Translate this page into:
Dye-sensitized solar cells constructed using titanium oxide nanoparticles and green dyes as photosensitizers
⁎Corresponding authors. AAHindi@pnu.edu.sa (Awatif A. Hendi), mmalenazy@pnu.edu.sa (Meznah M. Alanazi), mawad@ksu.edu.sa (Manal A. Awad),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Recently, researchers have taken a particular interest in dye-sensitized solar cells (DSSCs) based on titanium dioxide (TiO2) nanoparticles (NPs) due to their exceptional physico-chemical characteristics and excellent photoconversion efficiency. This research is dedicated to fabricating dye-sensitized solar cells (DSSCs) using TiO2NPs and green natural dyes. The average particle size appeared about 151.6 nm of the synthesized TiO2NPs, measured by dynamic light scattering technique. The nanostructured TiO2 was characterized optically with a UV–visible and X-ray fluorescence spectrophotometer, and structurally by using X-ray diffraction (XRD). Analysis of the particle size and morphology of TiO2NPs has been confirmed by Transmission Electron Microscopy (TEM), and the Energy-dispersive spectroscopy (EDS) analysis indicated the elemental composition. The TiO2NPs thin film of paste was spread on the transparent conducting glass as the substrate with copper metal attached to the surface using the doctor-blade method. Green dyes extracted from Lawsonia inermis (Henna) and spinach were used as sensitizers, iodine as electrolytes, and TiO2NPs as photoelectrode to fabricate dye sensitized solar cells (DSSCs). The DSSCs were evaluated with a fill factor of 0.09 and 0.37, which were obtained with an efficiency of 0.24 %, and 2.19 % for spinach and henna dyes, respectively.
Keywords
UV–vis
XRD
TiO2NPs
DSSCs
Fill factors
Efficiency
1 Introduction
Dye-sensitized solar cells (DSSCs) are from the family of photo-electrochemical cells. One of the main differences between DSSs and other kinds of solar cells is the method charges are separated (Luo et al., 2022). The DSSCs are promising since they are green energy photovoltaic devices, relatively inexpensive, and easy to manufacture. for supplying indoor lighting and electronic applications like wireless sensors, DSSCs are an effective photovoltaic technology. Their potential for affordable interior photovoltaics is highlighted by their plentiful supply of materials and reasonable cost, in addition to their ability to be produced as lightweight, flexible, and thin solar modules. They must, however, scale up their fabrication techniques for industrial manufacture in order to attaintormance stability and photovoltaic efficiency in typical indoor environments (Kokkonen et al., 2021). Two conducting glass electrodes are used in the DSSCS, which consists of porous nanocrystalline broad bandgap semiconductor-based metal oxide film coated with dye-adsorbed such as zinc oxide, and titanium dioxide nanoparticles. While the counter electrode is covered with graphite or platinum, and the dye is renewed by the redox couple-containing electrolyte solution (Agrawal et al., 2022; Wu et al., 2008). Considering that they offer a significantly large surface area for dye anchoring, the nanostructured metal oxide coatings are particularly alluring for DSSCs. (Mehmood et al., 2022).
The semiconductor component that composes the photoelectrode's (PE) core must be stable chemically and unaffected by the electrolyte species. To maximize the effective surface area for dye adsorption, it should be provided in nanostructure form by roughly a factor of a thousand, thereby increasing the effectiveness of sunlight harvesting and Its lattice structure should be appropriate for dye bonding. For effective electron injection, it should have a conduction band that is just below the dye's LUMO level (Zhou et al., 2022). TiO2 has a lengthy history of application in photoanodes among semiconductor metal oxides, including notable studies by Honda and Fujishima, in addition to the Chen research group (Fujishima and Honda, 1972). Following the first DSSCs in 1991, the use of this material increased significantly, according to (O’Regan and Grätzel, 1991) a study on high surface area nanoparticle TiO2 films sensitized with a dye that injects electrons after photoexcitation. TiO2 is a semiconductor with a broadband gap, physical characteristics, and high electronic mobility, which is beneficial for electron transport. The direct band gap is (3.69 eV) with low recombination loss and high exciton binding energy (Li et al., 2022). A critical feature of the DSSC process is the dye that is employed as a photosensitizer. The dye's absorption spectrum and how well it is anchored to the semiconductor surface have a significant impact on cell efficiency (Ahmed and Anwar, 2022). To increase photon absorption and, consequently, electron injection and conversion efficiency, dyes' chemical structure is crucial. In addition to dyes, we also discussed the impact of the sandwich, monolithic, and honeycomb DSSC structural shapes on the DSSCs performance (Mujtahid et al., 2022).
