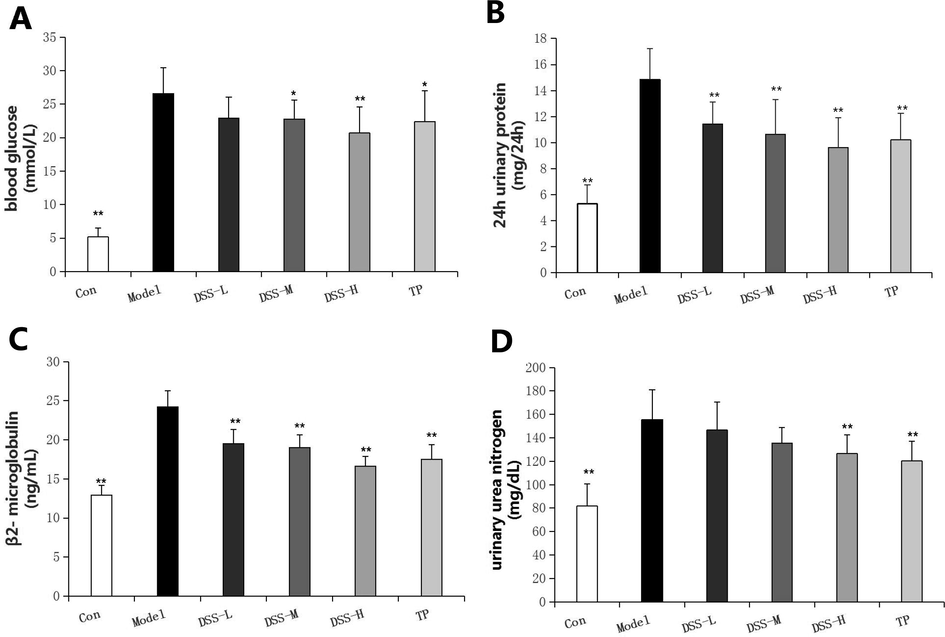
Effect of DSS on the expression of blood glucose, 24 h urinary protein, β2-microglobulin and urinary urea nitrogen in the control and experimental groups of rats. *P < 0.05, **P < 0.01 vs model.
There was a significant difference in the level of 24 h urinary protein and β2-microglobulin between the control rats and the model rats (P < 0.01), while the treatment of DSS could reduce urinary protein and β2-microglobulin in the diabetic rats conspicuously (P < 0.01) (Fig. 1B and C).
The urinary urea nitrogen content of the model rats was notably higher than that of the control rats (P < 0.01). In addition, the use of DSS-H can reduce the urea nitrogen content notably (P < 0.01) (Fig. 1D).
3.2 Renal histopathological changes
In the control rats, the renal corpuscles were intact structurally, the glomerular structures of capillary globules were clear, the renal tubules were regular and their epithelial cells were arranged neatly. No infiltration of inflammatory cell was observed. However, renal pathological changed in DN such as increased glomerular volume, increased mesangial cells and mesangial matrix, as well as thickening of the glomerular basement membrane and balloon wall. And some areas had focal glomerular sclerosis. In addition, we can observe irregularities in the renal tubules around the glomeruli, individual tubular atrophy and infiltration of focal inflammatory cells. Other than that, PAS staining explained that the glomeruli and renal tubules exhibited different degrees of glycogen deposition in the model rats. And the capillary basement membrane and mesangial areas of the glomerulus were more pronounced. After the administration of DSS or TP, these (above-mentioned) pathological changes in each group were all improved, with the improvement in the DSS-H group being the most significant (Fig. 2).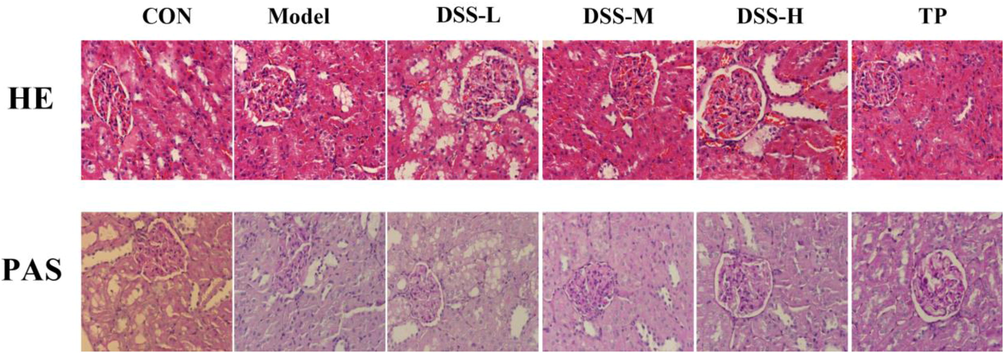
Effect of DSS on the renal histology of DN model rats.
