Translate this page into:
Does pre-culture in sugar-rich media affect carbohydrate content and post-thawing recovery rate of cryopreserved potato (Solanum phureja) shoot tips?
⁎Corresponding author at: Department of Natural and Life Sciences, Faculty of Exact Sciences and Natural and Life Sciences, University of Tebessa, 12000 Tebessa, Algeria. chenchouni@gmail.com (Haroun Chenchouni)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
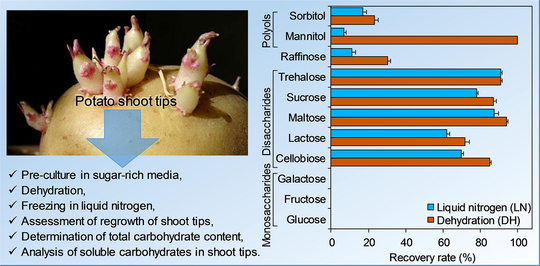
The cryopreservation by encapsulation-dehydration of in vitro-cultivated shoot tips of potato showed that maltose and trehalose were the most effective cryoprotectants. Shoot tips pre-cultivated in sucrose, trehalose or glucose, indicated an increase in total soluble sugars, especially when sucrose is applied as cryoprotectant.
Abstract
Shoot tips of Potato (Solanum phureja) were cryopreserved with the encapsulation-dehydration method, including: coating of shoot tips in calcium alginate beads, pre-culture in a solution containing 0.75 M of sugar (0.1 M of sucrose +0.65 M of another cryoprotectant sugar), and dehydration (for 4.5 h) on silica gel to reduce water content to 21–27%. Regrowth of shoot tips was assessed after freezing in liquid nitrogen. After cryopreservation, coated shoot tips were cultivated on a medium enriched with growth substances to measure concentrations of soluble carbohydrates. Maltose and trehalose ensured a better cryopreservation (88% and 91% of regrowth, respectively). Regrowth rates of potato shoot tips in the presence of polyols were very low and no recovery was obtained after freezing of shoot tips that were pre-cultivated in monosaccharides. Concentration of soluble carbohydrates increased in shoot tips pre-cultivated in sucrose, trehalose or glucose, especially when sucrose was applied as the cryoprotectant. The application of sucrose helped potato shoot-tips to acquire a certain tolerance to dehydration and cryofreezing. The analysis of soluble carbohydrates in potato shoot tips confirmed the accumulation of sugars during the pre-culture process, principally in the form of sucrose. The statistical analysis showed that soluble carbohydrate contents in shoot tips increased when total carbohydrates increased in the cultivation medium. The modelling approach indicated that the contents of soluble carbohydrates increased when pre-cultivation of potato shoot-tips was conducted in sucrose media compared to the control and other sugars.
Keywords
Cryopreservation
Pre-culture
Soluble carbohydrates
Liquid nitrogen
Coated shoot tips
Encapsulation-dehydration
Potato
1 Introduction
In vitro culture was developed as a tool for the conservation of a wide range of plant species. Large scale propagation, somatic embryogenesis, and production of metabolites are other important fields of application (Bouafia et al., 1995; Mehalaine and Chenchouni, 2020). Nevertheless, the use of in vitro culture methods for long-term conservation can induce physiological, genetic and epigenetic changes (Muthusamy et al., 2007; Yang and Ye, 2013). Other limitations of in vitro culture conservation are high maintenance cost, risk of contamination and mixture of genotypes. The preservation at very low temperatures “cryopreservation”, at −196 °C, is currently the sole technique that allows safe long-term preservation of biological materials (Engelmann, 2004). The low temperature of liquid nitrogen stops cell metabolism and can thus ensure a long-term preservation of pathogen-free genotypes without the risk of genetic drifts.
Several studies tried to identify the main factors that induce variations in regrowth of cryopreserved shoot tips of cultivated varieties and wild species of potato (Engelmann, 2004; Kim et al., 2006; Kaczmarczyk et al., 2008; Kaczmarczyk et al., 2011). Pre-culture, cultivation conditions of shoot tip donor plants, cooling, warming and post-culture treatments were identified as crucial factors for successful cryopreservation (Kaczmarczyk et al., 2011). Already cultivated potato varieties and wild potato species were successfully cryopreserved and showed high regrowth rates after thawing (Kim et al., 2006). However, the mode of action of cryoprotectants is poorly known, probably due to the presence of various complex mechanisms. Cryoprotectants with low molecular weight, generally penetrate into the cells and act in a colligative way, i.e. proportionally to the number of molecules per unit of water volume (Arakawa et al., 1990). Other cryoprotectant agents have a more specific action and are called non-colligative cryoprotectants, forming preferentially hydrogen bonds with membrane proteins and phospholipids (Rodrigues et al., 2008).
Potato is the basic food ingredient in most daily dishes of many countries worldwide, including Algeria (Oustani et al., 2015). Thus, achieving mass production of this crop and improving agriculture production in terms of quantity and quality is a national strategy in Algeria, which is adapted also by several developing countries (Fellah et al., 2018; Mihi et al., 2019). The modernization of agriculture depends on the improvement of scientific research in agricultural and agri-food sectors. The modernization of potato production system includes the application of techniques and tools for seed preparation using cryo-preservation protocols of plant genetic resources, as well as the use of appropriate methods of cultivation for planting and even best practices of irrigation and harvesting (Fellah et al., 2018; Boudjabi et al., 2015, 2019). So in order to achieve food sovereignty, a revolution in agricultural production systems, including potato cropping, must be rethought in this context. The present study aims at improving the recovery rates of cryopreserved potato shoot tips (Solanum phureja) using different cryoprotectant molecules. The effect of adding cryoprotectant agents to the pre-culture media was assessed with a set of eleven cryoprotectants. Total and soluble carbohydrate contents in shoot tips were determined using High-Performance Liquid Chromatography (HPLC) following Black et al. (1996), in order to better understand the mechanisms of sucrose action during dehydration and cryopreservation (Fabre and Dereuddre, 1987; Dumet, 1994; Folgado et al., 2015). Hereafter, total carbohydrates or total sugars refer to the sum of the sugars that were analysed.
