Translate this page into:
Effect of Artemisia annua on kidney in gentamicin-induced nephrotoxicity in mice through regulation of the COX-2, NF-κB pathway
⁎Corresponding author. o.saeeed@tu.edu.sa (Omaima Nasir)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background
This study aimed to examine the role of Artemisia annua in kidney functions in gentamicin-induced nephrotoxicity in mice.
Methods
In this study, 15 mice were used and divided into four groups. Each group has four mice; the first group is considered a control group with three mice due to receiving normal saline. Group II consists of an extract of Artemisia annua, group III consists of gentamicin, and Group IV consists of a combination of Artemisia annua and gentamicin. This process was continued for 15 days. All the mice were induced, and serum was extracted and used for biochemical parameters such as Creatinine, Urea, Uric acid, TNF-α, MDA, GSH, and Catalase (CAT) levels—additionally, histological and quantitative real-time PCR (qRT-PCR) analysis.
Results
The results of this study confirmed biochemical values such as creatinine, Urea, and UA values showed a positive association (p<0.05), and showed a nominal association with histological analysis (p > 0.05). The Gentamicin group has a strong association with COX-2, NF-κB, and TGF-β genes (p < 0.05).
Conclusion
This study confirms gentamycin has a role in kidney functions with nephrotoxicity in mice and the protective effect of Artemisia annua.
Keywords
Artemisia annua
Gentamicin
Mice and biochemical parameters
qRT-PCR analysis
1 Introduction
The Asteraceae plant family includes Artemisia annua L. (A. annua), which is native to Asia (primarily China, Japan, and Korea) after being imported to Poland, Brazil, Spain, France, Italy, Romania, the United States, and Austria, it was domesticated. Herbalists in China have been using it to cure various conditions since ancient times (Lee et al., 2023). Only artemisinin, a sesquiterpene trioxane lactone with an endoperoxide bridge required for bioactivity, is found in A. annua. Artemisinin and its derivatives demonstrated anticancer efficacy in human and animal cancer cell lines by inhibiting cell growth, inducing apoptosis, and inhibiting angiogenesis and metastasis (Salaroli et al., 2022).
The kidneys play several essential roles in the body. Their primary function is to regulate the fluid equilibrium of the body by filtering and secreting metabolites and minerals from the blood and excreting nitrogenous waste combined with water as urine. The kidneys control the body's blood pressure, glucose metabolism, and red blood cell production. The kidneys filter approximately 180 L of blood daily, roughly four times the amount that passes through any other organ. As a result, circulating pollutants can cause tissue damage in the kidneys. There is a high morbidity and mortality rate among those suffering from renal disease, making it the ninth largest cause of death worldwide. One of the most prevalent drug or toxin-induced kidney diseases is nephrotoxicity. Aminoglycoside antibiotics, chemotherapeutic agents, chemical reagents, and heavy metals are potent therapeutic drugs that can harm the kidneys and result in acute renal failure. Aside from medications, other factors such as aging, diabetes, hypertension, liver disease, and oliguria can cause acute renal failure. Medicinal herbs containing nephroprotective compounds can prevent and treat nephrotoxicity (Wannes and Tounsi 2022).
Gentamicin is a potent aminoglycoside antibiotic for gram-negative bacterial infections (Krause et al., 2016). Gentamicin, presumably through the transition of free radicals, ends up causing cellular damage to the kidney, liver, and organs of hearing or balance or the auditory nerve (Noorani et al., 2011; Pai et al., 2012). The most common gentamicin adverse effect is toxicity towards the renal system, which builds up in the nephron’s epithelial cells (Erdem et al., 2000). This was demonstrated further by increasing the formation of oxygen radicles, nephron-oxidative lipid degradation, and renal nitrogen monoxide synthesis (Kopple et al., 2002).
Currently, the utilization of plant-origin medicines has attracted researchers as their plentiful availability of bioactive components and very low to no side effects compared with synthetic drugs. Several studies have been conducted on the protective effects of medicinal herbs on the liver and kidney (Jacob Jesurun and Lavakumar 2016). Consequently, several preliminary and clinical trial researches have concentrated on antioxidants or medicines with the promise antioxidative, anti-inflammatory, and nephron-protective activities in the last ten years (Cao et al., 2019; Elfaky et al., 2019; Medić et al., 2019). Research has revealed that the antioxidant compounds existing in medicinal plants or herbs could preclude gentamicinintigate nephrotoxicity containing; Aegle marmelos L (A. marmelos) (Kore et al., 2011). Abutilon indicum L (A. indicum) (Jacob Jesurun and Lavakumar 2016). Boerhavia diffusa L. (B. diffusa), Phyllanthus Embilica L, (P. Embilica) (Olaleye et al., 2010). Ficus racemose L (L. F. racemose) (Gowda and Swamy 2012). Tribulus terrestris (T. terrestris). Further, it is documented a wide range of crude herbal extracts provide a rich supply of potentially beneficial novel components for treating renal issues (Abdel-Kader et al., 2016).
