Translate this page into:
Drought stress alleviation through nutrient management in Cyamopsis tetrogonoloba L.
⁎Corresponding authors at: Department of Botany & Microbiology, College of Science King Saud University, Riyadh 11421, Saudi Arabia. kperveen@ksu.edu.sa (Kahkashan Perveen)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Drought is one of the most serious issues since it reduces plant development and, as a result, yield significantly. This research is an attempt to provide an organic solution to combat the global issue of plant stress due to drought. To reduce the impact of drought on Cyamopsis tetragonoloba, the traditional preparation Panchagavya was used, and its effects on growth, photosynthetic and non-photosynthetic pigment synthesis, thylakoid protein composition, antioxidant activity, anatomy, and yield were investigated. Initially, drought had a negative effect on plant growth and other activities; however, as the duration of the treatment increased, the effect of drought decreased significantly. The plants that were under drought stress and were provided with nutrients, showed more plant growth and yield. Flavonoid and anthocyanin production was 50% higher in the plants under drought stress with nutrient supplementation than in the plants under drought stress and the control plants. The accumulation of 55, 47, 33, 27, and 20 kDa polypeptides of thylakoid protein also increased under nutrient conditions. Drought stress reduced overall fruit output by 10% relative to the control group (well irrigate plants). Nutrition management increased plant performance under stress conditions. Fresh & dry pod weights of control, drought stress, and drought with nutrients were recorded at 3.2 g & 0.6 g, 3 g & 0.52 g, and 4.5 g & 1.2 g, respectively. Under drought stress with nutrients, plants had more stomata per unit than control plants. This is the first study in which Panchagavya was used to alleviate drought. With the addition of beneficial nutrients, water use efficiency can be improved, and drought's negative impacts can be mitigated tangentially by activating plant physiological, biochemical, and metabolic processes. This study will add the crucial information required to determine the response of plants under drought stress conditions as well as how to alleviate its effects in a traditional organic way.
Keywords
Cyamopsis sp.
Drought stress
Growth parameters
Panchagavya
Secondary metabolites
Vermicompost
- ANOVA
-
Analysis of Variance
- DPPH
-
2,2-diphenyl-1-picryl-hydrazyl-hydrate
- FW
-
Fresh weight, ROS, reactive oxygen species
- SDS PAGE
-
Sodium dodecyl-sulphate polyacrylamide gel electrophoresis
- TCA
-
Tricarboxylic Acid
- UVB
-
Ultra Violet B Radiation
Abbreviations
1 Introduction
The abiotic stress of drought has many different effects on plants at different levels of organization. Crops in temperate and other tropical areas also experience seasonal water stress, particularly in the summer, even though drought or a shortage of water in the soil are more frequent in the semi-arid tropics. The severity, duration, and stage of development of water stress are factors that indicate how plants respond to it (Farooq et al. 2009).
Photosynthetic pigments are an essential factor in plant growth. Chlorophyll concentration is known as a means of detecting stress-related changes Chlorophyll content is an essential component of photosynthesis that is affected by drought stress. The photosystem, electron transport component, oxidative phosphate ATPase, stomatal closure, and chloroplast ultrastructure were also among the genes that discovered to have differential expression (Hu et al. 2023). Therefore, its depletion is considered an unstable factor in drought- and heat-resistant and sensitive plants. The concentration of chlorophyll and carotenoids is associated with the stress tolerance of plants (Qaderi et al. 2023).
An excess of anthocyanin accumulation increases the plant's resilience to these stresses (Nakabayashi et al., 2014). Conversely, plants have developed a range of acclimation mechanisms that enable them to grow and survive in dry environments. Examples include osmotic adaptation and antioxidant defence systems. Soluble sugars and proline build up as osmolytes in plants when they lack water to maintain the integrity of membrane proteins and increase their resistance to drought stress. Drought sensitivity varies by genotype, physiological stage, treatment duration, and the use of various compounds (Ors et al., 2021).
Additionally, plants can sustain severe internal water deficits and desiccation while still retaining enough metabolic activity to survive (Ennajeh et al., 2010).
Even in fields that have been fertilized, drought can cause nutrient deficiencies because the physiochemical characteristics of the soil can restrict the movement and absorption of specific nutrients (Amtmann and Blatt, 2009). However, it has been demonstrated that fertilising crops with micronutrients is a successful method for reducing the effects of drought on crops. The introduction of drought-tolerant plants with high nutrient utilization is one potential technical approach to address or at least ameliorate this issue (Ahmed et al. 2022). Strategies for reducing the damage caused by drought and the resulting nutrient deficit by providing exogenous fertilisers can be developed. It protects the plant from nutrient deprivation. Panchagavya and vermicompost are organic products that have the ability to promote plant development and immunity. Application of Panchagavya leads to increased plant growth, yield, and quality (Choudhary et al., 2014). This field study's objective was to assess Cyamopsis tetragonoloba L. reaction to drought stress in a semi-arid environment when used as a vermicompost input. We compared growth, the synthesis of photosynthetic and non-photosynthetic pigments, the stomata index, and morphological features under the fertiliser treatments in this study.