2 Materials and methods
2.1 In vitro culture
In vitro potato plants (Solanum phureja) were obtained from the GERMICOPA SA company (France). Mono-nodal stem cuttings of 1 cm of length were excised from 6 to 8 week-old in vitro potato plants on fresh “cutting” medium. The culture medium contained macroelements of Tendille and Lecerf (1974) i.e. (808.8 mg/L KNO3, 160.1 mg/L NH4NO3, 141.7 mg/L Ca(NO3)2.4 H2O, 123.3 mg/L MgSO4·7H2O, 104.4 mg/L KCl, 81.7 mg/L KH2PO4, 7.0 mg/L K2HPO4), microelements of Murashige and Skoog (1962): (6. 2 mg/L H3BO3, 0.025 mg/L CoCl2·6H2O, 27.8 mg/L FeSO4·7H2O, MnSO4·22.3 mg/L 4H2O, 0.83 mg/L KI, 0.25 mg/L Na2MoO4·2H2O, 8.6 mg/L ZnSO4·7H2O, 5.57 g/L FeSO4·7H2O, 7.45 g/L Na2-EDTA, 0.025 mg/L CuSO4·5H2O), vitamins of Morel and Wetmore (1951): (100 mg/L inositol, 1 mg/L thiamine.HCl, 1 mg/L nicotinic acid, 1 mg/L pyridoxine.HCl, 1 mg/L Ca pantothenate, 0.01 mg/L biotin, Na-Fe-EDTA, 30 g/L sucrose, 8 g/L agar, pH 5.8), 30 g/L of sucrose, and 8 g/L of Difco® Bacto agar. The pH was adjusted to 5.85 with NaOH (1 N) and HCl (1 N). Agar was added at a temperature of 100 °C, followed by autoclaving at 110 °C for 20 min.
2.2 Shoot tip excision
For propagation of shoot tip donor plants, mono-nodal stem cuttings were sub-cultured for seven days in deep petri dishes (Ø = 10 cm) and incubated under the above described conditions, under room temperature (20 ± 1 °C), relative humidity = 70 ± 5%, photoperiod = 16 h, and light intensity = 50 μmoles/m2/s. Potato shoot tips of a length of 0.5–1.0 mm (with few leaf primordia) were excised under the stereoscope.
2.3 Encapsulation procedures
Excised potato shoot tips were placed in liquid Ca-free medium, containing macroelements (500 mg/L Ca(NO3)2·4H2O, 125 mg/L MgSO4 7H2O, 1000 mg/L KCl, 125 mg/L KH2PO4, 1000 mg/L (NH4)2SO4) of Morel and Muller (1964), microelements of Heller (1953) i.e. (0.1 mg/L MnSO4·4H2O, 1 mg/L ZnSO4·7H2O, 1 mg/L H3BO3, 0.01 mg/L KI, 0.03 mg/L CuSO4·5H2O, 0.03 mg/L AlCl3, 0.03 mg/L NiCl2·6H2O, 1 mg/L FeCl3·6H2O), vitamins of Morel and Wetmore (1951), with EDTA-FeNa2, 5 mg/L of gibberellic acid (GA3), 0.001 mg/L of 1-naphtaleneacetic acid (NAA), 0.01 mg/L 6-benzylaminopurine (BAP), 34.2 g/L of sucrose (0.1 M), supplemented with 30 g/L of sodium alginate (3%). For encapsulation, shoot tips and solution were absorbed with a micropipette, and dropped in an alginate-free culture medium (same composition), supplemented with 14.7 g/L of calcium chloride (CaCl2, 2H2O). Grown beads have a diameter of 3–4 mm and contained 1–2 shoot tips per bead. Beads were rinsed twice with liquid Ca-free shoot tip medium and pre-cultured on semi-solid shoot tip medium, supplemented with different types of cryoprotectants (sugars and polyols).
2.4 Cryoprotection (pre-culture)
A standard concentration of 0.1 M of sucrose was maintained for the encapsulation and pre-culture media. A final osmolarity of 0.75 M was obtained, adding the equivalent of 0.65 M of each cryoprotectant to the pre-culture media (Bouafia et al., 1995). Eleven different cryoprotective pre-culture treatments were assessed: 0.1 M of sucrose plus 0.65 M of different monosaccharides (glucose, fructose and galactose), disaccharides (trehalose, cellobiose, maltose and lactose), triholoside (raffinose), mannitol, sorbitol and sucrose. Coated shoot tips were pre-cultivated for two days under the previously described conditions (see subsection: 2.1. In vitro culture).
2.5 Dehydration on silica gel and freezing in liquid nitrogen (LN)
Pre-cultured shoot tips were dehydrated for 4.5 h on silica gel at a temperature of 20 °C. Shoot tips’ water content was reduced to 0.20–0.22 g of water/g DM. Shoot tips were transferred into cryobiological tubes, directly plunged in LN and maintained for minimum 1 h in LN. Water content of beads [in %] was calculated as (fresh weight−dry weight)/fresh weight, which represents weight loss.
2.6 Rewarming and plant recovery
Shoot tips were rewarmed at room temperature (∼20 °C), directly placed on shoot tip medium, and incubated at 20 ± 1 °C and a relative humidity of 70 ± 5%, with a photoperiod of 16 h and light intensity of 50 μmoles/m2/s, provided by True lite Durolux fluorescent tubes.
