Translate this page into:
Diversity and correlation of entomopathogenic and associated fungi with soil factors
⁎Corresponding author. qayyum.mirza@mnsuam.edu.pk (Mirza Abdul Qayyum)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Ecological consideration is of key importance in finding fungi and other entomopathogens for managing insect pests. The probability of finding entomopathogenic fungi is increased by knowing the soil characteristics supporting fungal survival and diversity. Many opportunistic fungi are closely associated with EPF in soil. Diversity and occurrence of fungi were carried out from soil samples (1 4 5) and dead insects (2 2 5) collected from natural and cultivated areas of south Punjab. The relative research for the presence and abundance of EPF in samples of soils collected from cultivated to non-cultivated hilly lands show that fruit orchid can be considered as a richer in these fungal species. The EPF was mainly isolated from the collected (2 2 5) insect cadavers belonging to six insect orders out of which only 94 were positive for any category of fungus isolated. Insects from Coleoptera were reported with maximum occurrence (44.68%) for harboring any kind of the fungus followed by Lepidoptera (36.17%). Aspergillus niger (27.50%) was the most occurring taxa among all isolates, while Fusarium oxysporium was dominantly occurring specie (17.02%). It can be concluded that orchard soils that are least disturbed (tillage, weeding, etc) and supplied with ample moisture should be preferred for sampling in order to isolate the entomopathogens. Furthermore, insect cadavers from coleoptera and lepidoptera should be preferred for collection for the sake of entomopathogenic fungi.
Keywords
Aspergillus niger
Coleoptera
Entomopathogenic fungi
Lepidoptera
Fusarium oxysporium
1 Introduction
Microorganisms play a key role in the natural ecosystem, particularly in the soil environment, enhancing productivity, improving soil structure and ecosystem functioning, and the health of the plants (Acosta-Martinez et al., 2007). In soils the development of microorganisms mainly depends upon the physical (soil texture) and chemical properties (pH, E.C., C/N ratio), agrotechnical factors, fertilization and organic matter content which are the main source of nutrients and energy for microorganisms (Johansson et al., 1999). One of the best alternatives to synthetic insecticides is entomopathogenic fungus adapted to local conditions. Entomopathogenic fungus control offers a successful, cost-effective, and less labor-intensive which makes it a better alternative to chemical control.
Insect pathogenic fungi abundance, biological activity, and epizootics mainly depend on climatic conditions (Stuart et al., 2006; Karar et al., 2021). EPF such as Beauveria bassiana, Metarhizium anisopilae, and Isaria furmosea are well studied and have great potential against insects (Evans et al., 2018). As an ideal microbial control agent, these fungi have some special approaches against fruit fly including repellency, oviposition deterrence, and high mortality (Karar et al., 2021; Dimbi et al., 2009). The effectiveness and consistency of performance are strongly relying on the abiotic and biotic factors (Fernandes et al., 2010). Entomopathogenic fungi formulation contains nutrition, inert material adjuvants offer a high degree of pathogenicity against different limiting abiotic factors (Barreto et al., 2016), desiccation, and ultraviolet radiation (Fernandes et al., 2007).
The occurrence and distribution of EPF in various regions of Pakistan are poorly understood. The present study was planned to isolate native EPF isolates from the soils of cultivated, non-cultivated habitats, forest and hilly areas. The survey was conducted in different regions of Punjab on different habitats (forest, fruits, vegetables, and crop fields). In this survey insect cavaders and soil samples are collected were EPF isolates where isolated from insect bait method using Galleria mellonella. The present study aimed at isolating entomopathogenic fungi from local soils and insect cadavers providing a potent strain with greater adaptability and virulence.
2 Materials & methods
2.1 Insect sampling and isolation
Insect cadavers with fungal growth were collected from vegetables, orchids, and non-cultivated fields near Multan from November 2019 to March 2020. Cadavers were treated from the sodium hypochlorite 0.3% for the 60 s, rinsed it with dH2O for 4–5 times. After that cadavers were placed on the Potato Dextrose Agar @39 g in dH2O per liter. Inoculated petri dishes were placed in an incubator at 25 °C for 3–5 days. The fungal growth which developed around the cadavers were identified and purified through repeated culturing on PDA. The nature of entomopathogenic or opportunistic fungi was determined after macroscopic examination, and fungal spore and colony characters were further considered for identification. Any fungal growth observed was preserved for morphological identification (based on slide characters) under suitable magnification using taxonomic keys (Bartnet and Hunter, 1999).