2 Material and methods
2.1 Plant growth and experimental treatment
Certified seeds of C. tetragonoloba, purchased from the Department of Agriculture, Chennai, are presented in the experimental fields. The seeds were immersed in running water overnight. The seeds were planted in the experimental fields of Pachaiyappa's College. During the trial, they were watered regularly and precautions were taken to avoid microbial or insectoid contamination. The plants are treated at the first leaf stage.
When the seedling was 5 days old, the following treatment was administered:
-
One group of plants was kept as control and irrigated.
-
The second group of plants was exposed to water stress on alternate days.
-
The third group of plants was subjected to water stress using the recommended amount of vermicompost and panchagavya. An amount of 30 ml of panchagavya, mixed with one litre of water, was given to the plants. For the experiment, only panchagavya were given to plants; they were not treated with any biofertilizer or pesticides.
Panchagavya is a traditional nutrient mixture for plants prepared with cattle by-products and other local organic products. The ingredients in it were cow by-products, milk, curd, jaggery, ghee, banana, tender coconut, and water (Choudhary et al. 2014).
2.2 Determination of growth
The uprooting of the seedlings was followed by measurements of their shoot length and fresh weight. After drying the plants for 24 h at 90 °C, the dry weight is recorded.
2.3 Estimation of pigments
2.3.1 Chlorophylls
The absorbance at 645 and 663 nm and 470 nm was used to calculate the total amount of chlorophyll and carotenoids, respectively, present in the 80% acetone extract (Mackinney 1941; Maclachlan and Zalik, 1963). The following formula was used
Chlorophyll a (mg/l): (12.21 × A663) − (2.81 × A645)
Chlorophyll b (mg/l): (20.13 × A645) − (5.03 × A663)Total chlorophyll (mg/l): 7.18 × A663 + 17.32 × A646
2.3.2 Carotenoids
2.3.3 Flavonoids
Pieces of fresh leaves weighing 100 mg were incubated in 5 ml of 80% acidified methanol (80:20:1 of methanol:water:HCl) at 4 °C for a whole night. The flavonoid concentration was calculated as a function of the leaf's fresh weight using the absorbance at 315 nm after centrifugation to remove debris (Mirecki and Teramura, 1984).
2.3.4 Anthocyanins
The leaves were ground in acidified methanol (80%) to extract the anthocyanins (80:20:1 of methanol:water:HCl). According to Mancinelli et al., (1975) the clear extract was centrifuged, and the absorbance at 530 and 657 nm was used to calculate the quantity of anthocyanin A/g fresh weight: (A530) − (0.3 × A657).
2.4 Analysis of stomatal density
After taping off the peltate trichomes, the abaxial epidermis was painted with nail polish so that the stomatal density (SD) could be determined. The dried coating of nail polish was pulled off using adhesive tape, mounted on a microscope slide, and examined using a light microscope and a camera linked to a computer. The ratio of stomata to leaf area and trichome scars to leaf area were then determined. Each treatment had ten leaves chosen from it, and those leaves were counted three times by three different people.
2.5 Leaf anatomy analysis
Using the free hand sectioning technique, the anatomical characteristics of leaves were examined.
2.6 Isolation of thylakoid membrane
Freshly collected leaf samples were homogenized in a semi-frozen isolation buffer containing 50 mM Tricine, pH 6.9, and 400 mM sucrose for 10–20 s at maximum speed in a Sorvall homogenizer. The homogenate was filtered through eight layers of cheesecloth after being spun for five minutes at 3,000 g at 4 °C. The pellet was resuspended in a pH 7.5 solution of 100 mM sucrose, 5 mM MgCl2, and 20 mM Tris following a five-minute centrifugation at 10,000 g. The resulting pellet was a representation of thylakoid membranes.
2.7 Protein analysis
SDS-PAGE analysis of thylakoid polypeptides was performed on a 15% acrylamide slab gel. At 20°Celsius and an SDS-Chl ratio of 20:1, samples were solubilized in 2% SDS containing 60 mM DTT and 8% sucrose for 5 min.
2.8 DPPH radical scavenging assay
The following equation, which is a modified version of the method that was previously presented (Brand-Williams et al. 1995), was used to estimate the radical-scavenging capacity of each fraction at various concentrations. The DPPH radicals EC50 value is equal to mg of extract mol1. To confirm the calibration curve at 514 nm (y = 1.145E−2–4.192E−3, r = 0.9999, where y = absorbance and x = concentration of DPPH), a value of 92.18 mol/L of DPPH was measured in the reaction system. Every investigation went through three separate runs.
2.9 Hydrogen peroxide scavenging assay
The concentration of hydrogen peroxide was 2 ml, TCA, when 100 mg of fresh tissue was soaked in 0.1% trichloroacetic acid. 0.5 ml of the supernatant were combined with 0.5 ml of 10 mm of potassium phosphate buffer (pH 7.0) and 1 mm of potassium iodide (1 ml) after the homogenate was centrifuged at 12,000g for 15 min. At 390 nm, a reasonable top in absorbance was seen (Velikova et al. 2000).