2.7 Determination of total and soluble carbohydrates in shoot tips
Total carbohydrate content was measured in both untreated (taken directly from the mono-nodal stem cuttings) and coated shoot tips. Shoot tips were pre-cultured for 2 days on shoot tip culture media, supplemented with 0.65 M of trehalose, glucose and sucrose (total osmolarity = 0.75 M). Pre-culture conditions were the same as previously described (see subsection: 2.1. In vitro culture). After pre-culture, shoot tips were extracted from the coating using a scalpel and rinsed with demineralized water. Shoot tips were fixed in 0.5 mL of ethanol (96% vol.) and stored at −25 °C. The experiment was repeated three times with a sample size of 20–25 shoot tips per treatment.
Shoot tips were crushed in the presence of 1 mL of ethanol (96% vol.) and centrifuged at 2000g for 10 min (at 4 °C). The supernatant was collected (ethanolic extract), the pellet was resuspended in 1 mL of water, crushed and centrifuged again to obtain the aqueous extract. Supernatants were stored at −25 °C after that the ethanolic and aqueous extracts were vacuum evaporated for 2–5 h. The dry extract was diluted in 200 µL of distilled water. Charged molecules were removed from the extract solution using an ion exchange resin mini-column. Samples of 20 µL of extract solution were analysed using high performance liquid chromatography (HPLC). Quantitative measurement was carried out with the integration method. Two reference solutions were used, the first contained sucrose, glucose and fructose, and the second enclosed the same carbohydrates plus trehalose.
2.8 Data analysis
Variation in fresh (FMW) and dry weight matter (DMW) of empty beads as well as FMW/DMW ratio and weight loss in % (FMW-DMW/FMW) was assessed among a set of 11 cryoprotectants using one-way analyses of variance (ANOVA) followed by Tukey’s post hoc tests. Normal distribution of data points was tested using the Shapiro test. The effect of the cryoprotectant molecular mass on weight loss and fresh and dry weights of encapsulated potato shoot tips (in beads) was analysed using a generalized linear model (GLM), including Gaussian error family and identity link. Raw data used in computations is available online at: https://dx.doi.org/10.6084/m9.figshare.4542454.
The effect of dehydration (-LN), liquid nitrogen exposure (+LN) and pre-culture with different cryoprotectants on the shoot tips’ recovery rate was analysed with a two-way ANOVA test. Separated ANOVA tests were performed for two cryoprotectant categories (disaccharides, trisaccharides and polyols). Variations of concentrations of total soluble carbohydrates and individual sugars in potato shoot tips, pre-cultured on three different cryoprotectants (sucrose, trehalose and glucose), were analysed with Tukey’s HSD test. The variation of different soluble carbohydrate concentrations following total carbohydrates of different pre-culture treatments was tested using GLM with Gaussian distribution error and identity link. Statistical analysis was carried out using R software (R Core Team, 2019).
3 Results
3.1 Fresh and dry matter weights of pre-cultured beads
Pre-cultured empty beads (without shoot tips) were used to determine if the cryoprotectant treatment altered the water retention potential of the beads. After pre-culture in liquid monosaccharide-rich culture media (glucose, fructose and galactose), empty calcium alginate beads showed DMWs ranging from 145.5 to 158 mg. FWM and DMW of empty alginate beads increased significantly after pre-culture in disaccharide-rich culture media. Beads pre-cultured in sucrose- and trehalose-rich media had FMWs of 439.3 ± 3.3 mg and 266.6 ± 2.1 mg, respectively. Beads pre-cultured in maltose-, cellobiose- and lactose-rich culture media showed FMWs of 282.0 ± 2.4 mg, 336.9 ± 3.2 mg, and 335.2 ± 3.0 mg, respectively (Table 1). The highest DMWs were observed when beads were pre-cultured in culture media containing raffinose (318.6 ± 2.6 mg) or sucrose (345.6 ± 2.8 mg). Low DMWs were observed in beads pre-cultured in mannitol- (138.6 ± 2.1 mg) and sorbitol-rich (150.1 ± 0.2 mg) culture media.
Cryoprotectants
MM [g/mol]
FMW [mg]
DMW [mg]
FMW/DMW ratio
Weight loss [%]
Glucose
180
207.2 ± 0.8c
158.0 ± 0.2c
1.311 ± 0.004d
23.55 ± 0.39 bc
Fructose
180
214.8 ± 1.2d
157.3 ± 0.4c
1.366 ± 0.011e
26.78 ± 0.58 a
Galactose
180
198.1 ± 1.8b
145.5 ± 0.6b
1.361 ± 0.015e
26.54 ± 0.78 a
Maltose
342
282.0 ± 2.4f
221.0 ± 2.4 e
1.276 ± 0.003ab
23.73 ± 0.21b
Cellobiose
342
336.9 ± 3.2g
257.5 ± 3.4 g
1.308 ± 0.007cd
26.1 ± 0.33 a
Sucrose
342
439.3 ± 3.3i
345.6 ± 2.8 i
1.271 ± 0.002a
21.63 ± 0.19 de
Lactose
342
335.2 ± 3.0g
247.7 ± 2.1f
1.353 ± 0.006e
23.26 ± 0.08 bc
Trehalose
342
266.6 ± 2.1e
206.3 ± 2.1 d
1.292 ± 0.003bc
21.26 ± 0.21 e
Raffinose
504
404.6 ± 2.2 h
318.6 ± 2.6 h
1.270 ± 0.003a
22.97 ± 0.14 bc
Mannitol
182
180.6 ± 2.5a
138.6 ± 2.1 a
1.303 ± 0.001cd
21.33 ± 0.13 e
Sorbitol
182
194.9 ± 0.6b
150.1 ± 0.2b
1.298 ± 0.002cd
22.61 ± 0.16 cd
Overall
278.2 ± 87.0
213.3 ± 70.0
1.310 ± 0.035
23.61 ± 1.98
F(10, 22)
4659
3817
88.9
96.71
P-value
<0.0001
<0.0001
<0.0001
<0.0001
The ANOVA test showed significant differences for DMWs, FMWs, FMW/DMW ratio and weight loss between the assessed cryoprotectants (P < 0.001). Following Tukey test, the lowest values of FMW/DMW ratio were recorded with sucrose (1.271) and raffinose (1.27) whereas the highest scores were observed with fructose (1.366) and lactose (1.353). Regarding the weight loss, it was the lowest in mannitol and trehalose with an average of 21%, and the highest in fructose, galactose and cellobiose with about 26% (Table 1).