Artemisia annua is a plant species indigenous to East Asia, specifically China, Korea, and Mongolia (Rath et al., 2004). Artemisinin, an antimalarial compound, was isolated from the plant's extract and has since gained widespread recognition. Artemisinin and its derivatives are useful in treating viral, bacterial, fungal, and malarial infections (Lappan and Peacock 2019). Previously, artemisinin and its product were subjected to therapies for treating respiratory disorders such as asthma and certain tumors through powerful anti-inflammatory effects. Not only the substances that were isolated but also an extract of the plant has been shown to have anticancer, anti-obesity (Efferth et al., 2001), and anti-rheumatoid arthritis effects (Efferth et al., 2001). Based on this evidence of Artemisia annua health advantages, we used a male mouse model of C57/BL6J gentamicin nephrotoxicity to investigate Artemisia annua influence on kidney and liver function activity.
2 Materials and methods
2.1 Chemicals
Gentamicin (80 mg/2 ml), was obtained locally, SPIMACO, Saudi Arabia, as were reduced glutathione (GSH), trichloroacetic acid, thiobarbituric acid (TBA), bovine serum albumin (BSA), and Bradford reagent from Sigma Aldrich Chemical Company (St. Louis, MO, U.S.A).
2.2 Experimental design with animals
C57/BL6J pathogenic free male mice weighing 20–25 g were approved by the Institutional Review Board of King Fahd-Medical Research Center, King Abdulaziz University, Jeddah, Saudi Arabia. All of the experiments were carried out at the biology department of the University College of Turabah. All mice were fed standard granulated food and kept in standard conditions (22–24 °C, 50–70% humidity, and a 12-hour light/dark cycle) (C1310, Altromin, Heidenau, Germany).
2.3 Artemisia annua preparation
The leaves of Artemisia scoparia were collected from Wadi Turabah at Turabah city southwestern of Saudi Arabia. An authentic person from the biology department, The University of Taif, Saudi Arabia, identified the plant. The leaves were cleaned with clean water, dried darkly, and roughly ground. The cold extract was used to get the crude extract, which was generated by placing 200 g powders in 500 ml of 95% ethanol in a clean glass jar for seven days at room temperature before filtering. The solvent was evaporated, and the resultant dried extracts were weighed and kept in a refrigerator at four °C till used.
2.4 Experimental design
Mice were randomly divided into four experimental groups (n = 4 in each group).
Group-1: The control group- received normal saline for a consecutive span of 15 days as positive control.
Group-2: Artemisia annua extract (1%) orally for a consecutive span of 15 days as positive control, (Eteng et al., 2013).
Group-3: Gentamicin 80 mg/kg intraperitoneally for a consecutive span of 15 days as negative control, (Avdagić et al., 2008).
Group-4: Gentamicin+ Artemisia annua for a consecutive span of 15 days.
2.5 Sample collection
Diethyl ether was used to euthanize the animals 24 h after the last drug administration, and blood samples were taken from the jugular vein. Serum was isolated from blood samples by centrifugation at 1500 rpm for 10 min at four °C and stored at 20 °C until analysis. After that, the animals were slaughtered, and the kidney and liver organs were harvested. The kidneys and liver were washed with saline and fixed in 10% phosphate-buffered formalin for histological studies. The kidneys and liver were immediately cleaned in ice-cold saline and cut in half. For biochemical estimation, one portion was homogenized (1/10 w/v) in ice-cold Tris-HCl buffer (0.1 M, pH 7.4) and stored in the refrigerator at 20 °C. The other part was kept in liquid nitrogen at -80 °C for real-time PCR experiments. The Bradford technique was used to determine the protein content of all homogenates samples, with BSA serving as the standard (Bonjoch and Tamayo 2001).