In terms of health and the environment, organic dyes have been developed to replace carcinogenic dyes in order to provide low-cost, ecologically friendly electronics. As a result, natural dyes photosensitizers extracted from plant parts may be excellent alternatives; thus, many studies have been actively performed to adopt natural dyes as a photosensitizer of DSSCs to realize eco-friendly DSSCs; for example, (Ghann et al., 2017) discovered that the highest power-conversion efficiency was found by 2 % for their DSSCs using Pomegranate as dyes sensitizer. Furthermore, according to (Khammee et al., 2021), the greatest efficiency of natural pigments recovered from Inthanin bok leaves is 1.138 % 0.018 under the condition of 1 layer of TiO2 nanoparticles.
The objective of this study was to present the synthesis of titanium dioxide nanoparticles (TiO2NPs) and characterized what was achieved using different devices such as an electron microscope, Fluorescent, UV, XRD, TEM, and EDS. In the development of dye-sensitized solar cells (DSSCs), the possibility of naturally occurring dyes as sources of molecular sensitizers has been explored. In addition to that, the green dye solar cell has been fabricated and its efficiency and electrical parameters have been measured and calculated.
2 Experimental methods
2.1 Characterization of nanoparticles
The TiO2NPs of cubic shape and average edge size ∼ 21 nm, in powder form, were bought from Sigma Aldrich (St. Louis, MO, USA) and used as paste material to coat the substrate. The optical properties of TiO2NPs were examined by the UV–Vis spectrophotometer (Shimadzu UV 2450 UV–vis spectroscope, Shimadzu Corporation, Kyoto, Japan) and X-ray fluorescence (XRF, RF5301PCS spectrofluorophotometer, Shimadzu Corporation, Japan). The internal structure, morphological details, and compositional analysis of TiO2NPs were analyzed using transmission electron microscopy/TEM (JEM-1011, JEOL, Japan) and energy-dispersive spectroscopy (EDS, JSM-7610F, JEOL, USA). The crystallinity of TiO2NPs was studied using powder X-ray diffraction/XRD (Bruker D8 Advance, Germany). To measure the electrical parameters of the prepared solar cells for photoelectric characterization, their current–voltage (I-V) measurements were taken using a KEITHLEY Model 2400 sourcemeter (voltage/current: 200 V/1.0 A with a power of about 20 W). All measurements were carried out under direct sunlight illumination.
2.2 Method of green natural dyes extraction
To extract the pigments from Lawsonia inermis (commonly called Henna) and Spinach, their fresh leaves were bought from the local market of Riyadh, KSA. First, the Henna leaves were washed several times using fresh water to remove any dust from their surfaces and then converted into a paste by grinding in a mortar and pestle system, following the addition of a small quantity of distilled water. The paste was soaked overnight in a certain amount of distilled water. Finally, it was filtered and the natural dye filtration was kept in the refrigerator till further use. The same process was repeated for Spinach extraction.