The used General Linear Model showed a significant positive relationship between changes in FMWs and DMWs of empty alginate beads and the molecular mass of the respective cryoprotectant. The use of cryoprotectants with heavy molecular mass induced a significant increase in FMW (GLM: F = 88.4, P < 0.001) and DMW (GLM: F = 93.0, P < 0.001) of the pre-cultivated empty beads (Fig. 1). However, weight loss decreased significantly as the molecular mass of cryoprotectants increased (GLM: F = 13.4, P < 0.001).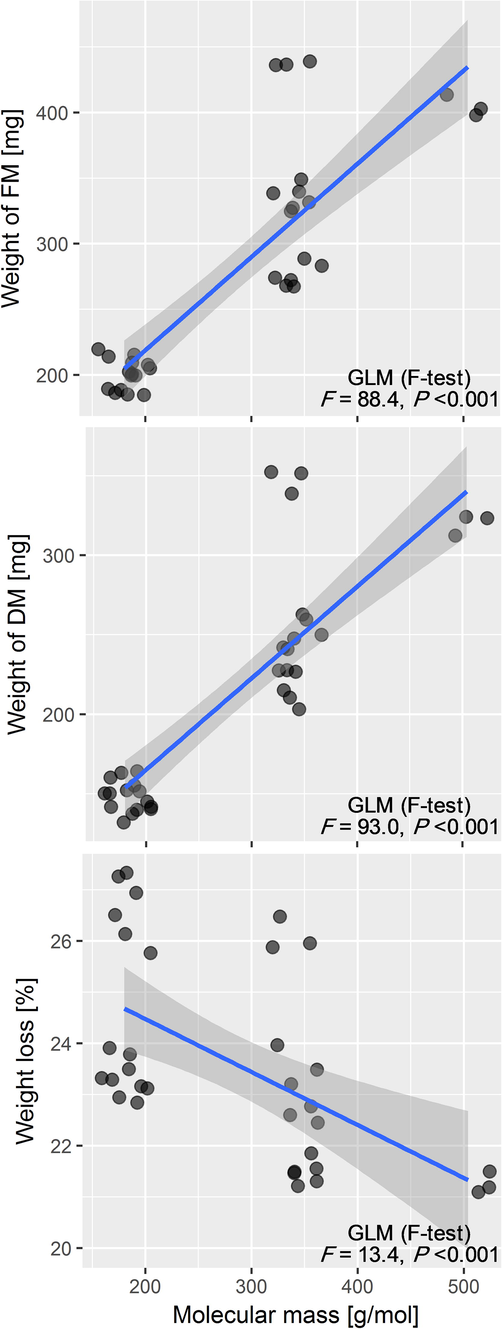
Relationship between the molecular mass of cryoprotectants and weigh loss, weights of fresh (FM) and dry matter (DM) of empty beads of potato. The lines represent a linear regression with a GLM fit (generalized linear model) and 95% confidence region in light grey. F (F-statistics) and P (P-value) are results of GLMs (Gaussian distribution error and ‘Identity’ link).
3.2 Tolerance to pre-culture, dehydration and freezing
Coated pre-cultured shoot tips showed an average recovery rates (rr) of 53% without liquid nitrogen exposure (-LN) and average 39% for in liquid nitrogen frozen samples (+LN). Dehydration with mannitol showed the highest recovery rate of 100% (-LN). No regrowth was observed when shoot tips were dehydrated in monosaccharide-rich culture medium (water content of beads: 21–27%). The pre-culture of shoot tips in disaccharide-rich culture media considerably increased its tolerance to dehydration (-LN) and freezing in liquid nitrogen (+LN). Highest post-thawing recovery rates were observed for shoot tips pre-cultured in culture media containing trehalose (rr = 91%), maltose (rr = 88%), and sucrose (rr = 78%). The ANOVA test showed significant variations for disaccharide cryoprotectants and treatments (Table 2). Shoot tips pre-cultured in culture media supplemented with polyols showed lower recovery rates after LN exposure. The ANOVA showed significant differences for the recovery rates of shoot tips pre-cultured in trisaccharide- and polyol-rich culture media (P < 0.001) (Fig. 2, Table 2).
Variables
DF
SS
MS
F
P
All cryoprotectants
Treatment
1
3462
3462
2218
<0.001
Cryoprotectants
10
87,153
8715
5585
<0.001
Treatment × Cryoprotectants
10
10,811
1081
693
<0.001
Residuals
44
69
2
Disaccharides
Treatment
1
496
496
266
<0.001
Cryoprotectants
4
2427
607
325
<0.001
Treatment × Cryoprotectants
4
182
46
24
<0.001
Residuals
20
37
2
Trisaccharides + polyols
Treatment
1
7041
7041
2697
<0.001
Cryoprotectants
2
4379
2189
838
<0.001
Treatment × Cryoprotectants
2
6553
3277
1255
<0.001
Residuals
12
31
3
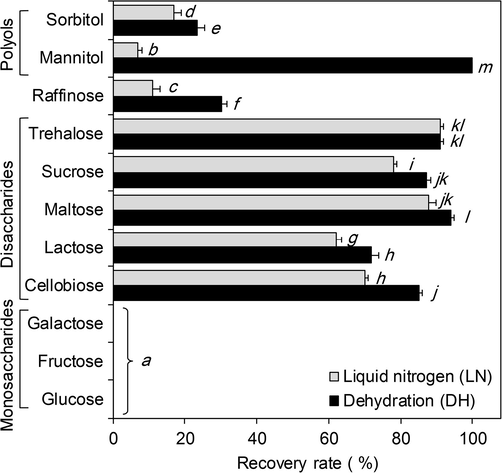
Recovery rates of shoot tips pre-cultivated in various cryoprotectant-based culture medium after dehydration and freezing in liquid nitrogen. Italic letters indicate results of Tukey's HSD tests, where the same letter are not significantly different at P > 0.05.