2.2 Soil sampling
Soil samples were collected from November 2019 to March 2020. Sampling was carried out using the soil auger at the depth of 5–20 cm after the removal of small plants and soil litter from the top layer. Randomized sampling was done by taking 5 sub-samples, mixing these to form a composite sample. Soil samples were shade dried and sieved to remove any debris. When the moisture was removed, the soil was ground into a fine powder and sieved again and preserved for further use.
2.3 EPF isolation
Finely powdered soil, 10 g was shifted into rearing vials (1.5 × 6 cm) and 5 larvae of Galleria mellonella were buried into this soil for 5 days. The soil in vials (5 replications) was incubated at 20–22 °C in dark. After 5 days, G. mellonella larvae shifted on the moist filter paper for fungal sporulation. Any growth on the larvae was observed with microscopic examination. Larvae were then transferred to PDA plates to permit any fungal particle to grow into a colony. The resultant culture was purified by repeated culture.
2.4 Statistical analysis
The incidence of EPF in changed habitats was associated using chi-squred test. The pathogenicity in bioassay, and death percentage in control test was very little (0.08%). so, it was excluded from analysis. The mortality percentage of the cavaders from every isolate was corrected by using Abbott’s (1925) formula. The Minitab v13.2, which is a statistical software was used for the analysis of the data (Minitab 2002 Software Inc., Northampton, MA, USA).
3 Results
Soil is one the best reservoir that harbors several fungal taxa, including entomopathogenic as well as opportunistic fungi. Entomopathogenic fungi have a great tendency to thrive in soil especially low in soil pH, EC, and temperature while high in moisture contents (Table 1). However, some opportunistic fungi can also survive in soils. The insect pathogenic fungus requires good moisture content for its viability. The 145 soil samples from 29 sites in Punjab which were collected during the survey, exhibited the great diversity of fungi. Those fungi belong to different genera (Table 2) and among them 40 were pathogenic to the insect.
Field category
Sample code
Crop
Temperature
Soil Characters
Soil Texture
Geographical quadrats
Air
Soil
Moisture%
pH
E.C. (mS)
Clay %
Silt%
Sand%
Altitude (m)
Latitude
Longitude
Field crops
JC209
Cotton
31
33.1
9.98
8.28
0.38
13.63
31.81
54.54
126
30.04276
71.85388
MC109
Cotton
28
29.1
10.73
6.89
1.62
6.67
16.67
76.67
122
30.14935
71.45893
JDC209
Desi cotton
34
33.3
4.2
5.9
0.22
35
20
45
126
30.05056
71.87638
MSo99
Sorghum
30
27.8
6.87
6.47
1.61
12.5
9.37
78.12
122
30.15461
71.44747
JSoii209
Sorghum
34
32.6
13.83
8
0.28
10
30
60
126
30.08371
71.85750
JSoi209
Sorghum
29
32.1
23.1
6.8
0.19
36.36
22.72
40.9
126
30.04102
71.85751
MR109
Rice
28
26.4
22.3
7.4
0.98
4
36.9
59.1
122
30.15469
71.47980
JRi209
Rice
31
32.4
22.69
8.4
0.44
6.25
37.5
56.25
126
30.04176
71.85990
JRii209
Rice
34
33
20.41
7.8
0.76
5.5
33.33
61.11
126
30.08382
71.89574
MRo109
Rose
28
26.6
19.76
6.91
1.71
12.12
18.18
69.69
122
30.15399
71.47667
JMa209
Maize
34
33.8
11.17
6.5
0.55
4.54
22.72
72.72
126