2.3 Fabrication of dye-sensitized solar cells
Cutting the transparent conductive oxide/TCO is the first step in preparing the substrate. i.e. fluorine-doped tin oxide/FTO films with a size of 2 × 2 cm2. Each substrate is cleaned while immersing sequentially in water, acetone ((CH3)2CO) 70 %, ethanol (C2H5OH) 70 %, and finally in the deionized water using a sonication procedure for a few minutes each. After that, the substrates were transferred into a petri dish and a hairdryer was used to dry them (see Fig. 1).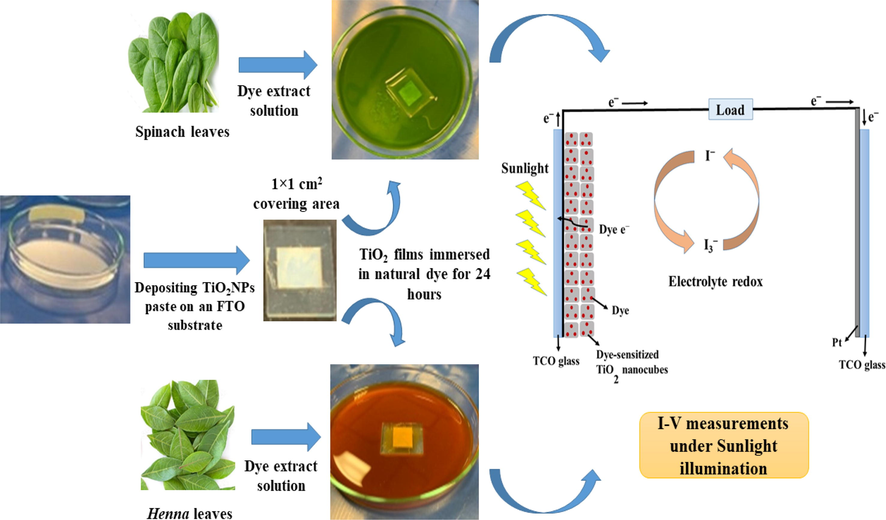
Schematic illustration of the fabrication of dye-sensitized TiO2NPs based photoelectrode, including the basic structure of the DSSC device.
The DSSCs assembly was done according to the modified previously reported methods (Hasoon et al., 2015; Al-Attafi et al., 2017; Al-Attafi et al., 2021) in which the FTO conductive glass sheets were gently washed in an ethanol and detergent solution before being heat dried. The TiO2NPs paste was created by adding a few drops of diluted nitric acid to the TiO2NPs powder and carefully blending the two by grinding them together in a mortar and pestle. Next, we used the doctor-blading technique to deposit the TiO2NPs paste on the FTO conductive glass to create a TiO2 film with a thickness of 10 µm and an area of 1.0 cm2. The TiO2 films were sintered at 400 °C for 30 min, and after they were cooled, the TiO2 electrodes were each immersed for 24 h in as-prepared green natural dye solutions. By sandwiching an iodine-based redox electrolyte between a dye-sensitized TiO2 electrode and a carbon dust counter electrode, a solar cell was created. As-fabricated DSSCs were sealed on all sides to avoid any electrolyte solution leaking. For the photoelectric characterization of these DSSCs, the I-V measurements were carried out under direct Sunlight illumination, and based on I–V measurements, the fill factor (FF) of both DSSCs is calculated (Maadhde et al., 2021; Islam et al., 2020., (Bang et al., 2012)).
3 Results and discussion
The photon characteristics are crucial to the investigation of optical materials because they indicate how many photons will be absorbed upon exposure to light. In essence, UV–vis spectroscopy is used to examine the electrical structure and optical characteristics of the produced nanostructures. UV–Vis absorption analysis of TiO2NPs was directed at NTP in the wavelength range 200–800 nm. Fig. 2 shows a broad absorption band of TiO2NPs at 322 nm, it corresponds to the change from the O2p state (valance band) to the Ti3d state (conduction band). When compared to uncapped TiO2, the absorption edges in the bio-capped TiO2NPs displayed a little blue shift, which may be caused by the quantum confinement effect. (Samanta et al., 2017). Eq. (1), has been used to determine the relevant band gap energy (
),
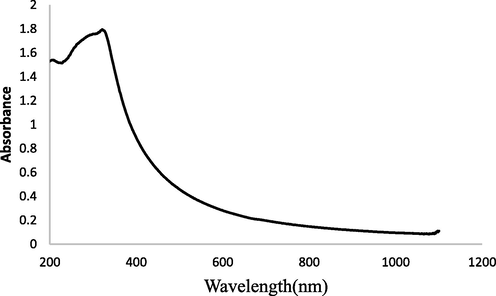
Absorption spectrum of TiO2NPs.