2.6 Serum biochemical assays
The serum in the ordinary vial was sorted in a cooled centrifuge at 4 °C for fifteen minutes. The technique of determining serum creatinine described earlier was used, and the method of determining serum urea used was the approach of utilising commercial kits. The amount of serum uric acid (UA) was determined by the use of the Fossate et al. (the enzymatic colorimetric method), although with some minor adjustments (Fossati et al., 1980) using kits provided by (Biodiagnostic, Giza, Egypt). Additionally, we have examined TNF-α, MDA, GSH, and Catalase (CAT) levels as described in our recent publication (AlThobaiti 2023) using kits and reagent provided by (Glory Science Co., Hangzhou, China).
2.7 Quantitative real-time PCR and gene expression analysis
Trizol reagent was used to extract total RNA from 15 mice used in this study (Invitrogen, Life Technologies, Carlsbad, CA, USA). Extracted genomic ribonucleic acid was quantified with NanoDrop (Alsaif et al., 2022), and finally, reverse transcription was carried out using the kit (Fermentas, MA, USA.). ABI SYBR kit and 7500 ABI RT-PCR equipment were used for quantitative real-time PCR (qRT-PCR). The complete protocol and relative gene expression were calculated as per (Song et al., 2022). The typical temperature profile consisted of a high of 95 °C for 5 min, followed by lows of 56 and 72 °C for 30 s each in 45 cycles. Afterward, the Ct for each sample is calculated by deducting the Ct for -actin from the Ct for each sample. The target gene's elevated signals were normalized by the housekeeping gene β-actin. 2 -ΔΔCT methods were used to the analysis of amplification data (Livak and Schmittgen 2001).
2.8 Histopathological examination of renal tissues
Tissue cells were fixed with 10% formaldehyde before being dehydrated in escalating graded ethanol, cleaned with xylene, and finally embedded in paraffin. Following that, hematoxylin and eosin dye were used to stain paraffin slices of kidney cut to a thickness of 5 micrometers with a microtome (hemotoxin and eosin).
2.9 Statistical analysis
The mean and standard deviation were used to represent all results. A one-way ANOVA test conducted multiple comparisons between groups, followed by the LSD test for biochemical parameters and the Dunnett T3 test for real-time RT-PCR findings. SPSS statistics programme was used for statistical analyses (SPSS; version 20). Shapiro-Wilk and Levene tests confirmed variance normality and homogeneity. The P-value threshold for statistical significance between groups was established at 0.05. Graphs were created using version 8.0.2 of GraphPad Prism on Windows.
3 Results
3.1 Biochemical analysis
The results confirmed a significant association with Creatinine, Urea, Uric Acid, and glutathione (p < 0.0001); both TNF-α and MDA levels showed a nominal association, and CAT showed a negative association. Fig. 1 describes the biochemical parameters present in mmol/l for all seven parameters. The mean of serum parameters such as creatine (2.23 ± 0.09), urea (36.98 ± 1.44), UA (10.13 ± 0.4), TNF-α (636.83 ± 24.8) and MDA (49.15 ± 1.91) levels were found to be high in the group-III. Finally, GSH (4.89 ± 0.17) and CAT (200.12 ± 6.89) levels were found to be high in the control group (group-I).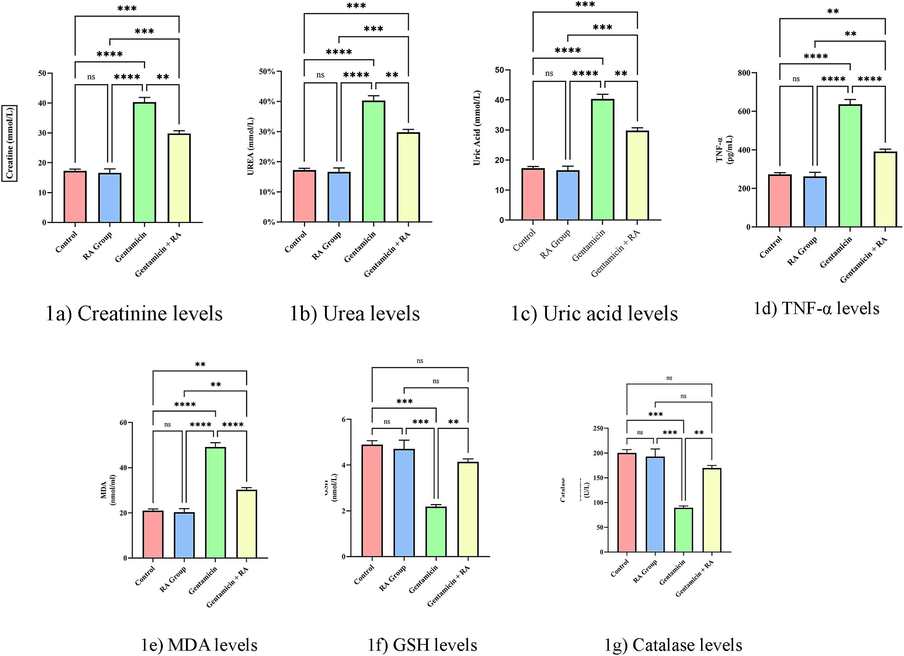
List of biochemical parameters examined in this study. Data shown as mean ± SE** p < 0.01, *** p < 0.001 and **** p < 0.0001 vs. gentamicin treated group. Statistical analysis was done with One-way ANOVA, Tukey’s post hoc test multiple comparisons.