2.10 Determination of proline
The amount of proline was calculated using Bates et al (1973) technique. Using a mortar and pestle, the leaves (0.2 g) were ground with 3 ml of sulphosalicylic acid (3% w/v), and then centrifuged the mixture at 18,000g for 15 min. An acidic ninhydrin solution and 2 ml of glacial acetic acid were then added to the supernatant in the test tube. The test tubes were warmed to room temperature after being incubated at 100 °C for one hour in a water bath. A vortex mixer was used to combine the 4 cc of toluene for 20 s. After carefully pipetting the toluene phase into a glass tube, the absorbance at 520 nm was measured using a UV–Vis spectrophotometer. A proline standard curve was used to calculate the quantity of proline, which was then represented as µg/g FW.
2.11 Yield analysis
The yield was determined by harvesting pods from mature plants. The following data were recorded: number of pods produced by each plant; number of seeds in each pod; weight of 100 seeds; length of pod; fresh and dry weight of seeds. Data was recorded from 100 plants at various times of the year.
3 Results
In this study, the effects of drought stress on shoot growth traits were investigated in plants with and without nutrients. The results were compared with plants that were well irrigated (control). All irrigation regimes showed significant differences. Plants that were well watered and exposed to drought stress with nutrients grew faster than plants that were exposed to drought stress. Height increased at all stages of development. There was a significant difference between the treatments on the 20th and 25th days. The greatest increase in height was observed in the drought-stress treatment with fertilizer plants (Figs. 1 and 2A).
Morphological variations of Cyamopsis plants cultivated in well irrigated (1), Drought (2) and Drought with nutrient conditions (3).
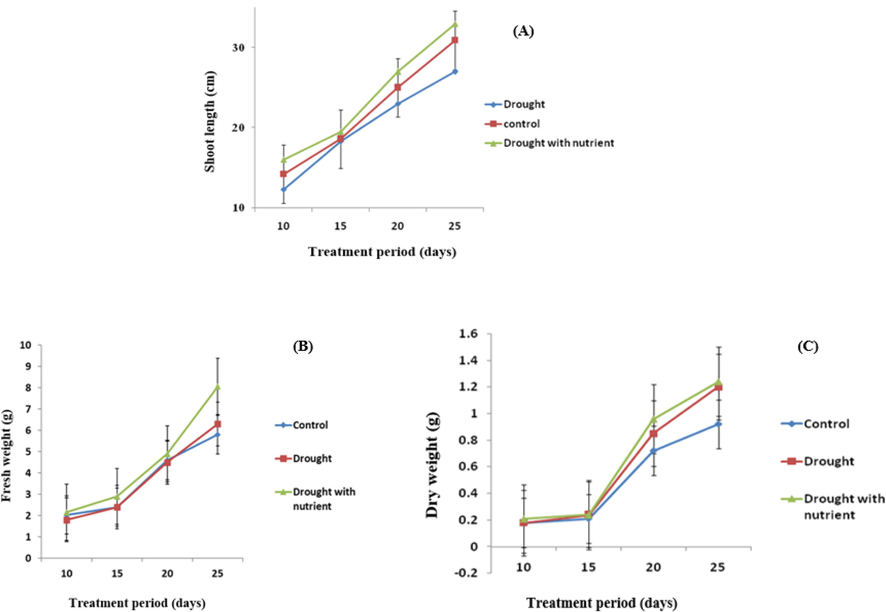
Alterations in the Shoot length (A) fresh weight (B) and dry weight (C) of Cyamopsis plants grown in well irrigated (control), Drought and Drought with nutrient conditions. Data is mean ± SE for n = 3.
As the water deficit increased, the root-to-shoot weight ratio increased. The extremely stressed seedlings had much higher values than the well-watered plants. Compared to the control plants, the development of water stress and water stress with nutrients was more widespread (Fig. 2B and C).
Under water stress, the supply of nutrients increases the total chlorophyll content of Cyamopsis throughout the growth cycle. Chlorophyll production was 50% higher in plants supplemented under water stress compared to other plants and peaked after 22 days. Chlorophyll synthesis was greater in the early stages of the drought-stressed plants, but later it was similar to that of the control plants. The results show that plants receiving drought and nutrients produce more chlorophyll than plants receiving only drought (Fig. 3A).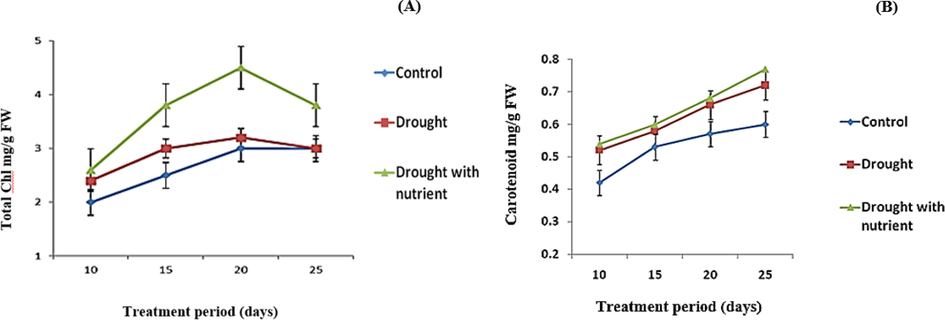
Variations in the total Chl (A) and Carotenoid (B) of Cyamopsis plants cultivated in well irrigated (control), Drought and Drought with nutrient conditions. Data is mean ± SE for n = 3.