XRD was used to characterize the anatase and rutile phases. The patterns are displayed in Fig. 3. Consequently, the sharp peaks obtained at a 2θ angle observed at 25.3, 37.77, 48.08, 54.02, 55.08°, indexed to the miller indices (hkl) values as (1 0 1), (0 0 4), (2 0 0), (1 0 5), (2 1 1), respectively, point to the structure of nanocrystalline anatase. The peaks that were seen lined up with the (COD 2300113) standard planes, which made the tetragonal structure of the TiO2NPs abundantly obvious. The high crystallinity of the nanoscale TiO2 is clearly implied by the sharp peaks, which are thought to be beneficial for photocatalytic activity (Singh et al., 2020).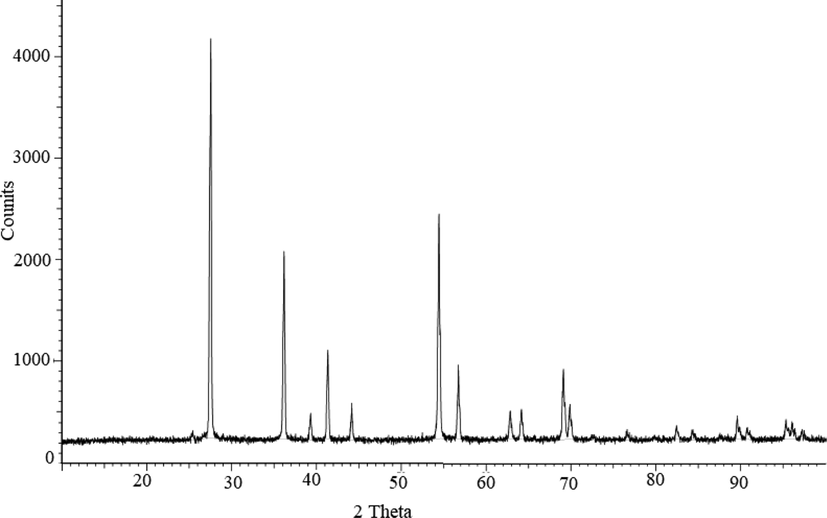
The XRD pattern of TiO2NPs.
TEM images of the TiO2NPs powders and natural dyes utilized in the construction of DSSCs were used to examine the morphology of such structures are presented in Fig. 4. These nanoparticles clearly demonstrate a tendency to self-assemble into