3.2 Histopathological analysis
Fig. 2 depicts the results of a microscopic examination of kidneys, which showed that the control and RA treatment groups had renal corpuscles with glomeruli (G) surrounded by capsular space within the renal cortex (see text). The broad capsular gap surrounding G was seen in the gentamicin group (white arrowhead), as was degradation and separation of the tubular epithelium (black arrow), hyaline cast (black arrow), and edema in the intestinal tissue (white arrow). Last, gentamicin-treated groups displayed PT and DT in addition to intact G, with modest congestion in both glomerular capillaries and intestinal blood vessels (shown by arrowheads). H&E staining was present in all areas; Bar = 50 µm.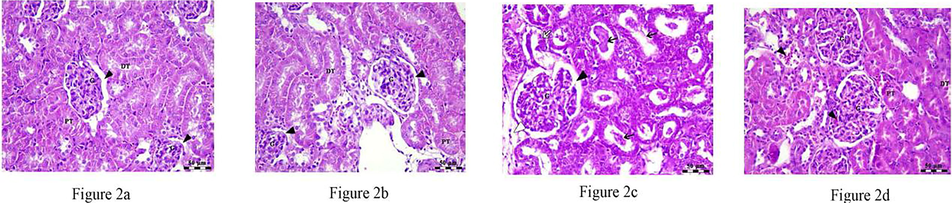
a–d: Photomicrograph of renal cortex of control group and RA treated group showing intact renal corpuscles with glomeruli (G) surrounded with capsular space (arrow heads) in addition to normal proximal (PT) and distal convoluted tubules (DT), whereas, gentamicin treated group showed G surrounded by wide capsular space (black arrow head), degeneration and separation of tubular epithelium (white arrow), hyaline cast (black arrow) and edema in the interstitial tissue (white arrow head). Finally, gentamicin treated group showed PT and DT in addition to intact G with mild congestion in both glomerular capillaries and interstitial blood vessels (arrow heads). All 2a-2d Stains show H&E, Bar = 50 μm.
3.3 qRT-PCR analysis
The expression of COX-2, NF-κB, and TGF-β genes was detected by qRT-PCR analysis, shown in Figs. 3–5. The COX-2 gene negatively affects both the control and RA groups. However, the gentamicin group showed a positive association. In contrast, the combination of gentamicin and RA-treated groups showed a negative association with brown stains in the renal corpuscles and tubular epithelium (Fig. 3). The NF-κB gene, on the other hand, exhibits mild expression in the control group, negative association in the RA-treated group, diffuse positive expression in the gentamicin group, and finally, weak positive expression in the gentamicin and RA-treated groups (Fig. 4). TGF-β was found to have a mildly positive expression with renal cortex in controls, a negative association in the RA-treated group, a significant increase in the gentamicin-treated group, and decreased levels when the gentamicin and RA-treated groups were combined (Fig. 5).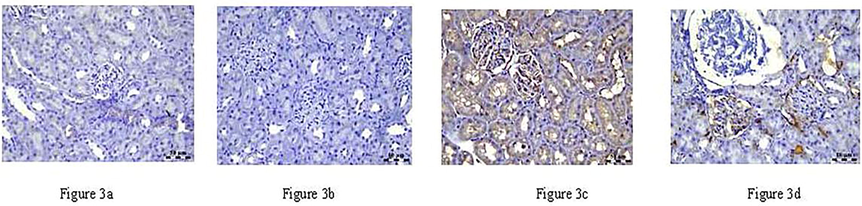
a–d: The COX-2 gene in control and RA treated groups showed negative association, where in gentamicin treated group showed increase and combination of gentamicin and RA treated groups showed decrease in COX-2 expression in both renal corpuscles and tubular epithelium with brown stain.