In the current study, when the total chlorophyll content increased, the carotenoid concentration remained constant during the treatments. On day 20, the chlorophyll content of the C. tetragonoloba leaves increased, and the high concentration of carotenoid content changed at the same time. Compared to the plants treated with drought, the well-watered plants have only a low amount of carotenoids (Fig. 3A and B).
Estimates were made regarding the flavonoid content of the leaves of plants that were given adequate water, drought, and nutrient-rich drought. The total flavonoids that were found in crude (unhydrolyzed) extracts of C. tetragonoloba plant leaves (Fig. 4A) demonstrated that the plants subjected to drought stress and given nutrients had a higher total flavonoid content than the control plants and the plants subjected to drought stress. Flavonoid accumulation throughout the growing season was the positive response of the plants subjected to drought stress and supplemented with nutrients. Flavonoid production was 50% higher in the plants under drought stress with nutrient supplementation than in the plants under drought stress and the control plants. Flavonoid production was only slightly different in the control plant and the drought-stressed plant. As can be seen in Fig. 4B, anthocyanin content was increased in drought stress with nutrient plants. From the initial stage, its production gradually increased. It was found that productivity was 50% higher.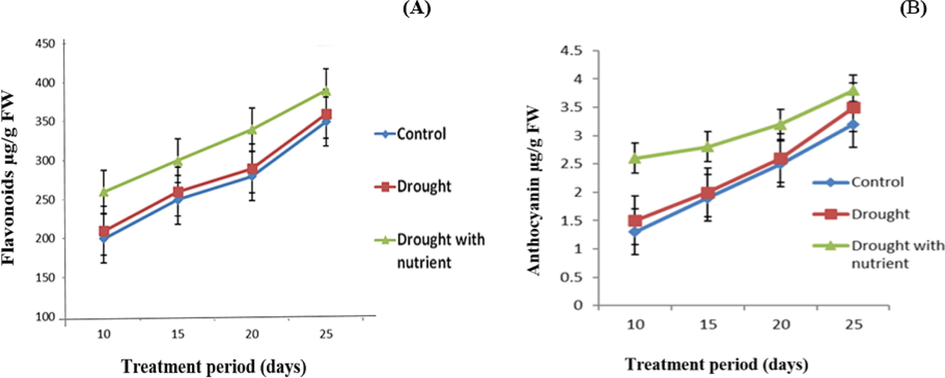
Variations in the amount of flavonoids (A) and anthocyanin (B) in Cyamopsis plants grown under well irrigated (control), Drought and Drought with nutrient conditions. Data is mean ± SE for n = 3.
DPPH and hydrogen peroxide activities respond positively to the severity of drought stress (Fig. 5A and B). Nevertheless, this antioxidant activity was higher in plants receiving drought with nutrients and 30% lower in plants receiving drought only. The study proves that the level of antioxidants in the control plant was normal and contributes to the plants' ability to withstand stress conditions.Proline was produced in large quantities when the plant was exposed to drought. Proline production was highest during drought in plants with nutrient supplementation (Fig. 6).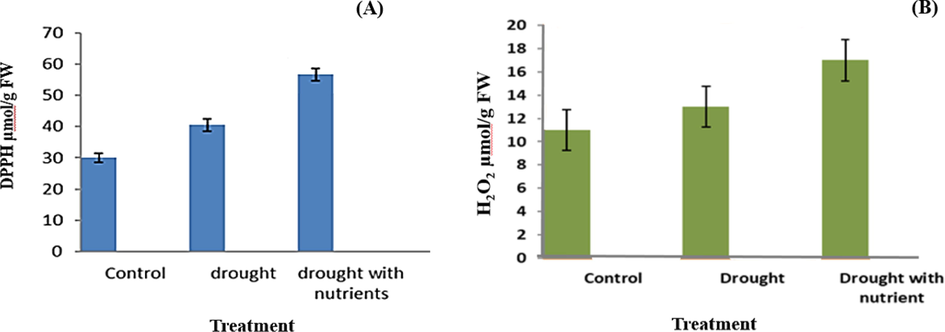
Variations in the DPPH (A) and hydrogen peroxide (B) activity of Cyamopsis plants grown under well irrigated (control), Drought and Drought with nutrient conditions. Data is mean ± SE for n = 3.
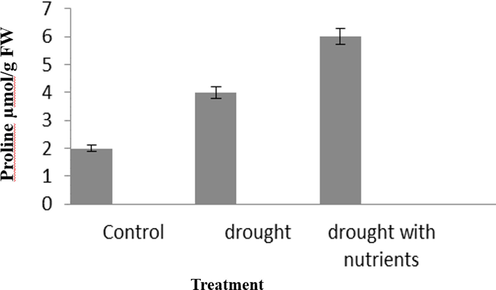
Variations in the proline content of Cyamopsis plants grown in well irrigated (control), Drought and Drought with nutrient conditions. Data is mean ± SE for n = 3.