dense, close-packed, well-crystallized, nearly uniform, unevenly sized, and irregular sub nano aggregates, these results with in agreement with (Singh et al., 2022; Conti Nibali et al., 2022).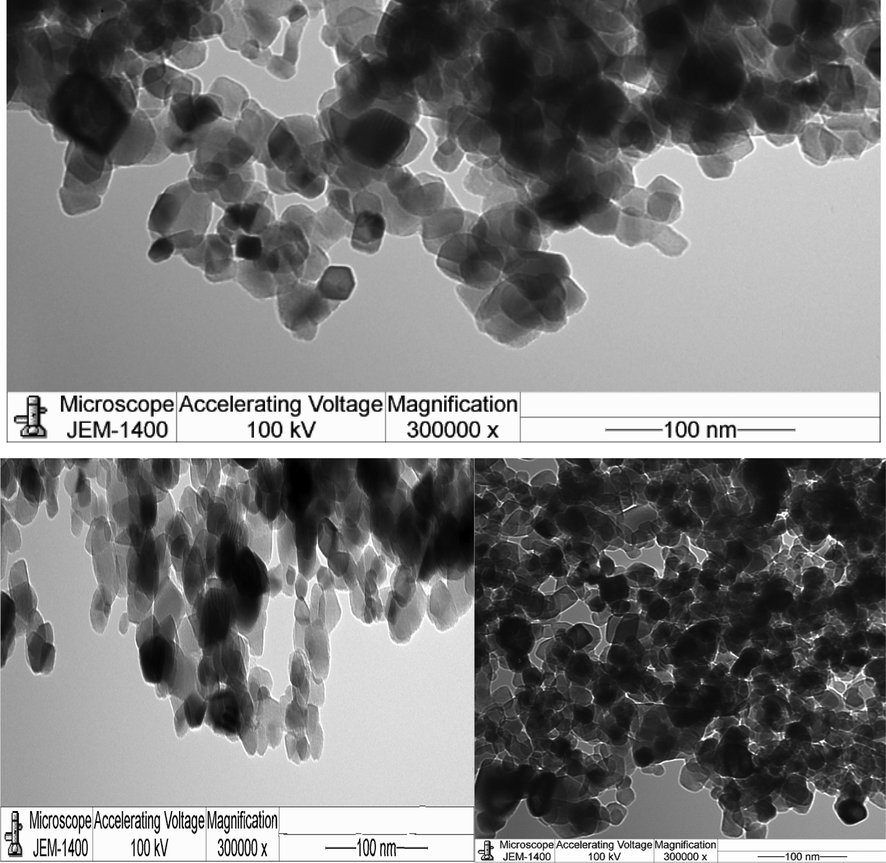
The TEM images of TiO2NPs.
The elemental composition of nanomaterials, which is a working mode of SEM, is determined using energy dispersive X-ray spectroscopy, which also offers details on the percentage that each element occupies in the materials. The sample's EDS compositional mapping is displayed in Fig. 5. It exhibits titanium and oxygen peaks from the sample. The existence of pure titanium dioxide nanoparticles was confirmed by the presence of oxygen and titanium. Additionally, the findings of the EDS analysis research demonstrate that the particles are indeed metallic TiO2NPs and are crystalline in nature. (Tarafdar et al., 2013; Mahalakshmi and Vijaya, 2021).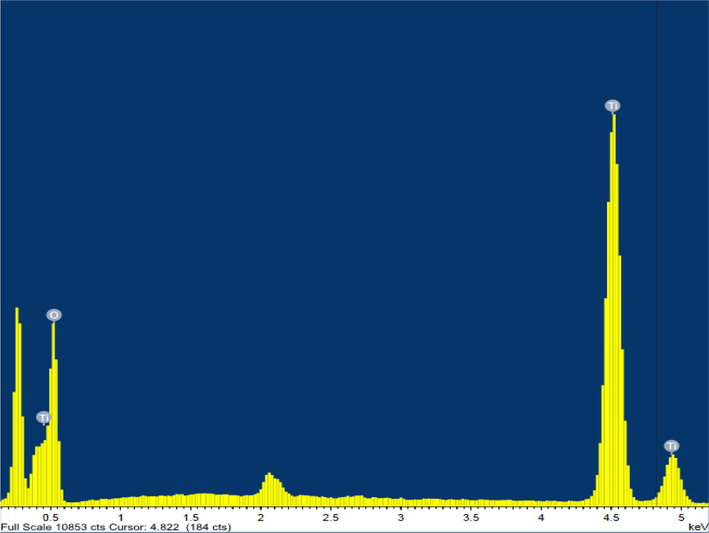
EDS mapping showing the chemical composition of the TiO2.