3.3 Expression of ED-1, MCP-1, TLR4 in kidney tissue
In order to observe the status of renal macrophages, we examined renal macrophage-specific antigen ED-1 and chemokine MCP-1, and observed the expression of TLR4 receptor.
As Fig. 3(B–D) showed, the protein expression levels of ED-1, MCP-1, and TLR4 in the model rats were up-regulated evidently in comparison to the control rats, while DSS decreased the levels of ED-1, MCP-1, and TLR4 in a dose-dependent manner.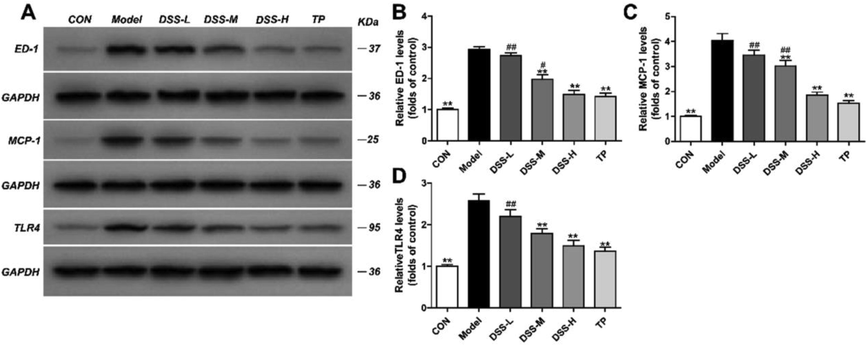
Influence of DSS on the expression of ED-1, MCP-1, TLR4 in the DSS groups. Protein levels of ED-1, MCP-1 and TLR4 in each group were examined by western blot assay. GAPDH was used as a loading control (A). Quantification of ED-1 (B), MCP-1 (C) and TLR-4 (D) protein expression. The results were presented as mean ± SD (n = 5). *p < 0.05, **p < 0.01 vs Model group, #p < 0.05, ##p < 0.01 vs TP group.
3.4 Expression of ED-1+/TLR4+ in rat kidney
To explore whether DSS inhibits the TLR4 receptor of macrophages, we examined renal macrophage-specific antigen ED-1+/TLR4+.
As Fig. 4 (B) showed, compared with the control group, the protein expression of ED-1+/TLR4+ was increased in the model group. After being treated by DSS or TP, the level of ED-1+/TLR4+ displaced reduced remarkably compared with the model rats (p < 0.05).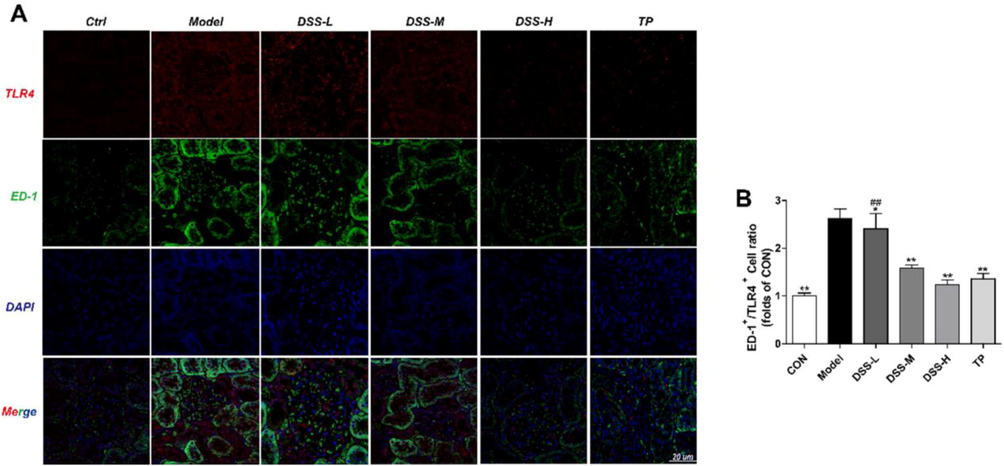
Influence of DSS on the expression of ED-1+/TLR4+ in the DSS groups. The expression of ED-1+ (green)/TLR4+ (red) in each group were detected by immunofluorescence (A-B). The results were presented as mean ± SD (n = 3). *p < 0.05, **p < 0.01 vs Model group, #p < 0.05, ##p < 0.01 vs TP group.