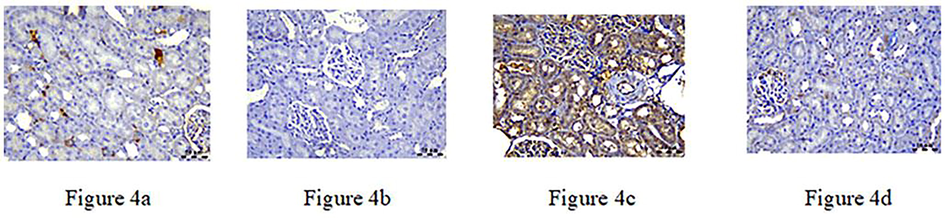
a–d: NF-κB gene shows mild expression in control group, negative expression in RA treated group, diffuse positive expression in gentamicin group and weak positive expression in combination of gentamicin and RA treated groups.
![a–d: Display the renal cortex of control group shows mild positive expression in TGF-β, negative association in RA treated group, significant increase in gentamicin treated group and in combination of gentamicin and RA treated groups, it shows significant decrease [IHC, Bar = 50 μm].](/content/185/2023/35/7/img/10.1016_j.jksus.2023.102813-fig5.png)
a–d: Display the renal cortex of control group shows mild positive expression in TGF-β, negative association in RA treated group, significant increase in gentamicin treated group and in combination of gentamicin and RA treated groups, it shows significant decrease [IHC, Bar = 50 μm].
4 Discussion
The biochemical parameters including Creatinine, Urea, Uric acid, TNF-α, MDA, GSH and CAT parameters, were studied in this study. Anova analysis confirmed a positive association with Creatinine, Urea, UA, and GSH (p < 0.05). The histological analysis showed intermediate results obtained in the four groups. However, staining was present in all areas; Bar = 50 µm. Finally, qRT-PCR analysis showed COX-2 gene was positively associated with the gentamicin group, diffuse positive expression in the gentamicin group in the NF-κB gene, and a significant increase in the gentamicin-treated group in the TGF-β gene.
The kidneys play a crucial role in human health by filtering the blood and eliminating metabolic byproducts and harmful waste. Nephrotoxicity refers to the rapid decline in kidney function due to the toxic action of medications and other substances. Nephrotoxicity refers to the detrimental effect of substances on renal function. Nephrotoxicity can occur due to several different pathways, such as those involving the kidneys' tubules, glomeruli, crystals, inflammation, and inflammatory responses. Molds and fungi, cancer-causing chemicals like cisplatin, antibiotics like aminoglycosides, and metals like lead, arsenic, and mercury are all potential causes of nephrotoxicity. Both inherited, and environmental factors have been linked to renal failure. Extrinsic variables include cardiovascular disease, obesity, diabetes, sepsis, lung failure, and liver failure.
In contrast, intrinsic factors are conditions like glomerulonephritis, polycystic kidney disease, tubular cell death, and stones that affect kidney function (Pathan). Involvement of the kidney in the metabolism of pharmaceuticals and other xenobiotics leads to nephrotoxicity. Since administering nephrotoxic medications is inevitable in healthcare, drug-induced nephrotoxicity remains a significant issue. According to numerous studies, between 1.8% and 16% of all acute renal failures (Dubiwak et al., 2022; Osman et al., 2022). The most common laboratory findings in drug-induced nephrotoxicity are Creatinine, Urea, and UA. The kidneys were tested by measuring urea and creatinine in the blood. These two values change when kidney nephrons are severely injured (Rahmat et al., 2014).
Gentamicin is an aminoglycoside antibiotic used to treat Gram-negative bacteria infections; however, nephrotoxicity and hepatotoxicity have been described as significant side effects, with ROS being the primary culprits in both cases. Up to 50% of patients experience nephrotoxicity in therapeutic doses, whereas toxic doses may cause lifelong kidney impairment. The kidneys are in charge of filtering and regulating the blood, among other things. The liver controls various critical processes, including detoxification, toxin removal, and the metabolic and biotransformation of multiple chemicals (Khalil et al., 2022).