3.3 Composition of shoot tips with soluble carbohydrates
The ANOVA showed significant differences for the total carbohydrate content of potato shoot tips pre-cultured in culture media containing three different types of cryoprotectants (F(3, 32) = 2556, P < 0.001). For the control treatment (without pre-culture), shoot tips showed a total carbohydrate content of 33 ± 1.2 mg/g FMW. The highest carbohydrate content was observed when shoot tips were pre-cultured in trehalose-rich medium (Fig. 3). Contents of total sugars (i.e. sum of the sugars that were analysed) in controls averaged 14.6 mg/g FMW of sucrose, 9.7 mg/g FMW of glucose and 8.7 mg/g FMW of fructose (Fig. 4). After pre-culture in 0.75 M sucrose-rich medium, the total soluble carbohydrate content was 48.4 ± 1.6 mg/g FMW (1.46 times higher than that for the control treatment). The total concentration of carbohydrates (78.3 ± 0.8 mg/g FMW) significantly increased after pre-culture in trehalose-rich culture medium. This increase was related to the high sucrose content (57.5 mg/g FMW), representing 73.4% of the total carbohydrates of shoot tips. The contents of glucose (14%) and fructose (12.6%) constituted only minor portions of the total carbohydrate content. For the glucose-rich pre-culture medium, shoot tips showed a total carbohydrate content of 58.4 ± 0.7 mg/g FMW. Glucose represented 33% (19.3 mg/g FMW), whereas sucrose was dominant with 50.5% of total sugars (29.5 mg/g FM) (Fig. 3, Fig. 4). Total soluble carbohydrate content was significantly different for pre-culture treatments (P < 0.004) and type of soluble carbohydrates (P < 0.001). The composition of the soluble carbohydrates (glucose, fructose and sucrose content) varied significantly following the cryoprotectant molecule used in pre-culture. The variation of soluble carbohydrate contents was higher in sucrose and glucose (P < 0.001) compared to fructose (P = 0.035) (Table 3).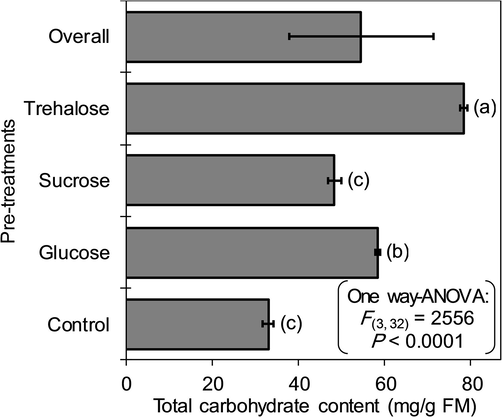
Contents of total carbohydrates measured in potato shoot tips directly pre-cultivated in three cryoprotectants “sucrose, trehalose and glucose” (n = 3, with each replicate composed of 20–25 shoots). Error bars represent standard deviations. The control included shoot tips not pre-cultivated. Letters between brackets indicate results of Tukey's HSD tests, where the same letter are not significantly different at P > 0.05.
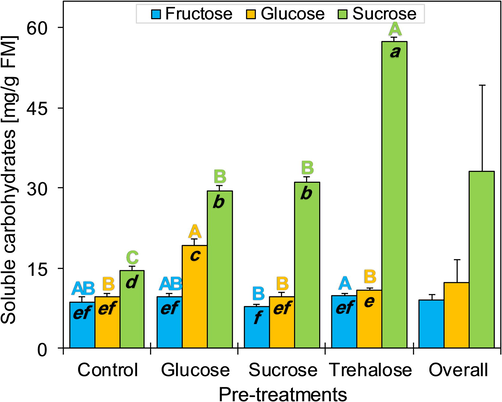
Variation of soluble carbohydrate contents in potato shoot tips pre-cultivated based on the application of various cryoprotectants “sucrose, trehalose and glucose” (n = 3, with each replicate composed of 20–25 shoots). The control included un-precultivated shoot tips. Vertical bars represent standard deviations. Italic lowercase letters in black colour indicate results of Tukey's HSD tests associated with two-way ANOVA (Table 3), where the same letter are not significantly different at P > 0.05. Capital and coloured letters (top of bars) are Tukey's HSD tests associated with one-way ANOVAs (comparisons of each soluble carbohydrate between pretreatments).
Variables
DF
SS
MS
F
P
All soluble carbohydrates (two-way ANOVA)
Pre-culture
3
1080.5
360.2
5.4
0.004
Type of carbohydrate
2
4104.4
2052.2
30.9
<0.001
Residuals
30
1995.2
66.5
Glucose contents (one-way ANOVA)
Pre-culture
3
194.4
64.8
101.5
<0.001
Residuals
8
5.1
0.6
Fructose contents (one-way ANOVA)
Pre-culture
3
7.1
2.4
4.7
0.035
Residuals
8
4.0
0.5
Sucrose contents (one-way ANOVA)
Pre-culture
3
2859.3
953.1
1311.6
<0.001
Residuals
8
5.8
0.7
3.4 Relationships between rich-media total carbohydrates and soluble carbohydrates in shoot tips
The GLM testing the variation of different soluble carbohydrate concentrations following total carbohydrates and pre-culture treatments showed that soluble carbohydrate contents increased when total carbohydrates increased in the media. However, this relationship was not significant (GLM: t = 0.71, P = 0.491) (Table 4). The model indicated also that the contents of soluble carbohydrates increased when the pre-cultivation of potato shoot-tips was carried out in sucrose compared to control and other sugars (Fig. 5). The GLM also revealed that regardless of total carbohydrate concentrations in pre-culture treatments, no differences (P > 0.05) were observed in concentrations of the soluble carbohydrates in shoot-tips (Table 4). (2.5% CI and 97.5% CI: lower and upper Confidence Intervals, SE: Standard Error).