3.5 Expression of ED-1+/PCNA+ in rat kidney
For the purpose of exploring whether DSS inhibits macrophage proliferation, we examined renal macrophage specific antigen ED-1+/PCNA+.
As Fig. 5(B) showed, compared with the control group, the protein expression of ED-1+/PCNA+ was increased in the model group. In contrast to the model rats, the level of ED-1+/PCNA+ in DSS-M was decreased clearly (p < 0.05), and they were reduced remarkably in the rats of DSS-H group and TP group (p < 0.01).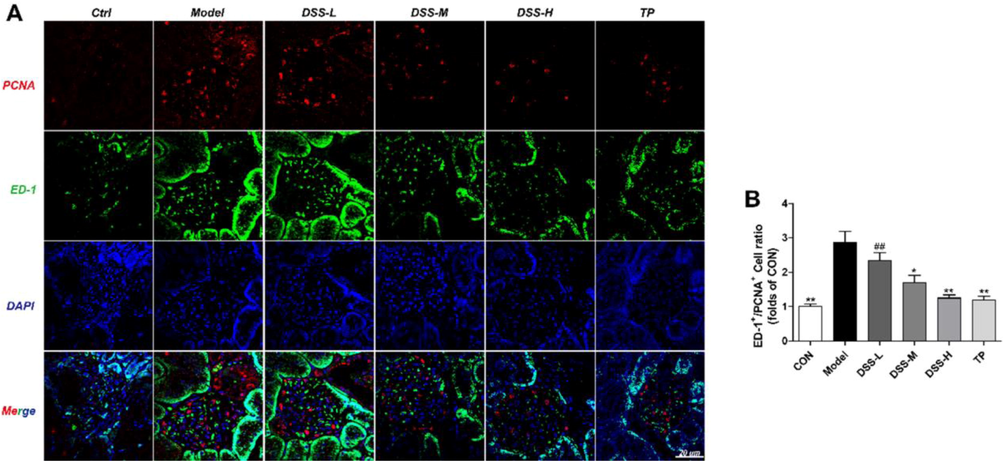
Influence of DSS on the expression of ED-1+/PCNA+ in the DSS groups. The expression of ED-1+ (green)/PCNA+ (red) in each group were detected by immunofluorescence (A-B). The results were presented as mean ± SD (n = 3). *p < 0.05, **p < 0.01 vs Model group, #p < 0.05, ##p < 0.01 vs TP group.
3.6 Expression of ED-1+/iNOS+ in rat kidney
With the aim to explore whether DSS inhibits macrophage activation, we examined renal macrophage-specific antigen ED-1+/iNOS+.