However, its clinical value is limited due to its severe effects on renal and liver functioning (Khalil et al., 2022). To put it simply, oxidative stress occurs when a tissue lacks antioxidants and an abundance of reactive oxygen species (ROS), more often known as free radicals. Drug-induced oxidative stress causes oxidative stress because it increases the production of free radicals at a higher rate than antioxidants can neutralize them. Increased expression of genes involved in inflammatory signaling, such as NF-kB and cytokines, contributes to this occurrence (Elsayed et al., 2022; Khalil et al., 2022). In particular, oxidative stress and inflammation triggered by GTN have been associated with its toxicity (Rho and Yoon 2017; Laaroussi et al., 2021). This ultimately results in cell apoptosis. Induction of hepatotoxicity by gentamicin has been documented by several prior researchers, with symptoms including hepatocyte degeneration and necrosis (Lukiswanto et al., 2022; Wijayanti et al., 2023).
The current study results were found to be engaging with gentamicin as well as Artemisia annua. The results fluctuated based on the groups and parameters used in this study.
5 Conclusion
In Conclusion, gentamicin and a combination of Artemisia annua and gentamicin showed elevated and associated levels with biochemical parameters such as Creatinine, Urea, UA, and GSH. qRT-PCR analysis showed a strong association in the gentamicin family. This study confirms gentamycin has a role in kidney functions with nephrotoxicity in mice and the protective effect of Artemisia annua.
Acknowledgement
The researchers want to thank the Deanship of Scientific Research Taif University for funding this work.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Nephroprotective and hepatoprotective effects of Tribulus terrestris L. growing in Saudi Arabia. J. Pharmacy Pharmacognosy Res.. 2016;4(4):144-152.
- [Google Scholar]
- Molecular screening via sanger sequencing of the genetic variants in non-alcoholic fatty liver disease subjects in the Saudi population: A hospital-based study. Metabolites. 2022;12(12):1240.
- [Google Scholar]
- Protective effect Spirulina against Monosodium glutamate-induced hepatic dysfunction: A biochemical, molecular, and histopathological study. J. King Saud Univ.-Sci.. 2023;35(2):102464
- [Google Scholar]
- Spirulina platensis protects against renal injury in rats with gentamicin-induced acute tubular necrosis. Bosn. J. Basic Med. Sci.. 2008;8(4):331.
- [Google Scholar]
- Protein Content Quantification by Bradford Method. Handbook of Plant Ecophysiology Techniques. Springer; 2001. p. :283-295.
- Combinational effect of curcumin and metformin against gentamicin-induced nephrotoxicity: Involvement of antioxidative, anti-inflammatory and antiapoptotic pathway. J. Food Biochem.. 2019;43(7):e12836.
- [Google Scholar]
- Amelioration of nephrotoxicity in mice induced by antituberculosis drugs using ensete ventricosum (Welw.) Cheesman Corm Extract. Int. J. Nephrol.. 2022;2022
- [Google Scholar]
- The anti-malarial artesunate is also active against cancer. Int. J. Oncol.. 2001;18(4):767-773.
- [Google Scholar]
- Development of a novel pharmaceutical formula of nanoparticle lipid carriers of gentamicin/α-tocopherol and in vivo assessment of the antioxidant protective effect of α-tocopherol in gentamicin-induced nephrotoxicity. Antibiotics. 2019;8(4):234.
- [Google Scholar]
- Testicular toxicity of cisplatin in rats: ameliorative effect of lycopene and N-acetylcysteine. Environ. Sci. Pollut. Res.. 2022;29(16):24077-24084.
- [Google Scholar]
- The protective effect of taurine against gentamicin-induced acute tubular necrosis in rats. Nephrol. Dial. Transplant.. 2000;15(8):1175-1182.
- [Google Scholar]
- Biochemical and haematological evaluation of repeated dose exposure of male Wistar rats to an ethanolic extract of Artemisia annua. Phytother. Res.. 2013;27(4):602-609.
- [Google Scholar]
- Use of 3, 5-dichloro-2-hydroxybenzenesulfonic acid/4-aminophenazone chromogenic system in direct enzymic assay of uric acid in serum and urine. Clin. Chem.. 1980;26(2):227-231.
- [Google Scholar]
- Histopathological and nephroprotective study of aqueous stem bark extract of Ficus racemosa in drug induced nephrotoxic rats. IOSR J. Pharmacy.. 2012;2(2):265-270.