Photovoltaic experiments on the manufactured DSSCs using these green dyes as sensitizers measured each cell's I-V curve under sun irradiation. Where the system consists of direct sunlight,cables, variable resistance, a solar cell and a voltmeter the cables were connected to the voltmeter, solar cell, and variable resistance, and the side of the TiO2/dye electrode was shined a light. Next, the resistance had set to its maximum value and recorded the voltage, then reduced the resistance and recorded the voltage. The operation was repeated until the voltage was nearly zero, after which the light was switched off. Then the corresponding current was calculated at each recorded point. And then it was calculated the fill factor and calculated the power conversion efficiency (η). Typical sandwich-type cells were used to assemble the dye-sensitized electrode. The area of the cell was 1 cm2 and the power conversion efficiency η of the DSSC is given by Eq. (2)
In Eq. (2),
,
, and
represent the open circuit photovoltage, the short-circuit photocurrent per unit area, and the incident light power (100 mW/cm2), respectively. Aside from this, the fill factor FF is determined by Eq. (3)
In Eq. (3), and represent the voltage and the current per unit area at the maximum output power point, respectively.
The I-V data in Table 1 were used to calculate the DSSCs output power. The current density for the DSSC sensitized by spinach and henna extracts is shown as a function of V in Fig. 6. The DSSC sensitized with spinach produced the lowest Pmax value, which might be attributed to weak bonding between the micro TiO2 particles and the dye molecule. Chlorophyll dye is less effective at converting electricity than henna dye in a DSSC because it depends on the availability of a disponible bond between the dye molecules and nano TiO2 particles, in which the electrons can be transported from excited dye molecules to nano TiO2, therefore, this is consistent with the findings of (Al-Attafi et al., 2021; Hower and Pratama, 2022; Moustafa et al., 2012; Mejica et al., 2022). A weak bond between the dye molecule and the TiO2 particles may be responsible for the maximum value obtained from the DSSC sensitized with spinach. Results from henna dye were twice higher as those from other dyes since plants contain several components that equally create various consequences. For instance, the flavonoid component found in the leaves of the henna plant is responsible for the orange-red hue, this may have influenced the experiment's findings (Etula, 2012; Backialakshmi and Gopinathan, 2018). Another explanation might be because chlorophyll dye absorbs a restricted range of light 400–450 wavelengths of visible light and has a high peak at 430 nm, which slows the rise in power conversion efficiency (Al-Alwani, M.A et al., 2015), while according to the lawsone pigment inherent in henna leaves, henna dye absorbs entire incoming light from 200 nm to 550 nm and has a broad absorption spectrum of visible light with decreased absorbance up to 700 nm. Furthermore, 550 nm corresponds to greenish yellow and 700 nm corresponds to red in the visible spectrum. (Kumar et al., 2020).
Dye
(V)
(
)
FF
Spinach
0.9552
2.8297
0.3456
0.731
0.24
0.09
Henna
0.7366
8.0411
0.5037
0.436
2.19
0.37
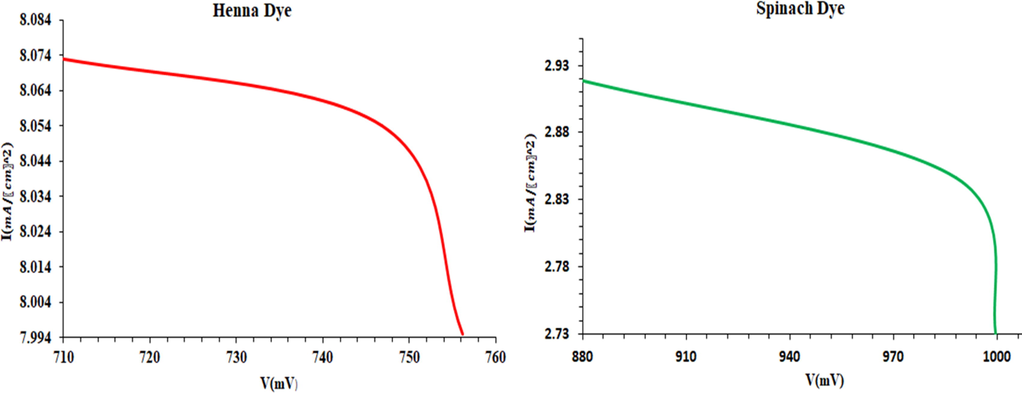
Photocurrent voltage characteristics of spinach and henna dyes.