30.04261
71.85388
Fruit crops
JMi209
Mango
29
34.5
11.98
5.9
0.3
10
30
60
126
30.04294
71.84362
MM99
Mango
30
28.1
13.52
5.5
0.58
6.67
13.33
83.33
122
30.15477
71.44756
JMii209
Mango
31
32.7
6.9
6.1
0.3
6.25
37.5
56.25
126
30.08381
71.37682
MD99
Date palm
30
26.2
10.79
6.51
1.79
6.06
18.18
75.75
122
30.15335
71.44866
JD209
Date palm
31
32.6
13.99
7.7
1.1
31.81
27.27
40.9
126
30.05962
71.87157
JL209
Lemon
29
33.1
14
6.5
0.21
13.63
31.81
54.54
126
30.05973
71.87362
JO209
Orange
29
32.7
5.68
6.78
0.33
20.83
33.33
5.16
126
30.05757
71.87625
MO109
Orange
28
28.5
14.64
6.61
1.72
10
20
70
122
30.15397
71.47662
Vegetable crops
JP209
Pumpkin
31
32.9
12.55
7.9
0.18
4.54
27.27
68.8
126
30.04151
71.36159
JCa209
Cauliflower
34
32.5
20.4
7.39
0.78
10
20
70
126
30.04261
71.85889
MRi109
Ridge gourd
28
27.1
10.34
6.62
2.03
3.33
23.33
73.33
122
30.15497
71.48085
MSp109
Spinach
28
27.6
16.94
6.97
1.85
12.25
25
62.5
122
30.15503
71.4808
Hilly area
JG209
Grass
34
32.4
21.43
7.8
0.56
16.6
33.33
50
126
30.04668
71.87269
JSe209
Sesbania
34
32
8.72
7.76
0.32
6.25
31.25
62.5
126
30.04913
71.87628
MSe109
Sesbania
28
28.5
18.33
5.93
1.67
9.09
12.12
78.7
122
30.15469
71.47988
MBl99
Wild Black berry
30
28.5
10.54
6.64
1.48
6.45
16.12
77.14
122
30.15125
71.44767
Nature of fungi
Fungal isolates
Field
Fruit
Vegetable
Hilly areas
χ2
P
Distribution frequency (%)
Total isolates (n)
Entomopathogenic
Beauveria bassiana
0
1
0
1
1.94
0.58
05.00
2
Metarhizium anisopliae
0
1
0
0
2.27
0.51
02.50
1
Trichoderma harzianum
1
1
1
2
1.06
0.78
12.50
5
Opportunistic
Aspergillus flavus
2
1
4
2
2.88
0.41
22.50
9
Asprgillus niger
2
2
5
2
1.91
0.58
27.50
11
Fuasrium oxysporum
1
1
1
0
0.97
0.80
07.50
3
Mucor varians
2
1
2
3
2.01
0.57
20.00
8
Penicillium chrysogenum
1
0
0
0
3.59
0.30
02.50
1
Total isolates
15
21
17
15
-
-
-
40
Percent occurrence (%)
37.50
52.50
42.50
37.50
-
-
100
-
The distribution and strength of EPF in soil samples cultivated from and non-cultivated mountainous lands showed that orchids had a more species diversity of these fungi. Sun and Liu (2008) found about 25 species in soil of field crops and 20 in orchard soils, indicating that the environment had a substantial effect on the occurrence of fungal species. The most common specie of all isolates was Aspergillus niger (27.50%), Aspergillus flavus (22.50%), and Mucor varians (21.50%).
The EPF were found in a significant number of cadavers obtained from the insect belonging to six insect orders. 225 insect cadavers were collected out of which only 94 were positive for any category of fungus isolated (Table 3). Insects from Coleoptera were reported with maximum occurrence (44.68%) for harboring any kind of the fungus followed by Lepidoptera (36.17%). The fungus Fusarium oxysporium was the most dominantly occurring species (17.02%) isolated, followed by F. solani (15.96%). Among the entomopathogenic fungi, B. bassiana was most occurring (7.45%) followed by the fungus M. anisopliae distributed with 4.26% occurrence (Table 4).
Nature of fungi
Fungal species
Insect count collected (n) from different orders
χ2
P
Distribution frequency (%)
Total isolates (n)
Lep.
Dip.
Col.
Hemi.
Hym.
Ortho.