Plant water uptake was determined by stomata control, which affects their ability to survive in a water-scarce environment. Drought stress increases leaf stomata density and stomata index. Under drought-stress nutrient conditions, stomata density, plant growth, density, and leaf area increased compared to the control. Under drought stress with nutrients, plants had more stomata per unit than control plants (Fig. 7).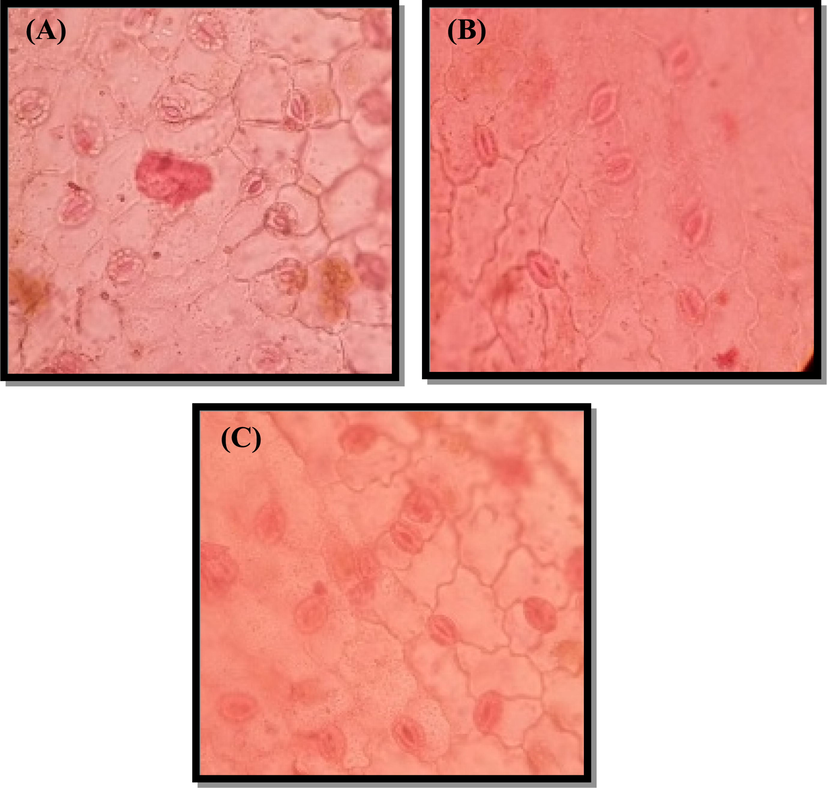
Modification in the stomatal density of Cyamopsis plants cultivated in (A): well irrigated (control), (B): Drought (C): Drought with nutrient.
The asymmetric, heterogeneous structure of a C. tetragonoloba leaf can be seen in cross-section (Fig. 8). There are two uneven palisades as well as a spongy parenchyma. The first consists of a single layer of compacted, elongated cells in contact with the upper epidermis, while the second consists of numerous layers of relatively elongated cells in contact with the lower epidermis. Variable leaf anatomical characteristics, well-watered plants, and drought stress (Fig. 8). Compared to drought and control plants, the length of palisades and spongy mesophyll increased more under drought stress with nutrients. It was 20% of the upper palisade and 50% of the spongy parenchyma layers.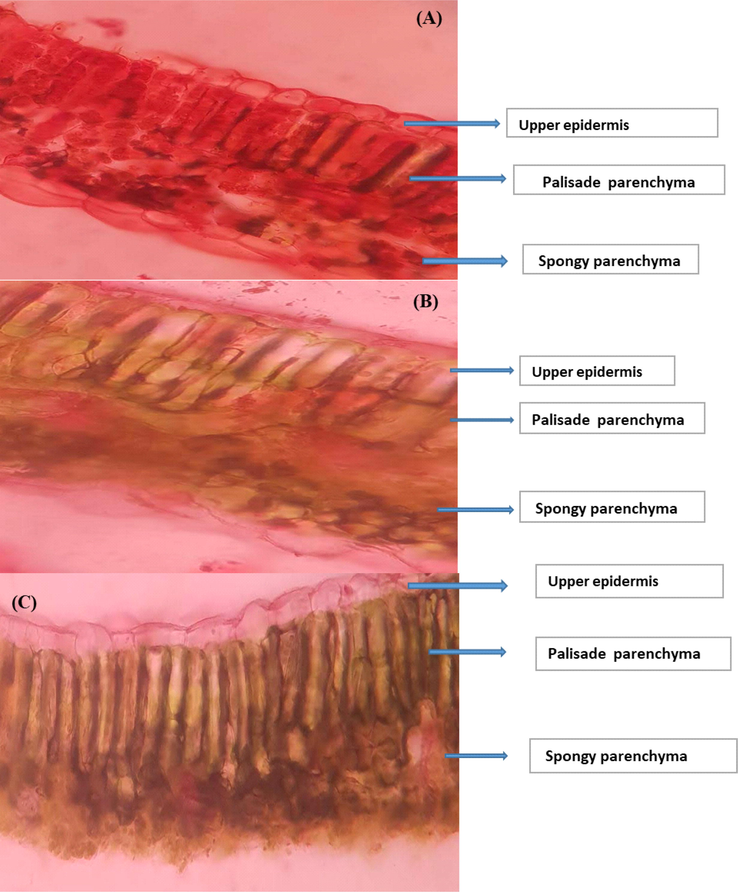
Cross-sections of the leaves of Cyamopsis plants cultivated in (A): well irrigated (control), (B): Drought (C): Drought with nutrient.