Whereas previous research has indicated the performance of natural dyes as a photosensitizer of eco-friendly DSSCs, the current study's findings using spinach and henna dyes show a similar percentage of conversion efficiency to those published by (Kim et al., 2021).
4 Conclusion
The usage of dye-sensitized solar cells as a simple, inexpensive source of renewable energy seems appealing. This photovoltaic device functions as an alternate to conventional solar cells operating by the p-n junction, it consists of a counter electrode, an electrolyte, and a photoanode. The photoanode is a crucial part of the DSSC. It serves as a scaffold for the adsorption of dye molecules. The operating electrode works as a conduit for photoexcited electrons from the dye to be collected and transported to an external electric circuit. In conclusion, in this work, the TiO2NPs have been successfully characterized using different technical methods such as XRD, UV, TEM and EDS. However, TiO2NPs were used in fabricating of DSScs as a photoanode, and iodine as an electrolyte. Natural dyes are predicted to be a promising alternative sensitizer for DSSCs due to their many features, including the simple manufacturing process and low cost. This study exposed that the dyes used to have a substantial impact on the photovoltaic feature of DSSCs. The DSSC sensitized by henna offered the highest two times conversion efficiency of 2.19 % among the DSSC spinach at 0.37 %. This work raises a lot of unanswered questions. The most interesting one has to do with enhancing cell photovoltaic performance.
Author contribution
All co-authors have contributed to this work and are aware of this submission.
Acknowledgments
Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2023R132), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Advancements, frontiers and analysis of metal oxide semiconductor, dye, electrolyte and counter electrode of dye sensitized solar cell. Sol. Energy. 2022;233:378-407.
- [Google Scholar]
- Application of natural dyes in dye-sensitized solar cells. In: Dye-Sensitized Solar Cells. Academic Press; 2022. p. :45-73.
- [Google Scholar]
- Effect of solvents on the extraction of natural pigments and adsorption onto TiO2 for dye-sensitized solar cell applications. Spectrochim. Acta A Mol. Biomol. Spectrosc.. 2015;138:130-137.
- [Google Scholar]
- Aggregated mesoporous nanoparticles for high surface area light scattering layer TiO2 photoanodes in Dye-sensitized Solar Cells. Sci. Rep.. 2017;7(1):10341.
- [Google Scholar]
- Solvothermally synthesized anatase TiO2 nanoparticles for photoanodes in dye-sensitized solar cells. Sci. Technol. Adv. Mate.. 2021;22(1):100-112.
- [Google Scholar]
- Backialakshmi, P., Gopinathan, C., 2018. Fabrication, optimization and characterization of TiO2 photoanode utilizing natural photosensitizerfor dye sensitized solar cell application. IJSRST. (4)5, 104–110.
- Effect of acetic acid in TiO2 paste on the performance of dye-sensitized solar cells. Ceram. Int.. 2012;38:S511-S515.
- [Google Scholar]
- TiO2 Nanoparticles dispersion in block-copolymer aqueous solutions: nanoarchitectonics for self-assembly and aggregation. J. Funct. Biomater.. 2022;13(2):39.
- [Google Scholar]
- Comparison of three Finnish berries as sensitizers in a dye-sensitized solar cell. Eur. J. Young Scientists Eng.. 2012;1:5-23.
- [Google Scholar]
- Electrochemical photolysis of water at a semiconductor electrode. Nature.. 1972;238(5358):37-38.
- [Google Scholar]
- Fabrication, optimization and characterization of natural dye sensitized solar cell. Sci. Rep.. 2017;7(1):1-12.
- [Google Scholar]
- Natural dye-sensitized solar cell based on zinc oxide. Int. J. Sci. Eng. Res.. 2015;6(5):137-142.