As Fig. 6(B) showed, compared with the control group, the protein expression of ED-1+/iNOS+ was increased in the model group. The level of ED-1+/iNOS+ was decreased evidently in the rats of DSS-H and TP groups in comparison to the model rats (p < 0.01).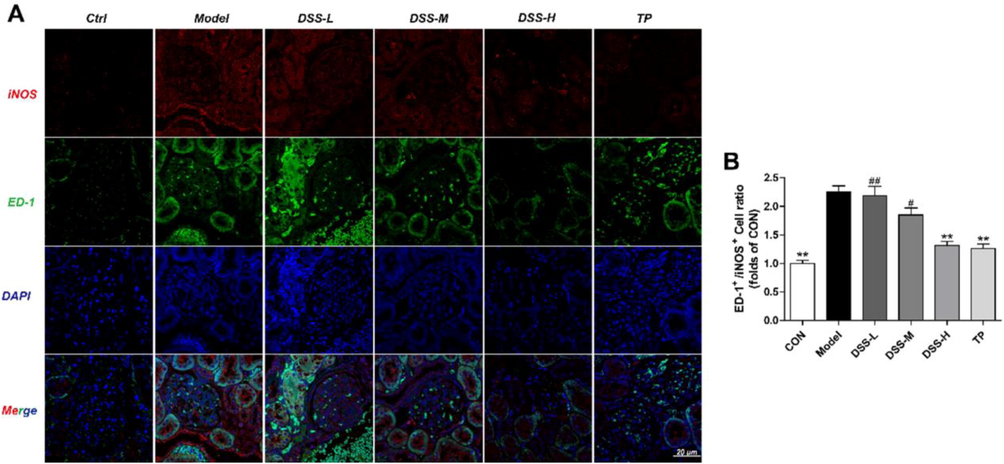
Influence of DSS on the expression of ED-1+/iNOS+ in the DSS groups. The expression of ED-1+ (green)/iNOS+ (red) in each group were detected by immunofluorescence (A-B). The results were presented as mean ± SD (n = 3). *p < 0.05, **p < 0.01 vs Model group, #p < 0.05, ##p < 0.01 vs TP group.
4 Discussion
Elevated blood glucose is one of the important clinical symptoms of diabetes. Our results indicated that DSS could ameliorate the blood glucose level of diabetic rats (Fig. 1). Moreover, the increase of urinary urea nitrogen and β2-MG can be used as an indicator to reflect the function of glomerular filtration (Kim et al., 2018; Turk et al., 2019). In our experiment, biochemical results indicates that the filtration function of glomeruli in diabetic rats was affected and DSS can improve renal function damage (Fig. 1). The results of HE staining and PAS staining indicates that the renal tissues structures of diabetic rats were destroyed and inflammatory cells infiltrated. However, DSS treatment has the function of improving renal pathological changes (Fig. 2). Our previous study found that DSS can reduce TGF-β1 (Li et al., 2013). TGF-β1 is a transforming growth factor secreted by macrophages mainly (Liu et al, 2018). Therefore, we further explore the relationship between DSS and macrophages.
We all know that ED-1 is a macrophage surface-specific marker antigen. It has been observed that ED-1 is expressed specifically on the surface of macrophages (Zhao et al., 2019a,b). Thus, the expression of ED-1 in kidney tissue can reflect the amount of macrophages. Monocyte chemotactic protein is the main chemokine that induces monocytes' chemotaxis. As a chemokine, MCP-1 has an influence on the accumulation and the function of macrophages (Ahn et al., 2019). Our results indicated that DSS inhibited the expression of ED-1 and MCP-1 remarkably, indicating that DSS can inhibit infiltration of macrophage in renal tissue (Fig. 3).
The TLR4 receptor is a type of Toll-like receptors. It not only recognizes foreign pathogens, but also recognizes endogenous substances and degradants. Studies have shown that inflammatory damage can be reduced by inhibiting the expression of TLR4 (Fei et al., 2019; Sivanantham et al., 2019; Zhang et al., 2019a,b,c). TLR4 is usually expressed in multiple cells and tissues (Jiang et al., 2019; Shi et al., 2019; Sivanantham et al., 2019; Van Maele et al., 2019; Xie et al., 2019; Zhang et al., 2019a,b,c; Zhao et al., 2019a,b). In our WB experiment, we detected the expression of TLR4 in renal tissues. The results showed that DSS can inhibit the expression of TLR4 in the kidney tissue of the diabetic rats (Fig. 3). However, we can’t know whether TLR4 has a connection with macrophages. So, we examined the expression of ED-1+/TLR4+ in renal tissue by immunofluorescence double-labeling experiments. The results proved that DSS can inhibit the expression of TLR4 in macrophages, indicating that DSS inhibits the activation of macrophages by inhibiting the expression of TLR4 receptor on the surface of macrophages (Fig. 4).