Variables
Estimate
2.5% CI
97.5% CI
SE
t-value
P-value
Intercept
−0.47
−25.75
24.81
12.90
−0.04
0.972
Soluble carbohydrate = Glucose ‘SCG’
18.90
−16.85
54.66
18.24
1.04
0.321
Soluble carbohydrate = Sucrose ‘SCS’
−1.42
−37.17
34.33
18.24
−0.08
0.939
Total carbohydrate content ‘TCC’
0.28
−0.49
1.04
0.39
0.71
0.491
Pre-culture in Glucose ‘PCG’
−29.89
−116.56
56.79
44.22
−0.68
0.512
Pre-culture in Sucrose ‘PCS’
0.94
−36.80
38.69
19.26
0.05
0.962
Pre-culture in Trehalose ‘PCT’
−17.66
−114.12
78.80
49.22
−0.36
0.726
SCG × TCC
−0.54
−1.63
0.54
0.55
−0.99
0.343
SCS × TCC
0.22
−0.86
1.30
0.55
0.40
0.695
SCG × PCG
−16.89
−139.46
105.69
62.54
−0.27
0.792
SCS × PCG
−10.41
−132.98
112.17
62.54
−0.17
0.871
SCG × PCS
−28.33
−81.71
25.06
27.24
−1.04
0.319
SCS × PCS
55.26
1.88
108.64
27.24
2.03
0.065
SCG × PCT
−5.86
−142.28
130.56
69.60
−0.08
0.934
SCS × PCT
99.49
−36.93
235.91
69.60
1.43
0.178
TCC × PCG
0.41
−1.21
2.02
0.82
0.49
0.630
TCC × PCS
−0.12
−1.08
0.83
0.49
−0.26
0.803
TCC × PCT
0.08
−1.33
1.49
0.72
0.11
0.915
SCG × TCC × PCG
0.68
−1.60
2.96
1.16
0.58
0.572
SCS × TCC × PCG
0.32
−1.96
2.60
1.16
0.28
0.787
SCG × TCC × PCS
0.78
−0.58
2.13
0.69
1.12
0.283
SCS × TCC × PCS
−0.86
−2.21
0.50
0.69
−1.24
0.240
SCG × TCC × PCT
0.39
−1.61
2.39
1.02
0.39
0.706
SCS × TCC × PCT
−0.87
−2.86
1.13
1.02
−0.85
0.413
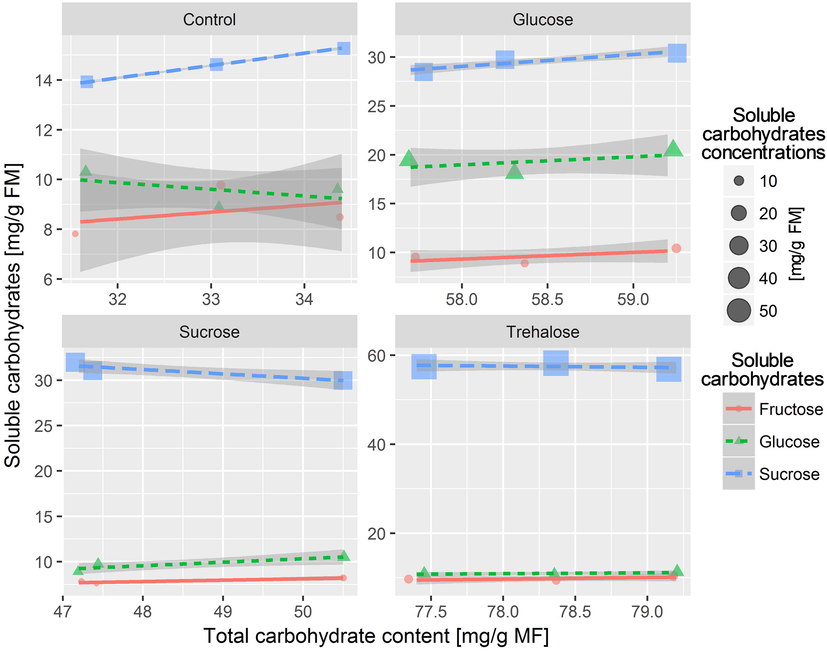
Relationship between total carbohydrate content and different soluble carbohydrate concentrations for various pre-culture treatments of cryopreserved potato shoot tips. Values are set to soluble carbohydrate concentrations and the shape and colour of points represent the type of soluble carbohydrate. The coloured lines represent linear regressions obtained by a Gaussian GLM fit (generalized linear model) with confidence regions in light grey.
4 Discussion
Regrowth of coated shoot tips of potato (Solanum phureja) after cryopreservation is strongly influenced by the pre-culture step. When shoot tips were pre-cultured for two days in a solution containing 0.75 M of sucrose, a post-thawing recovery rate of 78% was obtained (+LN). Natural tolerance to dehydration of some organisms is related to the modification of metabolism, including metabolism of sugars (Sun et al., 1994; Pinker et al., 2009; Folgado et al., 2015), as well as the accumulation of sucrose (Quain et al., 2009). Pre-culture of potato (Solanum tuberosum) shoot tips in sucrose-rich culture medium had a positive effect on plant regeneration (Folgado et al., 2014; Folgado et al., 2015). The penetration of sucrose into cells during the pre-culture stage can be explained by the accumulation of starch in the shoot tips of potato (Fabre, 1991) and date palm (Bagniol, 1992; Bagniol et al., 1992), but also in cell suspensions of Catharantus roseus (Bachiri, 1994). Pre-culture with sugar increases the shoot tips’ tolerance to dehydration and freezing in LN (Bouafia et al., 1996). Folgado et al. (2015) reported that pre-culture conditions improved plant recovery in the Solanum juzepcukii species.