- [Google Scholar]
- Hower, H., Pratama, F., 2022. Co-Action Performance of Two Natural Dyes As Photosensitizer In Dye-Sensitized Solar Cell (DSSC) Apllication, in: IOP Conference Series: Earth and Environmental Science, Vol. 995, No. 1, IOP Publishing, p. 012052.
- Fabrication and photovoltaic properties of organic solar cell based on zinc phthalocyanine. Energies. 2020;13(4):962.
- [Google Scholar]
- Natural dyes extracted from Inthanin bok leaves as light-harvesting units for dye-sensitized solar cells. Appl. Nanosci. 2021:1-13.
- [Google Scholar]
- Toward Eco-Friendly Dye-Sensitized Solar Cells (DSSCs): natural dyes and aqueous electrolytes. Energies. 2021;15(1):219.
- [Google Scholar]
- Advanced research trends in dye-sensitized solar cells. J. Mater. Chem. A. 2021;9(17):10527-10545.
- [Google Scholar]
- Biosynthesis of tin oxide nanoparticles using Psidium Guajava leave extract for photocatalytic dye degradation under sunlight. Mater. Lett.. 2018;215:121-124.
- [Google Scholar]
- Natural dye-sensitized solar cells using Lawsone pigment of Lawsonia inermis (henna leaves) as sensitizers. IJAEM. 2020;2(1):34-40.
- [Google Scholar]
- Application of nanostructured TiO2 in UV photodetectors: A review. Mater Adv. 2022:2109083.
- [Google Scholar]
- Photoelectrochemical polymerization for solid-state dye-sensitized solar cells. Macromol. Rapid Commun.. 2022;43(5):2100762.
- [Google Scholar]
- An investigation of the fill factor and efficiency of molecular semiconductor solar cells. In: Materials Science Forum. Vol vol. 1039. Trans Tech Publications Ltd.; 2021. p. :373-381.
- [Google Scholar]
- Evaluation of in-vitro biocompatibility and antimicrobial activities of titanium dioxide(TiO2) nanoparticles by hydrothermal method. Nano Biomed. Eng.. 2021;13(1):36-43.
- [Google Scholar]
- Semiconducting metal oxides-based electrodes as the photoanodes of dye-sensitized solar cells (DSSCs) In: Dye-Sensitized Solar Cells. Academic Press; 2022. p. :103-136.
- [Google Scholar]
- Anthocyanin pigment-based dye-sensitized solar cells with improved pH-dependent photovoltaic properties. Sustain. Energy Technol. Assess.. 2022;51:101971
- [Google Scholar]
- Green dyes as photosensitizers for dye-sensitized solar cells. J. Appl. Sci. Res.. 2012;8(8):4393-4404.
- [Google Scholar]
- Review effect of various types of dyes and structures in supporting performance of dye-sensitized solar cell TiO2-based nanocomposites. Int. J. Energy Res.. 2022;46(2):726-742.
- [Google Scholar]
- A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature. 1991;353(6346):737-740.
- [Google Scholar]
- Citrus limetta juice as capping agent in hydrothermal synthesis of ZnS nanosphere for photocatalytic activity. Mater. Res. Bull.. 2017;88:85-90.
- [Google Scholar]
- Structural, morphological, optical and photocatalytic properties of green synthesized TiO2 NPs. C R G S C.. 2020;3:100033.
- [Google Scholar]
- Green synthesis of TiO2 nanoparticles using Citrus limon juice extract as a bio-capping agent for enhanced performance of dye-sensitized solar cells. Surf. Interfaces. 2022;28:101652.
- [Google Scholar]
- Green synthesis of TiO2 nanoparticle using Aspergillus tubingensis. Adv. Sci. Eng. Med.. 2013;5(9):943-949.
- [Google Scholar]
- Progress on the electrolytes for dye-sensitized solar cells. Pure Appl. Chem.. 2008;80(11):2241-2258.
- [Google Scholar]
- Application of ultrathin TiO2 layers in solar energy conversion devices. Energy Sci. Eng.. 2022;10(5):1614-1629.
- [Google Scholar]