Proliferating cell nuclear antigen are indicators of cell proliferation. A number of studies have examined cell proliferation by detecting the expression of PCNA in cells (Ragy and Ahmed, 2019; Wang et al., 2018). Therefore, the expression of ED-1+/PCNA+ in kidney tissue of rats reflects the increase of macrophage. In addition, macrophages in tissues are classified into two types according to activation patterns and immune functions: classical activation (M1 type) and alternative activation (M2 type) (Bardi et al., 2018). Among them, M1 type macrophages can promote the synthesis of NO by inducing the production of reactive oxygen species, and finally release various inflammatory factors, such as iNOS. iNOS is a source of abnormal NO production. It can lead to nitrosation damage, endothelial dysfunction, and proteinuria and glomerular inflammation (Klessens et al., 2017). Both PCNA expression and iNOS expression can reflect the activation of macrophages. Therefore, we also detected changes in ED-1+/PCNA+, ED-1+/iNOS+ in the kidney by immunofluorescence double labeling assay. The outcomes exhibited that ED-1+/PCNA+, ED-1+/iNOS+ raised evidently in the model rats in comparison to the control rats, illustrating that macrophages were activated (Fig. 5 and Fig. 6). In addition, DSS significantly reduced the expression of ED-1+/PCNA+, ED-1+/iNOS+ (Fig. 5 and Fig. 6). In summary, all results indicate that DSS inhibits the activation of macrophages.
We finally draw the conclusion that DSS can improve renal tissue damage in a manner that inhibits the proliferation and activation of renal macrophages.
Acknowledgements
This study was funded by the National Natural Science Foundation of China (Grand No. 81603527), the Young Core Teacher of Henan Province, China (Grant No. 2016GGJS-080), Science and Technology Project of Henan Province, China (Grand No. 162102310466), Key Scientific Research Projects of Henan Province Colleges and Universities, China (Grand No. 17A360010).
Conflicts of interests
The authors declare that they have no conflicts of interests.
References
- Quantification of monocyte chemotactic activity in vivo and characterization of blood monocyte derived macrophages. J. Vis. Exp.. 2019;150
- [CrossRef] [Google Scholar]
- Melanoma exosomes promote mixed M1 and M2 macrophage polarization. Cytokine. 2018;105:63-72.
- [CrossRef] [Google Scholar]
- Eupatilin attenuates the inflammatory response induced by intracerebral hemorrhage through the TLR4/MyD88 pathway. Int. Immunopharmacol.. 2019;76:105837
- [CrossRef] [Google Scholar]
- Therapeutic approaches targeting inflammation for diabetes and associated cardiovascular risk. J. Clin. Invest.. 2017;127(1):83-93.
- [CrossRef] [Google Scholar]
- Inflammation and immunity pathways regulate genetic susceptibility to diabetic nephropathy. Diabetes. 2018;67(10):2096-2106.
- [Google Scholar]
- The activation of high mobility group Box1 and toll-like receptor 4 is involved in clopidogrel-induced gastric injury through p38 MAPK. Pharmazie. 2019;74(9):547-552.
- [CrossRef] [Google Scholar]
- AMPK activation regulates LTBP4-dependent TGF-β1 secretion by pro-inflammatory macrophages and controls fibrosis in Duchenne muscular dystrophy. Cell Rep.. 2018;25(8):2163-2176.
- [CrossRef] [Google Scholar]
- Supplementation of abelmoschus manihot ameliorates diabetic nephropathy and hepatic steatosis by activating autophagy in mice. Nutrients. 2018;10(11)
- [CrossRef] [Google Scholar]
- Macrophages in diabetic nephropathy in patients with type 2 diabetes. Nephrol. Dial. Transplant.. 2017;32:1322-1329.
- [CrossRef] [Google Scholar]
- The vicious cycle between transglutaminase 2 and reactive oxygen species in hyperglycemic memory-induced endothelial dysfunction. FASEB J.. 2019;fj201901358RR
- [CrossRef] [Google Scholar]
- Effect of modified angelica peony powder on inflammatory cytokine in patients with early diabetic nephropathy. Lishizhen Med. Mater. Med. Res.. 2013;24(6):1447-1448.
- [Google Scholar]
- TGF-β1 secreted by M2 phenotype macrophages enhances the stemness and migration of glioma? Cells via the SMAD2/3 signalling pathway. Int. J. Mol. Med.. 2018;42(6):3395-3403.
- [CrossRef] [Google Scholar]
- Protective effects of either C-peptide or l-arginine on pancreatic β-cell function, proliferation, and oxidative stress in streptozotocin-induced diabetic rats. J. Cell. Physiol.. 2019;234(7):11500-11510.
- [CrossRef] [Google Scholar]
- Monocytes promote crescent formation in anti-myeloperoxidase antibody-induced glomerulonephritis. Am. J. Pathol.. 2017;187(9):1908-1915.
- [CrossRef] [Google Scholar]
- Chitosan oligosaccharide-mediated attenuation of LPS-induced inflammation in IPEC-J2 cells is related to the TLR4/NF-κB signaling pathway. Carbohydr. Polym.. 2019;219:269-279.
- [CrossRef] [Google Scholar]
- Tannic acid prevents macrophage-induced pro-fibrotic response in lung epithelial cells via suppressing TLR4-mediated macrophage polarization. Inflamm. Res.. 2019;1–14
- [CrossRef] [Google Scholar]
- Comparison of signs on magnetic resonance image of shoulder between patients with stage 4 chronic kidney disease and hemodialysis patients with healthy controls. J. Back Musculoskelet. Rehabil.. 2019;1–6
- [CrossRef] [Google Scholar]
- Toll-like Receptor 4 signaling in hematopoietic-lineage cells contributes to the enhanced activity of the human vaccine adjuvant AS01. Eur. J. Immunol. 2019
- [CrossRef] [Google Scholar]
- The analysis of risk factors for diabetic nephropathy progression and the construction of a prognostic database for chronic kidney diseases. J. Transl. Med.. 2019;17(1):264.
- [CrossRef] [Google Scholar]
- Xueshuantong for injection ameliorates diabetic nephropathy in a rat model of streptozotocin-induced diabetes. Chin. J. Physiol.. 2018;61(6):349-359.
- [CrossRef] [Google Scholar]
- Dendrobium huoshanense polysaccharide regulates intestinal lamina propria immune response by stimulation of intestinal epithelial cells via toll-like receptor 4. Carbohydr. Polym.. 2019;222:115028
- [CrossRef] [Google Scholar]
- A glimpse of the mechanisms related to renal fibrosis in diabetic nephropathy. Adv. Exp. Med. Biol.. 2019;1165:49-79.
- [CrossRef] [Google Scholar]
- Catalpol ameliorates LPS-induced endometritis by inhibiting inflammation and TLR4/NF-κB signaling. J. Zhejiang Univ. Sci. B. 2019;20(10):816-827.
- [CrossRef] [Google Scholar]
- The metabolic regulator Lamtor5 suppresses inflammatory signaling via regulating mTOR-mediated TLR4 degradation. Cell. Mol. Immunol. 2019
- [CrossRef] [Google Scholar]
- Raltegravir attenuates experimental pulmonary fibrosis in vitro and in vivo. Front. Pharmacol.. 2019;10:903.
- [CrossRef] [Google Scholar]
- Increased impressions of TLR-2, ED-1 and IL-1β in children with minimal change nephrotic syndrome: A clinical report from renal biopsy. Chin. J. Integr. Trad. West. Nephrol.. 2019;20(5):402-405.
- [Google Scholar]
- Effect of Jiangbing Tiaozhi capsule on PKC and TGF-β1 expression in rats with early diabetic nephropathy. Chin. J. Basic Med. Trad. Chin. Med.. 2011;17(3):284-286.
- [Google Scholar]
- Inhibition of HIF-1a-mediated TLR4 activation decreases apoptosis and promotes angiogenesis of placental microvascular endothelial cells during severe pre-eclampsia pathogenesis. Placenta. 2019;83:8-16.
- [CrossRef] [Google Scholar]