- [Google Scholar]
- Nephroprotective effect of ethanolic extract of Abutilon indicum root in gentamicin induced acute renal failure. Int. J. Basic Clin. Pharmacol.. 2016;5:841-845.
- [Google Scholar]
- Chemical composition and valorization of broccoli leaf by-products (Brassica oleracea L. Variety: Italica) to ameliorate reno-hepatic toxicity induced by gentamicin in rats. Appl. Sci.. 2022;12(14):6903.
- [Google Scholar]
- Brassica oleracea L. var. botrytis leaf extract alleviates gentamicin-induced hepatorenal injury in rats—possible modulation of IL-1β and NF-κB activity assisted with computational approach. Life.. 2022;12(9):1370.
- [Google Scholar]
- l-carnitine ameliorates gentamicin-induced renal injury in rats. Nephrol. Dial. Transplant.. 2002;17(12):2122-2131.
- [Google Scholar]
- RP-HPLC method of simultaneous nephroprotective role of A. marmelos extract. Int. J. Res. Pharm. Chem.. 2011;1:617-623.
- [Google Scholar]
- Protective effect of honey and propolis against gentamicin-induced oxidative stress and hepatorenal damages. Oxidative Med. Cell Longevity. 2021;2021
- [Google Scholar]
- Corynebacterium and Dolosigranulum: future probiotic candidates for upper respiratory tract infections. Microbiol. Australia.. 2019;40(4):172-177.
- [Google Scholar]
- Discovery of artemisinin in Artemisia annua, its current production, and relevance to sub-Saharan Africa. S. Afr. J. Bot.. 2023;153:21-27.
- [Google Scholar]
- Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods. 2001;25(4):402-408.
- [Google Scholar]
- Protective effect of Moringa oleifera leaves extract against gentamicin induced hepatic and nephrotoxicity in rats. Iraqi J. Veterin. Sci... 2022;37(1)
- [Google Scholar]
- Pioglitazone attenuates kidney injury in an experimental model of gentamicin-induced nephrotoxicity in rats. Sci. Rep.. 2019;9(1):1-10.
- [Google Scholar]
- Noorani, A.A., Gupta, K.A., Bhadada, K. et al., 2011. Protective effect of methanolic leaf extract of Caesalpinia bonduc (L.) on gentamicin-induced hepatotoxicity and nephrotoxicity in rats.
- Antioxidant activity and hepatoprotective property of leaf extracts of Boerhaavia diffusa Linn against acetaminophen-induced liver damage in rats. Food Chem. Toxicol.. 2010;48(8–9):2200-2205.
- [Google Scholar]
- Comparative study between effects of ginkgo biloba extract and extract loaded on gold nanoparticles on hepatotoxicity induced by potassium bromate. Environ. Sci. Pollut. Res. 2022:1-10.
- [Google Scholar]
- Nephroprotective effect of ursolic acid in a murine model of gentamicin-induced renal damage. Int. Schol. Res Notices. 2012;2012
- [Google Scholar]
- Pathan, N., A systematic review on nephroprotective plants. Journal homepage: www. ijrpr. com ISSN. 2582 7421.
- Protection of CCl4-induced liver and kidney damage by phenolic compounds in leaf extracts of Cnestis ferruginea (de Candolle) Pharmacognosy Res.. 2014;6(1):19.
- [Google Scholar]
- Pharmacokinetic study of artemisinin after oral intake of a traditional preparation of Artemisia annua L. (annual wormwood) Am. J. Trop. Med. Hyg.. 2004;70(2):128-132.
- [Google Scholar]
- Chemical constituents of Nelumbo nucifera seeds. Nat. Prod. Sci.. 2017;23(4):253-257.
- [Google Scholar]
- Anticancer activity of an Artemisia annua L. hydroalcoholic extract on canine osteosarcoma cell lines. Res. Vet. Sci.. 2022;152:476-484.
- [Google Scholar]
- Ethanol extract of artemisia annua prevents LPS-induced inflammation and blood-milk barrier disruption in bovine mammary epithelial cells. Animals. 2022;12(10):1228.
- [Google Scholar]
- Protective effect of Moringa oleifera leaves extract against gentamicin induced hepatic and nephrotoxicity in rats. Iraqi J. Veterin. Sci.. 2023;37(1):129-135.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2023.102813.
Appendix A
Supplementary material
The following are the Supplementary data to this article:







