Translate this page into:
DNA damage in Cicer plant grown on soil polluted with heavy metals
*Tel.: +966 542140990 guddank@rediffmail.com (Sazada Siddiqui)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Available online 27 February 2015
Peer review under responsibility of King Saud University.
Abstract
In recent years industrialization is growing rapidly due to which the pollution load in water, air and soil is increasing day by day. Heavy metal pollution of the soil has raised concern in recent years due to its possible impact not only on human health but also on the plant system. To understand the consequences on plant systems, in the present study we cultivated the Cicer plant in soil polluted with heavy metals (Cd, Pb, Cr and Zn) collected from the Jhansi City of Uttar Pradesh, India with a geographical area of 502.75 thousand hectares. Seeds of Cicer were germinated in polluted soil sites such as T1 (Garden Soil, Control); T2 (Bharat Heavy Electrical Limited (BHEL)-Industrial); T3 (BHEL-Agricultural); T4 (Bijouli-Industrial); T5 (Bijouli-Agricultural). The effect of soil polluted with the heavy metals was analyzed by studying the percentage of seed germination, radicle length (RL), mitotic index (MI) and chromosomal aberrations (CAs) in root tip meristems. The results revealed that polluted soil with heavy metals T2 (BHEL-Industrial site) and T4 (Bijouli-Industrial site) had a significant impeding effect on the root meristem activity in Cicer as noticed by the reduction in seed germination percentage and RL compared to the control. Additionally, the variation in the percentage of mitotic abnormalities was observed. In general, increased percentage of chromosomal aberrations was observed in root tip cells of seedlings grown in polluted soil. Among these abnormalities laggards, bridges, stickiness, precocious separation and fragments were the most common. The obtained results demonstrated that heavy metal polluted soils led to a significant MI reduction and CA increase in root tip meristems of Cicer.
Keywords
Chromosomal aberrations
Genotoxicity
Mitotic index
Radicle length
Seed germination
1 Introduction
Soils are the main reservoirs for heavy metals generated by industrial activities e.g., metal finishing, paint pigment and battery manufacturing, leather tanning, mining activities, municipal wastewater sludges, urban composts, pesticides, phosphate fertilizers, or from atmospheric depositions. Manmade activities are primarily responsible for the increasing concentrations of heavy metals in agricultural land. Soil, especially those found in and around the industrial area are usually highly contaminated with the heavy metals, including, Zn, Ni, Cd, Cu, Pb, etc. (Adriano, 1986; Kabata-Pendias and Pendias, 1992). Metals are non- biodegradable and persist for a long period in aquatic as well as terrestrial environments. They may reach ground water through the soil and can be taken by plants causing profound effects on plant physiology and cytology (Balsberg Pahlsson, 1989; Siddiqui, 2013).
Plants are main components as they form the base of the food chain. Food chain contamination by heavy metals has become a serious issue in recent years because of their potential accumulation in biosystems through contaminated water, soil and air. Therefore, plant systems seem to be particularly important to analyze environmental issue on risk assessments (Sharma et al., 2004). In recent years, the consumption of Cicer arietinum, legume of the family Fabaceae, as an alternative source of food has been increased due to the fact that this plant contains high levels of proteins, vitamins and carbohydrates (Wang et al., 2010). In the last 20 years, the rapid increase in population in developing countries caused a massive food requirement and as a consequence planting fields for leguminosae and poaceae have continuously increased in the world.
The present investigation was aimed at evaluating the phytotoxicity and genotoxicity effects of different heavy metal contaminated soils on C. arietinum plant. For this purpose we analyzed C. arietinum Var.-C-18 plants cultivated in heavy metal polluted and in non-polluted soil samples collected from different areas of the Jhansi City, the well-known district of the Bundelkhand region in Uttar Pradesh, India with a geographical area of 502.75 thousand hectares.
2 Materials and methods
2.1 Site description
Soil samples were collected from five different sites of the Jhansi City, Uttar Pradesh, India. Jhansi is located at an elevation of 300 m above mean sea level (msl) and between the latitude of 24011′N–26027′N and longitude of 78017′E–81034′E (Fig. 1). The details of sites from which the soil was collected for the present study are as given below.
T1 = Garden Soil, Bundelkhand University, Main Camps
T2 = BHEL-Industrial
T3 = BHEL-Agricultural
T4 = Bijouli-Industrial
T5 = Bijouli-Agricultural
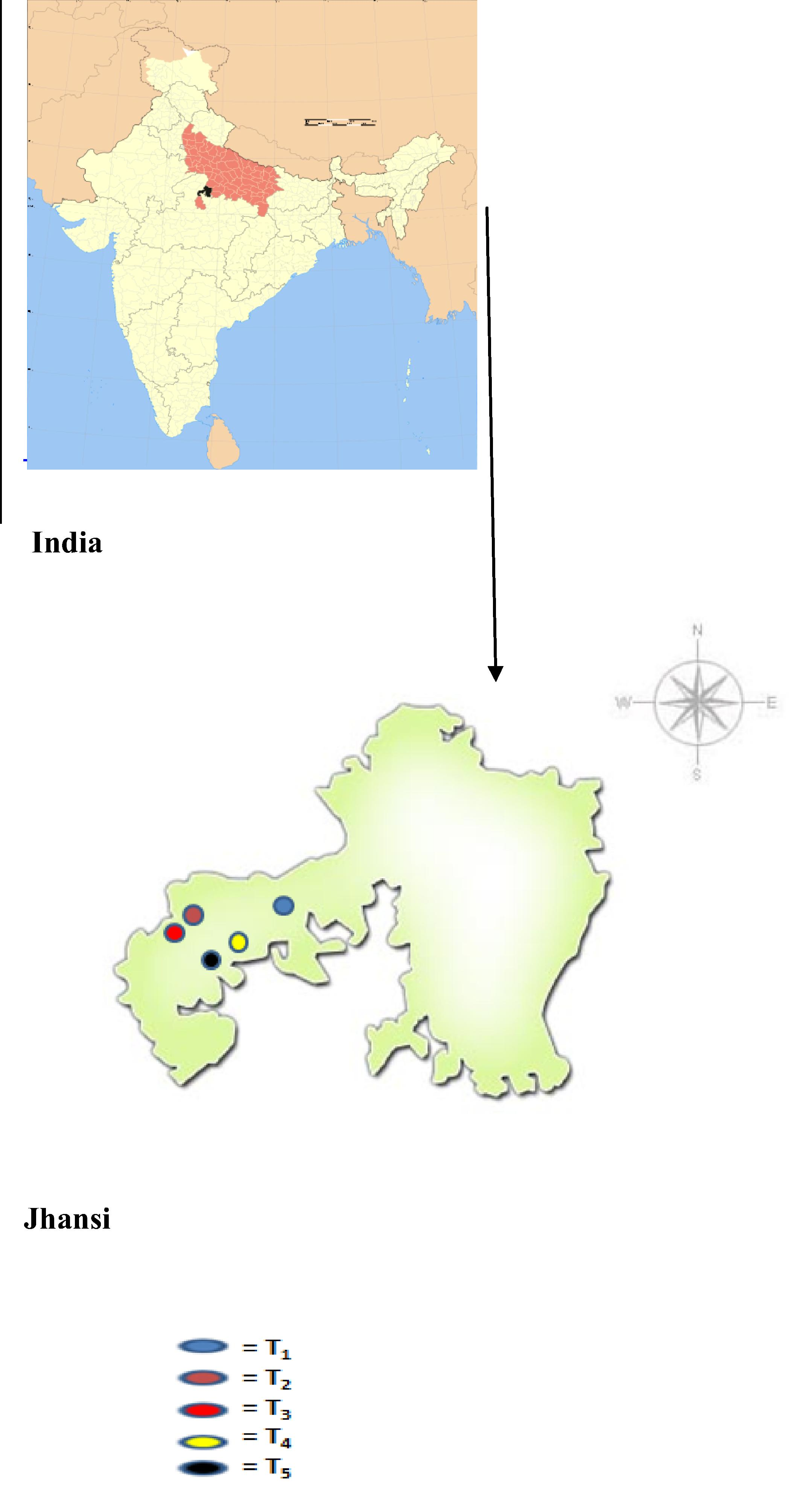
- Location map of study sites; T1 (Garden Soil); T2 (BHEL-Industrial); T3 (BHEL-Agricultural); T4 (Bijouli-Industrial); T5 (Bijouli-Agricultural).
The distance between the site T1 and T2 is 17 km; the distance between the T2 and T3 is 1 km; distance between site T1 and site T4 is 12 km; and the distance between site T4 and T5 is 1⧹2 km.
2.2 Collection of seeds
Healthy and uniform seeds of C. arietinum Var.-C-18 were collected from the Crop Research Farm, Govt. of Uttar Pradesh, Mauranipur, Jhansi, India.
2.3 Soil collection and analysis
The soil samples were collected from various parts of Jhansi as described above, air-dried at 20 °C, ground in a mortar, and then passed through a 2 mm plastic sieve. Well-fixed samples of 2 g each were digested with 8 ml of Aqua Regia on a sand bath for 2 h. After the samples were completely dried, the samples were dissolved with 10 ml of 2% nitric acid, filtered and then diluted to 50 ml with distilled water (Chen and Ma, 2001).Concentration of Cd, Pb, Cr, Zn was determined by using atomic absorption spectroscopy (iCEtm 3000, Waltham, MA, USA) and the results are shown in (Table 1). Sites: T1 (Garden Soil, Control); T2 (BHEL-Industrial); T3 (BHEL-Agricultural); T4 (Bijouli-Industrial); T5 (Bijouli-Agricultural).
Site
Cd (mg/kg)
Pb (mg/kg)
Cr (mg/kg)
Zn (mg/kg)
T1
0.33
42.8
27.0
115
T2
0.43
47.10
34.5
128
T3
0.37
41.20
28.60
119
T4
0.39
42.15
33.25
122
T5
0.32
36.50
27.10
116
2.4 Morphological analysis
Soils were collected from various sites with plastic spatulas and stored in polypropylene boxes. After removing the pebbles and twigs the soil samples were air-dried. Then they were passed through a 2 mm sieve and the soil was uniformly poured into sterilized Petri Plate. 30 seeds of C. arietinum were sown in duplicate. 30 seeds were surface sterilized with 0.5% sodium hypochlorite for 10 min, washed with distilled water several times to remove any excess of chemicals. Seeds were then soaked in double distilled water (DDW) for 12 h and then transferred to Petri Plates (15 cm diameter) containing 260 g of soil from the five different sites and placed in a Biological Oxygen Demand incubator (BOD-170, Pulse Life Science, Maharashtra, India) maintained at 24 ± 2 °C temperatures for further observation. Germination of seeds and radicle length (measured using a millimeter ruler) were analyzed at every 24 h interval. The experiment was repeated three times under the same conditions.
2.5 Cytogenetic analysis
Cytogenetic analysis was performed on Cicer root tip meristems fixed after seven days from seed germination on the different contaminated soils. Briefly, root tips were washed in water, fixed in Carnoy’s solution of (100% ethanol:glacial acetic acid 3:1) for 24 h and stored in 70% ethanol until further use. The fixed roots were hydrolyzed at 60 °C for 15 min, in 0.1 N HCl and stained in Leuco Basic Fuchsin for 10–20 min as described earlier (Siddiqui et al., 2007).
Root tips were squashed in 2% acetocarmine on slides, mounted and observed under oil immersion objective using a light microscope from Leica DMi1, Germany. All the slides were coded and examined unsighted. Mitotic index (MI) and chromosomal aberrations (Abs) in metaphase and anaphase plates were studied using a light microscope at a higher magnification (100×). From each slide, minimum of 1000 cells were scored and mitotic index was calculated. Chromosomal aberrations such as sticky chromosomes, precocious separation, fragments, and laggard were studied in a minimum of 50 metaphase and anaphase plates per slide and expressed in percentage.
2.6 Statistical analysis
Statistical analysis was performed employing a one way ANOVA test using the GPIS software 1.13 (Graph PAD, California, and USA) to detect the significance of differences of variables. (All values are expressed as mean ± SE).
2.7 Effect on seed germination of Cicer plant grown on soil polluted with heavy metals
Fig. 2 summarizes the germination potential of C. arietinum seeds grown in soils samples collected from various sites. In the seeds grown in Garden Soil (T1), germination potential was 10% at 24 h which increased to 15% and 27% at 48 h and at 72 h respectively. However, in seeds grown in T2 lower percentage of seed germination was observed compared to those grown in T1 (4%, 9% and 15% at 24, 48 and 72 h respectively). The difference was statistically significant (p < 0.05 and p < 0.01 at 24 and 72 h respectively) except at 48 h compared to T1 at respective times. A similar trend in seed germination was observed for the T4 soil. Furthermore, seed germination in soil from T3 and T5 was similar to that grown in Garden Soil.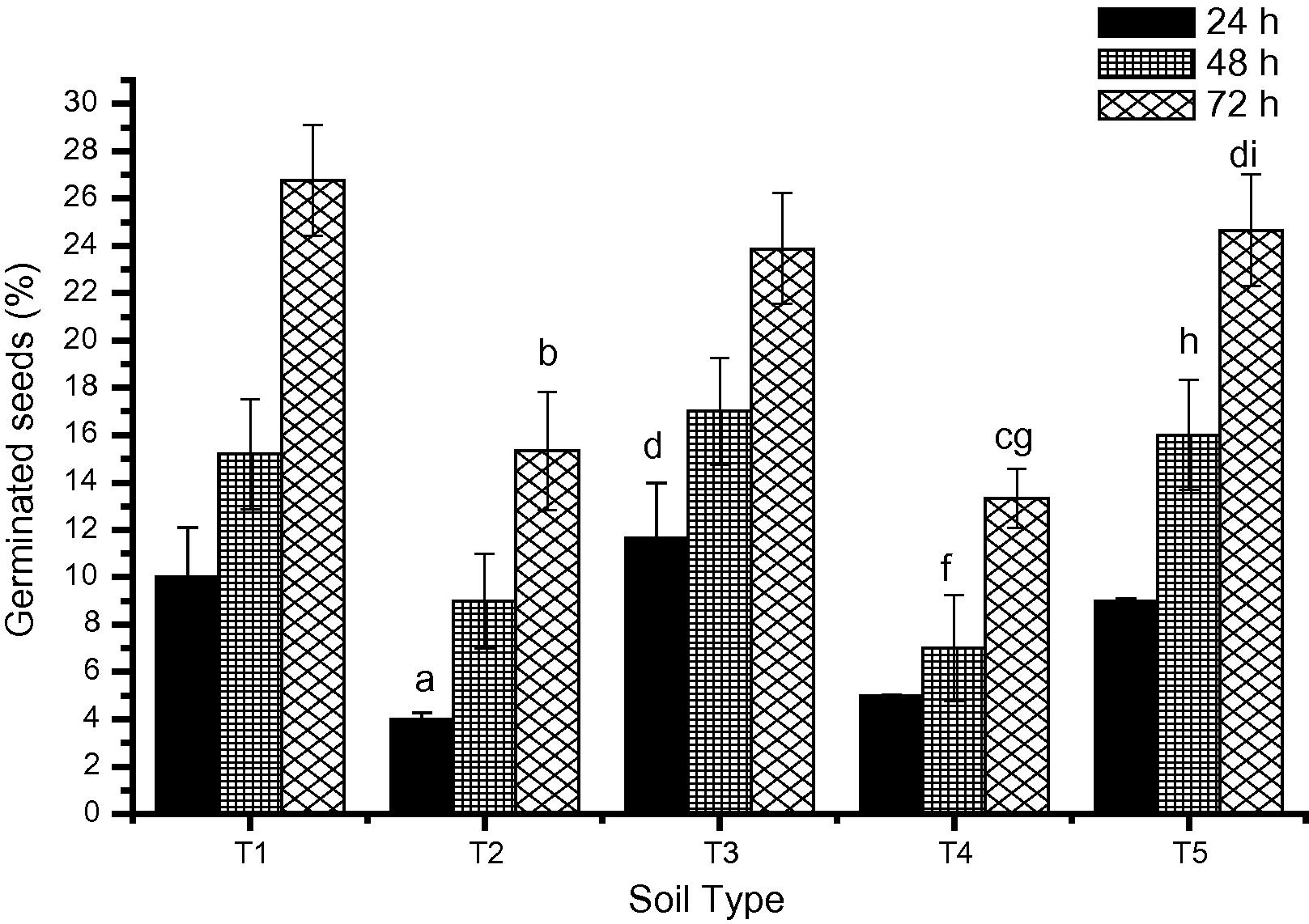
Effect on the seed germination of C. arietinum grown on soil polluted with heavy ap < 0.05, bp < 0.01, cp < 0.001 vs. T1 Garden Soil; dp < 0.01 vs. T2 BHEL Industrial; fp < 0.05, gp < 0.01 vs. T3 BHEL agricultural; hp < 0.05, ip < 0.01 vs. T4 Bijouli Industrial at respective time intervals.
2.8 Effect on radicle length of C. arietinum plant grown on soil polluted with heavy metals
Results of radicle length of the C. arietinum plant grown in the different soil samples is depicted in Fig. 3.In seeds grown in site T1, at 24 h, the radicle length was 1.00 ± 0.23 cm which increased to 1.36 ± 0.12 cm at 48 h and 2.19 ± 0.19 cm at 72 h. Even though radicle length was smaller than the seeds grown in T1, the difference was statistically not significant at 24, 48 or at 72 h. No significant difference was observed in radicle length of seeds grown in T3, T4 or T5 soils compared to the T1 soil. However, increased radicle length was observed in germinated seeds grown in site T3; root length was 1.41 ± 0.78 cm, 1.47 ± 0.12 cm and 2.4 ± 0.22 cm at 24, 48 and 72 h respectively. In addition, the radicle length was significantly higher in the T3 group at 48 and 72 h compared to the T2 (p < 0.05) group as well as the T4 group at 72 h (p < 0.05).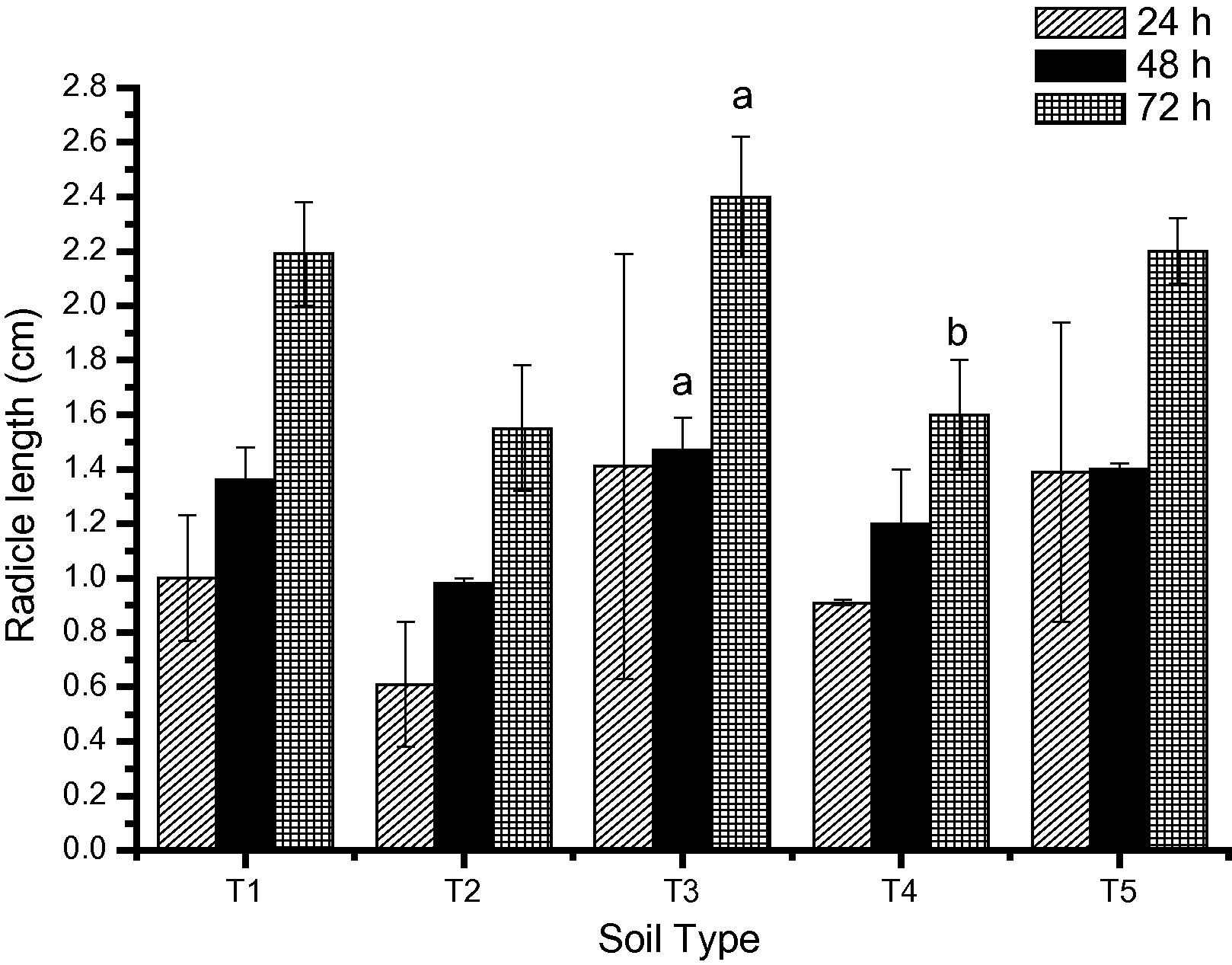
Effect on the radicle length of C. arietinum grown on soil polluted with heavy metals. ap < 0.05 vs. T2 BHEL Industrial at 48 and 72 h; bp < 0.05 T3 BHEL agricultural at 72 h.
2.9 Effect on mitotic index and chromosomal aberration of the Cicer plant grown in soil polluted with heavy metals
2.9.1 Mitotic index
Root meristems from seedlings grown in site T1 had a mitotic index of 27.78 ± 2.75 at 24 h, 25.12 ± 1.12 at 48 h and 23.54 ± 2.21 at 72 h (Fig. 4). Seedlings grown in site T2 exhibited a higher mitotic index at all the intervals (32.75 ± 1.25, 29.15 ± 2.15 and 27.78 ± 2.15 at 24, 48 and 72 h respectively) compared to those grown in other soil samples. However, the increase was statistically non-significant compared to Garden Soil. A similar trend was observed in the T4 group. A similar trend in mitotic index was observed for T1, T3 and T5 groups whereas, T2 and T4 had a similar trend with respect to mitotic index at various time intervals.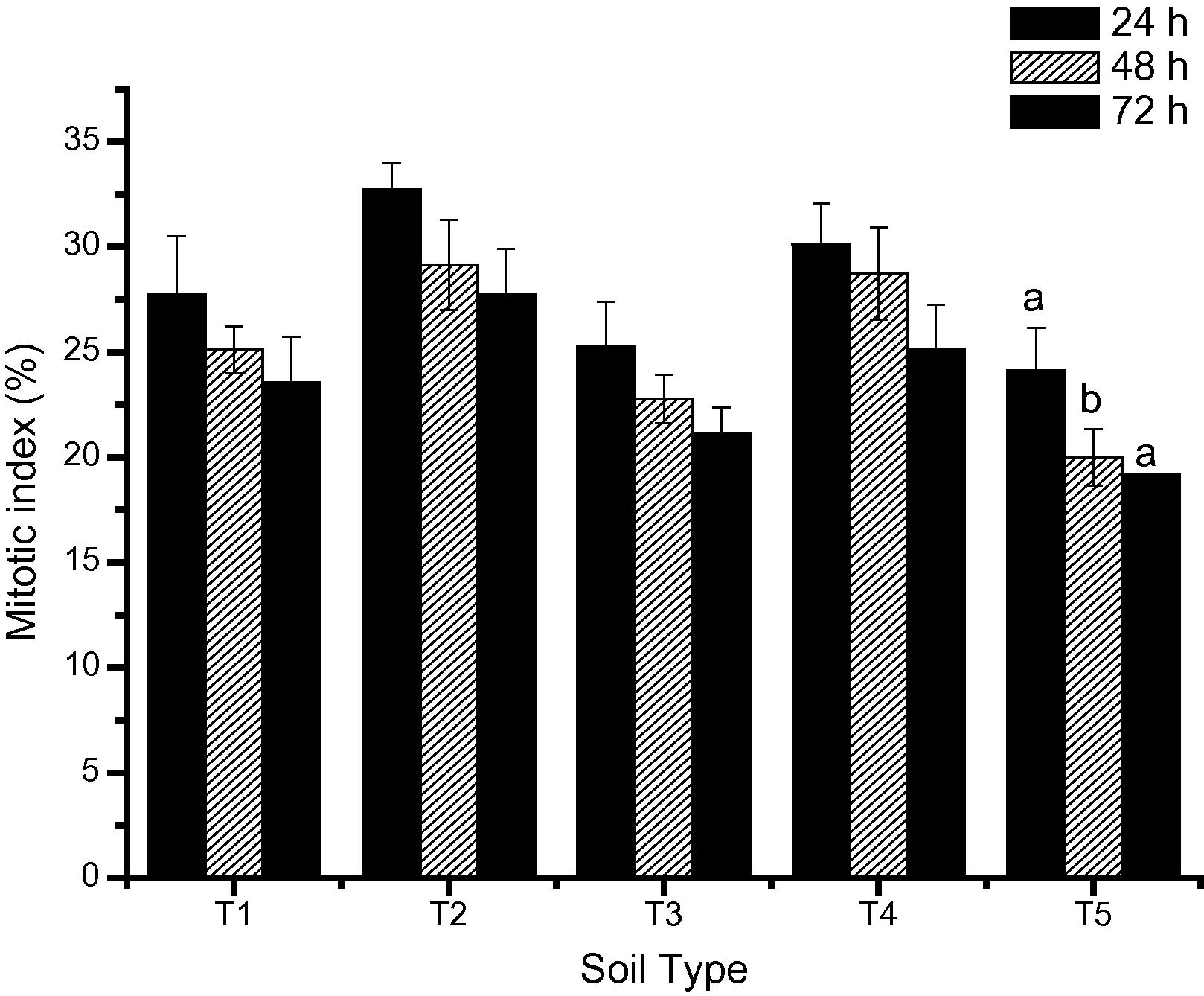
Effect on the mitotic index of C. arietinum grown on soil polluted with heavy metals. ap < 0.05 vs. T2 BHEL Industrial at 24 and 72 h, bp < 0.01 vs. T2 BHEL Industrial at 48 h.
2.9.2 Chromosomal aberration
Polluted soil induced a number of mitotic aberrations in C. arietinum (Table 2 and Plate 1). The incidence of abnormal metaphase–anaphase plate in root meristems from seedlings grown in soil from site, T1 was 0.41 ± 0.031 at 24 h, 0.79 ± 0.061 at 48 h and 1.17 ± 0.061 at 72 h. Precocious separation, in all phases of mitosis was the most frequent aberration, as they accounted for 80–85% of the total number of aberrations. Increased incidence of sticky chromosomes (0.33%), fragments (1.98%), precocious separation (3.15%) and laggard (3.0%) were reported in site T4. Similar trends were reported in T3 and T5 Sites. ap < 0.001 vs. T1 Garden Soil; bp < 0.001 vs. T2 BHEL Industrial; cp < 0.001 vs. T3 BHEL agricultural; dp < 0.001 vs. T4 Bijouli Industrial; cp < 0.05, dp < 0.001 vs. T3 BHEL agricultural; fp < 0.001 vs. T4 Bijouli Industrial; bp < 0.01, cp < 0.001 vs. T2 BHEL Industrial; dp < 0.01, fp < 0.001 vs. T3 BHEL agricultural; gp < 0.001 vs. T4 Bijouli Industrial.
Aberrations in 50 plates
Study sites
T1
T2
T3
T4
T5
24 h
% Sticky chromosome
0.00 ± 0.00
0.23 ± 0.001a
0.11 ± 0.002a,b
0.33 ± 0.02
0.15 ± 0.002a,b,c,d
% Fragment
0.01 ± 0.001
1.66 ± 0.04a
0.66 ± 0.03a,b
1.98 ± 0.03a,b,c
0.99 ± 0.05a,b,c,d
% Precocious separation
0.25 ± 0.01
2.33 ± 0.01a
0.99 ± 0.023a,b
3.15 ± 0.001a,b,c
1.25 ± 0.03a,b,c,d
% Laggard
0.15 ± 0.02
2.33 ± 0.01a
0.33 ± 0.03b
3.00 ± 0.21a,b,c
0.45 ± 0.03b,d
% Total aberrant plates
0.41 ± 0.03
6.55 ± 0.061a
2.09 ± 0.085a,b
8.46 ± 0.261a,b,c
2.84 ± 0.112a,b,c,d
48 h
% Sticky chromosome
0.01 ± 0.001
0.44 ± 0.04a
0.16 ± 0.001a,b
0.48 ± 0.002a,d
0.22 ± 0.001a,b,f
% Fragment
0.01 ± 0.02
3.66 ± 0.01a
1.26 ± 0.02a,b
2.15 ± 0.001a,b,d
1.12 ± 0.01a,b,d,f
% Precocious separation
0.44 ± 0.02
3.33 ± 0.07a
1.25 ± 0.23a,b
3.75 ± 0.07a,d
1.78 ± 0.01a,b,c,f
% Laggard
0.33 ± 0.02
3.15 ± 0.01a
0.66 ± 0.03a,b
3.89 ± 0.07a,b,d
0.66 ± 0.02a,b,f
% Total aberrant plates
0.79 ± 0.061
10.18 ± 0.13a
3.33 ± 0.281a,b
10.27 ± 0.14a,b,d
3.78 ± 0.09a,b,d,f
72 h
% Sticky chromosome
0.01 ± 0.001
0.68 ± 0.04a
2.10 ± 0.001a,c
0.48 ± 0.002a,c,d
0.27 ± 0.02a,c,f,g
% Fragment
0.15 ± 0.02
4.44 ± 0.07a
1.75 ± 0.04a,c
2.33 ± 0.07a,c,d
1.75 ± 0.02a,c,g
% Precocious Separation
0.66 ± 0.04
3.66 ± 0.08a
1.75 ± 0.04a,c
3.95 ± 0.03a,b,d
2.02 ± 0.05a,c,d,g
% Laggard
0.35 ± 0.02
4.25 ± 0.08a
0.77 ± 0.04a,c
4.00 ± 0.001a,b,d
0.99 ± 0.04a,c,d,g
% Total aberrant Plates
1.17 ± 0.061
13.03 ± 0.27a
6.37 ± 0.12a,c
10.76 ± 0.103a,b,d
5.03 ± 0.13a,c,d,g
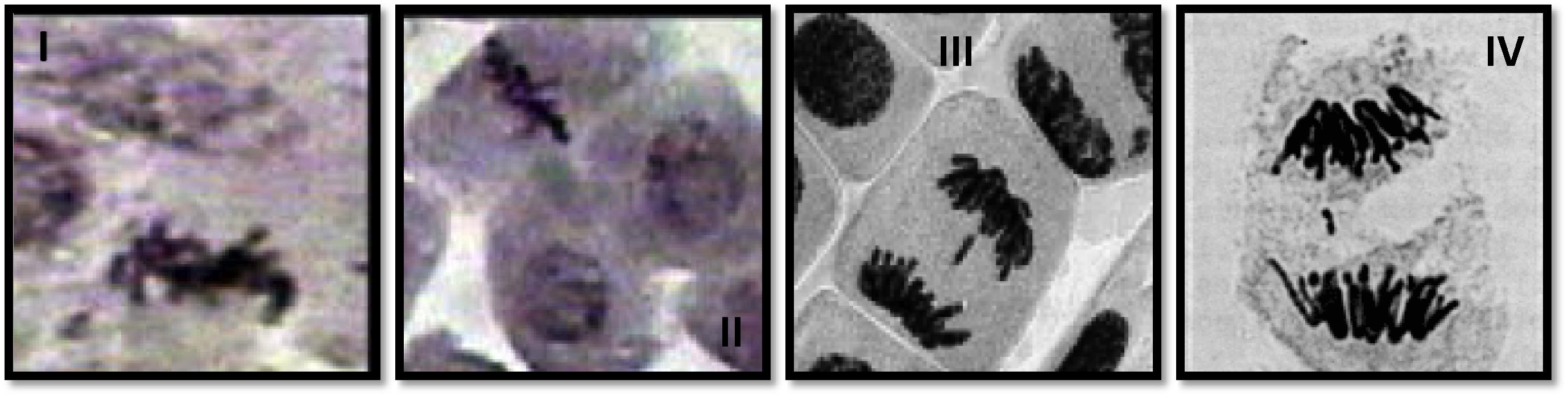
I. Sticky chromosome, II. Precocious separation, III. Anaphase with Laggard, IV. Anaphase with fragment.
3 Discussion
Toxic and mutagenic effects of heavy metal contaminated soil on the plant system have been reported by earlier studies (Sengar et al., 2008; Seregin and Kozhevnikova, 2006; Siddiqui et al., 2007, 2009, 2012). Significant decline in seed germination, slow development of radicles and increased chromosomal aberration observed in the mitotic cells of the Cicer plant grown in soil polluted with heavy metals in the present study further confirm that soil polluted by heavy metals can have genotoxic and phytotoxic effects.
The mechanism by which heavy metals induce these effects in the plant is complex. Heavy metals are known to interfere with nutritional elements of seedlings and plants, thus causing deficiency or abnormal ion distribution within the plant (Khan and Khan, 1983; Biddappa et al., 1987; Trivedi and Erdei, 1992). Aidid and Okamoto (1992, 1993) have shown that heavy metals inhibit plant growth by suppressing the elongation of the plant cells which may be due to the inhibitory effect on mitosis. Our group has made a similar observation earlier with heavy metals like cadmium and lead (Siddiqui et al., 2009; Siddiqui, 2012).Heavy metals may enter into the cell nucleus and may bind to purine and pyrimidine bases and proteins, denature spindles, and cause micronucleus MN formation as a result of the decrease in the chromosome number in the main nucleus (Jiang et al., 2001). Heavy metals can inhibit the DNA synthesis (Sudhakar et al., 2001) or may even block the cells in the G2_phase of the cell cycle preventing the cells from entering mitosis. Furthermore, heavy metals may also act on DNA-repair enzymes, either by modifying the protein structure of the enzymes, or by reducing the production of the enzymes at the transcription level which could also lead to chromosomal aberrations in mitotic cells. To support the above hypothesis, Cicer grown in soil polluted with heavy metals showed several chromosomal abnormalities in mitotic cells such as sticky chromosome, fragments, precocious separation and laggard. Among these abnormalities precocious separation (PS) was the most frequently observed chromosomal aberration in the Cicer. In addition the frequency of remaining abnormality is in the following order in different sites (Table 3). Sites: T1 (Garden Soil); T2 (BHEL-Industrial); T3 (BHEL-Agricultural); T4 (Bijouli-Industrial);T5 (Bijouli-Agricultural).
Sites increasing order chromosomal aberrations
T1 Precocious separation > Laggards > Fragments
(No sticky chromosomes were reported in T1 site)
T2 Precocious separation = Laggard > Fragment > Sticky chromosome
T3 Precocious separation > Fragment > Laggard > Sticky chromosome
T4 Precocious separation > Laggard > Fragments > Sticky chromosome
T5 Precocious separation > Fragment > Laggard > Sticky chromosome
Increase in the aberrant metaphase plates having sticky chromosomes might be due to the denaturing activity of heavy metals on nuclear proteins such as DNA topoisomerase II, which might also interfere with chromosome segregation (Panda and Panda, 2002). Heavy metals like Cd also caused sticky chromosomes in Pisum sativum (Fusconi et al., 2006, 2007; Siddiqui et al., 2009).
In the current study the incidence of precocious movement increased in sites T2 and T4. This precocious movement could appear because of the disturbed spindle activity (Umar and Singh, 2003) or due to the heavy metals breaking the protein moiety of the nucleoprotein backbone.
High incidence of fragments noticed in the root tips in the present study is probably formed by acentric chromosomes and also as a result of inversion. Fragmentation might have arisen due to stickiness of chromosomes and consequent failure of separation of chromatids to poles (Fusconi et al., 2006, 2007). In addition to this, DNA double stranded breaks induced by reactive oxygen species can lead to chromosome fragments. Earlier studies have shown that heavy metals increase the free radical level in cells (Weckx and Clijsters, 1997) which has several deleterious effects on the crucial macromolecules of the cell such as DNA.
The most common type of aberrations in the present investigation was the appearance of lagging chromosomes at metaphase and anaphase plates The induction of laggard could be attributed to the failure of the spindle apparatus to organize and function in a normal way rather than inhibition of these spindle fibers and this may lead to irregular orientation of chromosomes (Mansour, 1984; Grant, 1978; El-Abidin Salam et al., 1993).
The results of the present investigations revealed that industrial sites T2 and T4 have considerable inhibitory effects on seed germination and radical length and increased chromosome aberrations. In addition, in agricultural sites T3 and T5 seed germination radicle length increased and incidence of chromosomal aberration decreased. Based on the results of T2 and T4 sites and on the increased incidence of various kinds of chromosomal abnormalities as reported in this study, perhaps it might be logical to conclude that heavy metals exhibit genotoxic effects in polluted sites such as T2 and T4. However, the major drawback of this study is that levels of various heavy metals in the soils collected are not analyzed which could have further supported the genotoxic effects induced by soil samples collected from industrial sites. In conclusion, the results of the present study indicate that the soil quality has a significant effect on the plant development and the genetic integrity of the developing plant. Further studies in this direction are necessary to understand the association between the concentration of heavy metals in soil and their consequences on the development and phenotype of plants grown in such soils.
Acknowledgements
Authors are grateful to Dr. A.K. Giri, the the Head, Department of Botany, Bundelkhand University, Jhansi, for providing necessary laboratory facilities. Thanks are also due to the person in Charge of the soil testing laboratory, Department of Agriculture, Government of Uttar Pradesh, Jhansi, for rendering help in soil analysis.
References
- Trace Elements in the Terrestrial Environment. New York: Springer-Verlag; 1986.
- Effect of lead, cadmium and zinc on the electric membranepotential at the xylem/symplast interface and cell elongation of Impatiens balsamina. Environ. Exp. Bot.. 1992;32:439-448.
- [Google Scholar]
- Responses of elongation rate, turgor pressure and cell wall extensibility of stem cells of Impatiens balsamina to lead, cadmium and zinc. Biometals. 1993;6:245-249.
- [Google Scholar]
- Toxicity of heavy metals (Zn, Cu, Cd, Pb) to vascular plants. Water Air Soil Pollut.. 1989;47:287-319.
- [Google Scholar]
- Effect of root feeding of heavy metals on the leaf concentration of P, K, Ca and Mg in coconut (Cocosnucifera L) Plant Soil. 1987;101:295-297.
- [Google Scholar]
- Comparison of three aqua regia digestion methods of twenty Florida soils. Soil Sci. Soc. Am. J.. 2001;65:491-499.
- [Google Scholar]
- The mutagenicity of gramoxone (paraquat) on different eukaryotic systems. Mutat. Res./Genet. Toxicol.. 1993;319:89-101.
- [Google Scholar]
- Effects of cadmium on root apical meristems of Pisum sativum L: cell viability, cell proliferation and microtubule pattern as suitable markers for assessment of stress pollution. Mutat. Res.. 2007;632:9-19.
- [Google Scholar]
- Effects of cadmium on meristem activity and nucleus ploidy in roots of Pisum sativum L. cv. Frisson seedlings. Environ. Exp. Bot.. 2006;58:253-260.
- [Google Scholar]
- Chromosome aberrations in plants as monitoring system. Environ. Health Perspect.. 1978;27:37-43.
- [Google Scholar]
- Hyperaccumulation of cadmium by roots, bulbs and shoots of garlic (Allium sativum L) Bioresour. Technol.. 2001;76:9-13.
- [Google Scholar]
- Trace Elements in Soils and Plants (second ed.). CRC Press; 1992. p. 365
- Influence of lead and cadmium on the growth and nutrient concentration of tomato (Lycopersicum esculentum) and egg plant (Solanum melongena) Plant Soil. 1983;74:387-394.
- [Google Scholar]
- Cytological effects of the herbicide Tribunile on Vicia faba L. Egypt. J. Bot.. 1984;27:191-198.
- [Google Scholar]
- Genotoxicity and mutagenicity of metals in plants. In: Prasad M.N.V., Strzalka K., eds. Physiology and Biochemistry of Metal Toxicity and Tolerance Inplants. Amsterdam, The Netherlands: Kluwer Academic Publishers; 2002. p. :395-414.
- [Google Scholar]
- Effect of lead on seed germination, seedling growth, chlorophyll content and nitrate reductase activity in mung bean (Vigna radiata) Res. J. Phytochem.. 2008;2:61-68.
- [Google Scholar]
- Physiological role of nickel and its toxic effects on higher plants. Russ. J. Plant Physiol.. 2006;53:257-277.
- [Google Scholar]
- Sharma, R.K., Madhoolika, A., Marshall, F.M., 2004. Effects of waste water irrigation on heavy metal accumulation in soil and plants. Paper presented at a National Seminar, Bangalore University, Bangalore, Abstract No. 7, p. 8.
- Lead induced genotoxicity in Vigna mungo Var. HD-94. J. Saudi Soc. Agri. Sci.. 2012;11:107-112.
- [Google Scholar]
- Exposure of Cu and Mn to Cicer arietinum L. Var. BGD-72 seeds induces morphological and biochemical changes in the plant. South Asian J. Exp. Biol.. 2013;3:31-36.
- [Google Scholar]
- Cytogenetic changes induced by Sodium azide (NaN3) on Trigonellafoenum graecum L seeds. South Afr. J. Bot.. 2007;73:632-635.
- [Google Scholar]
- Evaluating cadmium toxicity to the root meristem of Pisum sativum L. Acta Physiol. Plant. 2009;31:531-536.
- [Google Scholar]
- Mitotic abnormalities induced by silk dyeing industry effluents in the cells of Allium cepa. Cytologia. 2001;66:235-239.
- [Google Scholar]
- Effects of cadmium and lead on the accumulation of Ca2+ and K+ and on the influx and translocations of K+ in wheat of low and high K+ status. Physiol. Plant. 1992;84:94-100.
- [Google Scholar]
- Comparative analysis of meiotic abnormalities induced by gamma rays and EMS in Barley. J. Indian Bot. Soc.. 2003;82:19-22.
- [Google Scholar]
- Effect of cooking on the composition of beans (Phaseolus vulgaris L.) and chickpeas (Cicer arietinum L) Food Res. Int.. 2010;43:589-594.
- [Google Scholar]
- Zn phytotoxicity induces oxidative stress in primary leaves of Phaseolus vulgaris. Plant Physiol. Biochem.. 1997;35:405-410.
- [Google Scholar]