SDS-PAGE analysis of the thylakoid protein profile showed an increase in the content of various high- and low-molecular-weight polypeptides under conditions of drought stress with nutrient supplementation (Fig. 9). The control and drought-stress chloroplasts showed a significant decrease in the amount of polypeptides (55, 43, 33, 29, 27, 23, and 17 kDa). At the same time, the production of chloroplast protein content was higher in the plant supplemented with drought nutrients, especially at 55, 43, 33, 23, and 17 kDa polypeptides. Drought stress caused a general decrease in 55, 47, 33, 27, and 20 kDa polypeptides. The synthesis of these chloroplast protein polypeptides was twice as high in drought- and nutrient-stressed plants as in control plants. In particular, we found an increase in 33, 23, and 17 kDa polypeptides.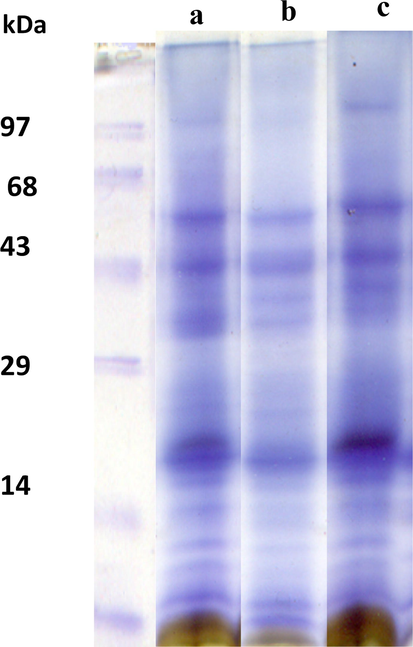
SDS –PAGE profile thylakoid protein in Cyamopsis plants cultivated in well irrigated (control) (a), Drought (b) and Drought with nutrient (c). Each well was injected with a 300 µg protein sample.
The overall effect of this plant was shown in its yield. Drought increased all the activities of this plant in the initial stage, but later the plant production started to decrease due to the effects of drought (Fig. 10). At the same time, drought and nutrient supplementation increased all the activities of the plant, as shown by the length of the pods, their fresh weight, dry weight, and size. Fresh & dry pod weights of control, drought stress, and drought with nutrients were recorded at 3.2 g & 0.6 g, 3 g & 0.52 g, and 4.5 g & 1.2 g, respectively. The seeds (100) weights of control, drought stress, and drought with nutrients were 3.5 g, 3 g, and 5.2 g, respectively (Fig. 10). The yield of the drought-stressed plants decreased by 10% compared to the control drought, but at the same time, it was 40% higher than the plants that received nutrient supplementation due to the drought.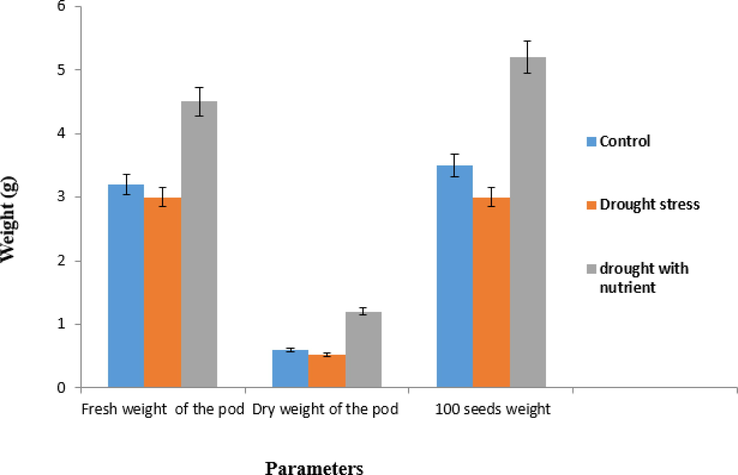
Quantitative changes in the fruit biomass and yield of the Cyamopsis plants grown under well irrigated (control), drought and drought with nutrient. Values represent average of 50 samples.
4 Discussion
The lack of available water is a major environmental barrier to farming. Losses in agricultural production due to drought are likely the most consequential, with the intensity and length of the stress being decisive factors. We have studied how drought stress affects plant development, photosynthesis, stomatal conductance, and yield.
The plants were newly collected at normal interval to evaluate the different elements. When compared to the control, C. tetragonoloba shoots' fresh and dry weights increased under drought stress. On the other hand, the treatment with panchagavya and exogenous vermicompost increased the fresh and dry weight of C. tetragonoloba shoots throughout the growing season. Changes in soil moisture outside of the optimal range can have an effect on yield and grain quality (Ahmed et al., 2017).
The study found that when plants were stressed by drought, their chlorophyll content was high in the early stages but decreased in later stages. However, when plants were stressed by drought and given nutritional supplements, their chlorophyll synthesis increased from the early stages to the later stages. Drought affects both the concentration of chlorophyll a and chlorophyll b. Carotenoids and total chlorophyll displayed a strong positive correlation. Under drought stress, chlorophyll levels either decreased or remained constant (Farooq et al. 2009). Franzoni et al. (2021) discovered a lower chlorophyll concentration in lettuce grown under water-stress conditions. Carotenoids, a photoprotective pigment, were shown to be present in greater amounts in plants exposed to drought stress, and their production was slightly higher in plants that received drought and nutrients. The main role of carotenoids is to inhibit the production of singlet chlorophyll by directly quenching triplet chlorophyll.