The efficacy of sucrose can be questioned when it is replaced by other cryoprotectants. Following a pre-culture with trehalose or maltose at a concentration of 0.65 M, the respective recovery rates of 91 and 88% (+LN) were higher than those obtained with sucrose (78%). Other disaccharides showed to be less effective, with recovery rates of 70% (cellobiose) and 60% (lactose). Shoot tips pre-cultivated on trehalose-rich culture medium (0.65 M of trehalose plus 0.1 M of sucrose) showed a significantly higher content of total sugars, which is principally due to an increase in trehalose and sucrose content (Fig. 3). A similar process was observed in buds of grapevines (Plessis, 1994) and eucalyptus (Monod, 1995).
In the current study, monosaccharides were demonstrated to be completely ineffective for the cryopreservation of potato shoot tips with the encapsulation-dehydration method. Similar results were reported by Bouafia et al. (1996). The relatively high water content in beads (21–27%) may be the main cause for the poor tolerance of potato shoot tips to cryopreservation. Nevertheless, in previous studies glucose was reported as a good cryoprotectant (Green and Angell, 1989). The cryoprotective effectiveness of glucose was tested on somatic embryos of carrot (Tessereau. 1993), oil palm (Dumet, 1994), chinaberry (Scocchi et al., 2007), maritime pine (Alvarez et al., 2012), as well on shoot tips of carnation (Fabre and Dereuddre, 1987), grapevine (Plessis, 1994), on membranes of thylakoids isolated from spinach (Santarius, 1996), and on potato (Bouafia et al., 1996). The tolerance of potato shoot tips to freezing in LN was negative when pre-culture was done in polyol-rich culture media. This may be explained by the low solubility of sorbitol, mannitol and raffinose. Indeed, beads pre-cultivated in mannitol-rich medium lost their translucency and become white during the dehydration process. Similar results were reported in earlier studies (Dumet, 1994; Monod, 1995). Pre-culture under cold conditions negatively affected the recovery rate of some potato species (Folgado et al., 2015).
As a cryoprotectant, sucrose plays a determining role in the acquisition of tolerance to dehydration and freezing in potato shoot tips. Other disaccharides like trehalose (recovery rate = 91%) and maltose (rr = 88%) showed inclusive to be effective as good as sucrose. The analysis of soluble carbohydrates in potato shoot tips confirmed the accumulation of sugars during the pre-culture process, principally in the form of sucrose, which increases recovery rates as the tolerance of cryopreserved plants to dehydration and cryofreezing improves compared to untreated plants.
5 Authors’ contributions
Samia Bissati and Christiane Morisset designed the study. Samia Bissati carried our laboratory work. Samia Bissati, Saliha Boudjenah and Haroun Chenchouni drafted the manuscript. Haroun Chenchouni analysed data and revised the article. All authors read and approved the manuscript.
6 Availability of data and materials
The datasets used and/or analysed during the current study are available online at: https://dx.doi.org/10.6084/m9.figshare.4542454, or from the corresponding author on a reasonable request.
Funding
This study was not funded by any sources.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Cryopreservation of somatic embryogenic cultures of Pinus pinaster: Effects on regrowth and embryo maturation. Cryo-Lett.. 2012;33:476-484.
- [Google Scholar]
- The basis for toxicity of certain cryoprotectants: a hypothesis. Cryobiology. 1990;27:401-415.
- [CrossRef] [Google Scholar]
- Première application de la technique d’enrobage-déshydratation à la cryoconservation d’une suspension cellulaire (Catharantus roseus. France: DEA thesis, Univ. Technologie de Compiègne; 1994.
- Cryoconservation d’apex prélevés sur des vitroplants de palmier dattier (Phoenix dactylifera L.). Mise au point du protocole et étude histo-cytologique des échantillons au cours de la congélation et de la remise en culture. Doctoral thesis, Univ. Paris. 1992;6 France
- [Google Scholar]
- Histo-cytological study of apices from in vitro plantlets of date palm (Phoenix dactylifera L.) during a cryopreservation process. Cryo-Lett.. 1992;13:405-412.
- [Google Scholar]
- Carbohydrate metabolism in the developing and maturing wheat embryo in relation to its desiccation tolerance. J. Exp. Bot.. 1996;47(2):161-169.
- [CrossRef] [Google Scholar]
- Cryopreservation of potato shoot tips by encapsulation dehydration. Potato Res.. 1996;39:69-78.
- [CrossRef] [Google Scholar]
- Effets d’une préculture sur la tolérance à la congélation dans l’azote liquide des apex axillaires de vitroplants de Solanacées tubérifères (Solanum phureja et Solanum tuberosum). Etude après enrobage et déshydratation. Acta Botanica Gallica. 1995;142:393-402.
- [CrossRef] [Google Scholar]
- Growth, physiology and yield of durum wheat (Triticum durum) treated with sewage sludge under water stress conditions. EXCLI J.. 2015;14:320-334.
- [Google Scholar]
- Sewage sludge fertilization alleviates drought stress and improves physiological adaptation and yield performances in Durum Wheat (Triticum durum): a double-edged sword. J. King Saud Univers. – Sci.. 2019;31(3):336-344.
- [CrossRef] [Google Scholar]
- Cryoconservation des massifs d’embryons somatiques de palmier à huile (Elaeis Guineensis Jacq.) par déshydratation-vitrification. Etude du rôle du saccharose pendant le prétraitement. Doctoral thesis. Univ. Paris 1994:6.
- [Google Scholar]
- Plant cryopreservation: Progress and prospects. Vitro Cell. Dev. Biol.-Plant. 2004;40:427-433.
- [CrossRef] [Google Scholar]
- Fabre J (1991). Cryoconservation d’apex de Solanacées tubérifères après encapsulation-déshydratation. Etude des modifications cellulaires au cours du prétraitement. Doctoral thesis, Univ. Paris 6, France.
- Effets de différentes substances (saccharose, glucose, sorbitol et mannitol) sur la résistance à la congélation dans l’azote liquide de méristèmes d’œillets (Dianthus caryophyllus L, var Eolo) cultivés in vitro. C.R. Acad. Sci. Paris. 1987;304:507-510.
- [Google Scholar]
- Effect of water regime on growth performance of durum wheat (Triticum Durum Desf.) During different vegetative phases. Irrigat. Drain.. 2018;67(5):762-778.
- [CrossRef] [Google Scholar]
- Unravelling the effect of sucrose and cold pretreatment on cryopreservation of potato through sugar analysis and proteomics. Cryobiology. 2015;71:432-441.
- [CrossRef] [Google Scholar]
- Changes in sugar content and proteome of potato in response to cold and dehydration stress and their implications for cryopreservation. J. Proteom.. 2014;98:99-111.
- [CrossRef] [Google Scholar]
- Phase relation and vitrification in saccharide-water solutions and the trehalose anomaly. J. Phys. Chem.. 1989;93:2880-2882.
- [CrossRef] [Google Scholar]
- Recherches sur la nutrition minérale des tissus végétaux cultivés in vitro. Ann. Sci. Naturel. Botan. et Biol. Végétales. 1953;14:1-223.
- [Google Scholar]
- Potato shoot tip cryopreservation. A review. Potato Res.. 2011;54:45-79.
- [CrossRef] [Google Scholar]
- Influence of alternating temperature preculture on cryopreservation results for potato shoot tips. Plant Cell Rep.. 2008;27:1551.
- [CrossRef] [Google Scholar]
- Cryopreservation of potato cultivated varieties and wild species: critical factors in droplet vitrification. CryoLett.. 2006;27:223-234.
- [Google Scholar]
- New insights for the sustainable production of medicinal plant materials: ex vitro and in vitro propagation of some valuable Lamiaceae species from northern Africa. Elsevier; 2020.
- Can palm date plantations and oasification be used as a proxy to fight sustainably against desertification and sand encroachment in hot drylands? Ecol. Indicat.. 2019;105:365-375.
- [CrossRef] [Google Scholar]
- Cryoconservation d’apex d’Eucalyptus gunnii (Hook F.) par enrobage-déshydratation. Effets du prétraitement et tolérance des glucides solubles en relation avec la tolérance à la déshydratation et à la congélation. Doctoral thesis, Univ. Paris. 1995;6 France
- [Google Scholar]
- La culture in vitro du méristème apical de la pomme de terre. C.R. Acad. Sci. Paris. 1964;258:5250-5252.
- [Google Scholar]
- A revised medium for rapid growth and bioassays with tobacco tissues cultures. Physiol. Plant.. 1962;15:473-497.
- [CrossRef] [Google Scholar]
- Effects of mutagens on somatic embryogenesis and plant regeneration in groundnut. Biol. Plant.. 2007;51:430-435.
- [CrossRef] [Google Scholar]
- Effect of poultry manure on the yield and nutriments uptake of potato under saline conditions of arid regions. Emirat. J. Food Agriculture. 2015;27(1):106-120.
- [CrossRef] [Google Scholar]
- Effects of sucrose préculture on cryopreservation by droplet-vitrification of strawberry cultivars and morphological stability of cryopreserved plants. Cryo-Lett.. 2009;30:202-211.
- [Google Scholar]
- Cryoconservation par enrobage-déshydratation de bourgeons de vigne (Vitis vinifera L.) cultivée in vitro. Effets du prétraitement des bourgeons, évolution des sucres solubles et comportement des solutions pendant la congélation. Doctoral thesis, Univ. Paris. 1994;6 France
- [Google Scholar]
- Sucrose treatment and explant water content: critical factors to consider in development of successful cryopreservation protocols for shoot tip explants of the tropical species Dioscorea rotundata (yam) CryoLett.. 2009;30:212-223.
- [Google Scholar]
- R Core Team (2019). R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. Available at www.r-project.org.
- Evaluation of trehalose and sucrose as cryoprotectants for hematopoietic stem cells of umbilical cord blood. Cryobiology. 2008;56:144-151.
- [CrossRef] [Google Scholar]
- Freezing of isolated thylakoid membranes in complex media. XI. The cryoprotective efficiency of combinations of bovine serum albumin with low-molecular-weight cryoprotectants. Cryo-Lett.. 1996;17:15-24.
- [CrossRef] [Google Scholar]
- Cryopreservation of somatic embryos of paradise tree (Melia azedarach L.) Cryo-Lett.. 2007;28:281-290.
- [Google Scholar]
- The role of sugar, vitrification and membrane phase transition in seed desiccation tolerance. Physiol. Plant.. 1994;90:621-628.
- [CrossRef] [Google Scholar]
- La multiplication végétative de l’asperge (Asparagus officinalis L.). Action de divers facteurs, en particulier de la nutrition minérale, sur le développement des méristèmes et sur la production de plantes adultes. Annal. de l’Amélioration des Plantes. 1974;24:269-282.
- [Google Scholar]
- Développement d’une méthode simplifiée de cryoconservation de tissus et d’embryons somatiques végétaux et étude de l’acquisition de la tolérance à la congélation. Doctoral thesis. Univ. Paris. 1993;6
- [Google Scholar]
- Trichomes as models for studying plant cell differentiation. Cell. Mol. Life Sci.. 2013;70:1937-1948.
- [CrossRef] [Google Scholar]