These findings demonstrate that adequate water shortages boost flavonoid synthesis and biomass accumulation. The concentration of flavonoids was also markedly elevated by the inclusion of nutrients. In comparison to the control, the plant with drought and nutrient addition and the plant under 10% drought stress had the highest flavonoid content, indicating that the rise in flavonoid production was brought on by biomass accumulation. Flavonoids, a kind of polyphenolic secondary metabolite, have been shown in prior studies to assist with drought relief (Nakabayashi et al., 2014). According to Amić et al. (2003) flavonoids' superior molecular qualities and antioxidant activity enable plants to adapt to a wide range of conditions. Flavonoids may be crucial in responding to different circumstances, such as drought. A key factor in drought resilience is flavonoids. Flavonoids prevent the closing of stomata by lowering the level of H2O2 in guard cells (Baozhu et al., 2022).
Plants produce anthocyanins as part of their defense mechanism in response to drought. The accumulation of anthocyanins in drought-stressed plants, potentially as a consequence of osmotic control and increased ABA content, suggests that these metabolites can be used as helpful indicators of drought stress (Li et al., 2019). The studies, however, do not rule out the possibility that anthocyanins have a photoprotective function and unquestionably demonstrate the primary driver of anthocyanin accumulation under drought stress or cold temperatures (Close and Beadle, 2003).
Under drought circumstances, stomatal density and stomatal duration showed a positive correlation. Under a gradient of drought, the stomatal density of the leaves of various jujube varieties also exhibits a similar pattern: first it rises, then it falls (Chen et al. 2014). Elevating antioxidant levels enhances plants' resistance to environmental stresses (Abdel Latef et al., 2019). Scavenging of plant free radicals has been postulated as a crucial component of the stress protection mechanism, and antioxidant enzyme activity is linked to osmotic resistance (Parida and Das. 2005). In general, adaptation to drought is dependent on the antioxidant system maintaining relatively low ROS levels (Rahimi et al., 2019). Regardless of the inherent resilience of plants to drought stress, an adequate and balanced supply of minerals can help mitigate the deleterious effects of drought.
Proline is an osmolyte that can develop intracellularly when plants are stressed. Osmolytes can help plants cope with water stress by balancing turgor pressure (Szabados and Savouré, 2010). The discovery that proline accumulation is triggered by drought in many plant species leads to suggest that an additional increase in proline accumulation could promote drought resistance (Bhaskara et al., 2015). Proline accumulates during drought or is administered exogenously to influence stress resistance in two ways: reducing tissue dehydration and protecting cell membranes and enzymes from the negative effects of dehydration (Per et al., 2017).
Under dry conditions, these changes should help C. tetragonoloba maintain vegetative growth and productivity while lowering the mesophyll layer. Compared to plants not under stress, the mesophyll layer became thicker under drought conditions. Palisade parenchyma, sponge parenchyma, stomatal density, and trichome density, in particular, could be viewed as important structural adaptations that contribute to the observed intra-specific diversity in olive tree responses to drought (Ennajeh et al., 2010).
When the chloroplast protein profile was examined, this finding was confirmed. Drought stress increases the ability of Cyamopsis to collect light by increasing chlorophyll content. Drought stress reduces the amount of photosynthetic pigment in the protein membrane that forms the thylakoid. It reduces the photon-capturing PSII proteins. Under drought, fertiliser caused their production to double. Drought has a significant influence on the chloroplast proteome, according to Tamburino et al. (2017).
When the plant we tested was subjected to dryness, it was discovered that it was drought resistant in the beginning, but that the effect deteriorated later and that the plant yielded badly compared to the control plant. A lack of water has a substantial influence on growth metrics such as number of leaves, dry root weight per plant (Ahmed et al., 2017). We noticed that the plant grew more prolific and drought resistant as a result of this small amount of nutrients. The amount of nitrogen in the plant affects how well the leaf performs photosynthetically Additionally, it has been demonstrated that sufficient nutrition levels allow metabolic processes to continue despite decreased tissue water potential, which helps to lessen the harmful consequences of drought stress (Farooq et al., 2009). According to several studies, plants' response to water scarcity can be tempered by providing enough nitrogen (de Freitas et al. 2022).
5 Conclusion
The drought stress slowed down the development of the plant, the formation of photosynthetic pigments, growth, yield, physiological process and morphological development. Application of panchagavya as a plant nutrient helped the plant overcome drought stress. The plants under drought stress and treated with panchagavya had higher plant growth, yield, secondary metabolites, and chlorophyll content. With that, more numbers of stomata, trichome palisade, sponge parenchyma, and thylakoid proteins were recorded. Also, Panchgavya application helped to maintain the high water potential of the tissue during drought and improve drought resistance through osmotic adjustment. The study suggests that the use of panchgavaya on drought-stressed plants may help plants increase their water use competence and minimize the detrimental properties of drought.
Acknowledgements
The authors are grateful to the Deanship of Scientific Research, King Saud University for funding through Vice Deanship of Scientific Research Chairs: Chair of Climate Change, Environmental Development and Vegetation Cover
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Extracts from yeast and carrot roots enhance maize performance under seawater-induced salt stress by altering physio-biochemical characteristics of stressed plants. J. Plant Growth Regul.. 2019;38:966-979.
- [Google Scholar]
- Impact of Putrescine and 24-epibrassinolide on Growth, Yield and Chemical Constituents of Cotton (Gossypium barbadense L.) Plant Grown under Drought Stress Conditions. Asian J. Plant Sci.. 2017;16:9-23.
- [Google Scholar]
- Impact of climate change on dryland agricultural systems: a review of current status, potentials, and further work need. Int. J. Plant Prod.. 2022;16(3):341-363.
- [Google Scholar]
- Structure-radical scavenging activity relationships of flavonoids. Croat. Chem. Acta. 2003;76(1):55-61.
- [Google Scholar]
- The flavonoid biosynthesis regulator PFG3 confers drought stress tolerance in plants by promoting flavonoid accumulation. Environ. Exp. Bot.. 2022;196:104792
- [Google Scholar]
- Rapid determination of free proline for water-stress studies. Plant Soil. 1973;39:205-207.
- [Google Scholar]
- Dynamic proline metabolism: importance and regulation in water limited environments. Front. Plant Sci.. 2015;6:484.
- [Google Scholar]
- Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol.. 1995;28(1):25-30.
- [Google Scholar]
- Response of relative sap flow to meteorological factors under different soil moisture conditions in rainfed jujube (Ziziphus jujuba Mill.) plantations in semiarid Northwest China. Agric. Water Manag.. 2014;136:23-33.
- [Google Scholar]
- Effect of foliar application of panchagavya and leaf extracts of endemic plants on groundnut (Arachis hypogaea L.) Legume Res.-Int. J.. 2014;37(2):223-226.
- [Google Scholar]
- Strategies to deal with drought-stress in biological nitrogen fixation in soybean. Appl. Soil Ecol.. 2022;172:104352
- [Google Scholar]
- Comparative impacts of water stress on the leaf anatomy of a drought-resistant and a drought-sensitive olive cultivar. J. Hortic Sci. Biotech.. 2010;85(4):289-294.
- [Google Scholar]
- Plant drought stress: effects, mechanisms and management. Sustain. Agric. 2009:153-188.
- [Google Scholar]
- Effect of glutamic acid foliar applications on lettuce under water stress. Physiol. Mol. Biol. Plants. 2021;27:1059-1072.
- [Google Scholar]
- Effects of drought stress on photosynthetic physiological characteristics, leaf microstructure, and related gene expression of yellow horn. Plant Signal. Behav.. 2023;18(1):2215025.
- [Google Scholar]
- ABA mediates development-dependent anthocyanin biosynthesis and fruit coloration in Lycium plants. BMC Plant Biol.. 2019;19:1-13.
- [Google Scholar]
- Plastid structure, chlorophyll concentration, and free amino acid composition of a chlorophyll mutant of barley. Can. J. Bot.. 1963;41(7):1053-1062.
- [Google Scholar]
- Photocontrol of anthocyanin synthesis: III. The action of streptomycin on the synthesis of chlorophyll and anthocyanin. Plant Physiol.. 1975;55(2):251-257.
- [Google Scholar]
- Effects of ultraviolet-B irradiance on soybean: V. The dependence of plant sensitivity on the photosynthetic photon flux density during and after leaf expansion. Plant Physiol.. 1984;74(3):475-480.
- [Google Scholar]
- Alternation of flavonoid accumulation under drought stress in Arabidopsis thaliana. Plant Signal. Behav.. 2014;9(8):e29518.
- [Google Scholar]
- Interactive effects of salinity and drought stress on photosynthetic characteristics and physiology of tomato (Lycopersicon esculentum L.) seedlings. S. Afr. J. Bot.. 2021;137:335-339.
- [Google Scholar]
- Salt tolerance and salinity effects on plants: a review. Ecotoxicol. Environ. Saf.. 2005;60(3):324-349.
- [Google Scholar]
- Salicylic acid and nutrients interplay in abiotic stress tolerance. Salicylic Acid: Multifaceted Hormone 2017:221-237.
- [Google Scholar]
- Environmental factors regulate plant secondary metabolites. Plants. 2023;12(3):447.
- [Google Scholar]
- The influence of chemical, organic and biological fertilizers on agrobiological and antioxidant properties of Syrian Cephalaria (Cephalaria syriaca L.) Agriculture. 2019;9(6):122.
- [Google Scholar]
- Chloroplast proteome response to drought stress and recovery in tomato (Solanum lycopersicum L.) BMC Plant Biol.. 2017;17:1-14.
- [Google Scholar]
- Oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective role of exogenous polyamines. Plant Sci.. 2000;151(1):59-66.
- [Google Scholar]
Further Reading
- The water-water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annu. Rev. Plant Biol.. 1999;50(1):601-639.
- [Google Scholar]
- Morpho-physiological and biochemical response of rice (Oryza sativa L.) to drought stress: A review. Heliyon. 2023;9(3):e13744
- [Google Scholar]