Entomopathogenic
Beauveria bassiana
5
0
0
0
1
1
13.1
0.0224
7.45
7
Metarhizium anisopliae
2
0
1
0
1
0
3.2
0.6691
4.26
4
Paecilomyces lilacianus
1
0
1
0
0
1
3.62
0.6053
3.19
3
Opportunistic
Aspergillus flavus
1
1
2
1
1
1
0.51
0.9917
11.70
11
Aspergillus fumigatus
1
2
2
1
1
1
1.29
0.9359
12.77
12
Asprgillus niger
4
2
1
1
1
3
7.01
0.2202
12.77
12
Fusarium oxysporium
3
2
1
1
2
3
5.4
0.3639
17.02
16
Fusarium solani
2
0
3
2
1
2
4.05
0.5429
15.96
15
Penicillium capsulatum
3
1
1
1
2
1
1.67
0.8931
9.57
9
Rhizopus stolonifers
2
1
1
0
0
1
3.35
0.6455
5.32
5
Total
34
23
42
19
31
15
100.00
94
Percent occurrence (%)
36.17
24.47
44.68
20.21
32.98
15.96
Species
Visual
Characters
Aspergillus flavus
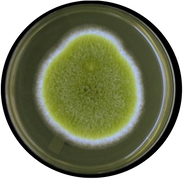
Colony color is greenish-yellow to olive and has white borders; Spores have a thick mycelial mat, septate hyphae having large conidiophores sizes of 3-6 µm.
Aspergillus fumigatus
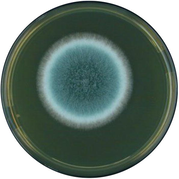
Colony color is bluish-green with multiple shades ending in a white border; spores are velvety and powdery; hyphae are septate with smooth walled conidiophores.
Asprgillus niger

The colony appears to be in pale yellow starting from white at the start; producing radial fissures and mat like mycelial growth.
Fusarium oxysporium
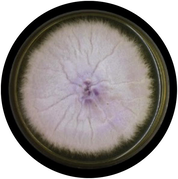
The initial colony color is white; turns greyish red to brown, septate hyphae, mycelia are aerial, and sizes ranges from 8 to 14 µm.
Fusarium solani
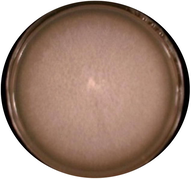
Colonies are white and cottony in the start later turn into blue-green or bluish brown. Microconidia shapes are oval, reniform, elongated oval, size 44–78 × 3.3–5.6 μm.
Rhizopus stolonifers

The colony is whitish to brown, fluffy, and cottony and hyphae are septate sparsely septate.
Beauveria bassiana

Colony color is bone white to pale yellow; the texture is velvety; powdery to cottony, hyphae are narrow and septate and conidiophores are single or aggregate with dense clusters.
Metarhizium anisopliae

Colony color white to green spores is olivaceous green, cylindrical, and 2.5-3.5 μm long.
Paecilomyces lilacianus
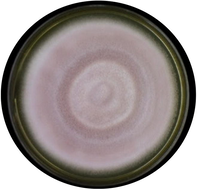
Colony color appears in faint violet color; septate hyphae and conidiophores size from 3 to 4 µm.
Penicillium chrysogenum
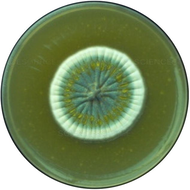
Spores are dry forming chains with filamentous hyphae; usually colorless and branched with septate hyphae and brushed shape conidiophore.
Mucor varians
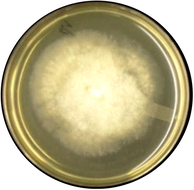
Hyphae of Mucor is filamentous, aseptate or coenocytic.
Trichoderma harzianum

They formed 1-2 concentric rings with green conidial production. Conidia (typically 3 to 5 µm in diameter).
4 Discussions
Soil inhabiting EPF plays a major role in managing many soil-associated insect populations and pervasive element of many terrestrial ecosystems (Quesada Moraga et al., 2007; Meyling and Eilenberg, 2007). The same fungal isolates perform the role as a potential biocontrol agent under cultivated agro-systems (Meyling and Eilenberg, 2006). Ecological and soil characteristics had a greater contribution to the richness of entomopathogenic taxa. Soil properties like soil texture, pH, E.C., and temperature play a key role in the occurrence of EPF. The probability of occurrence of I. fumosorosea and M. anisopliae is increased in sandy soils, while clay soil also offer the similar habitat for I. fumosorosea, M. anisopliae and B. basssiana (Tkaczuk et al., 2014). Organic soils provide the particular environment to promote the diversity of entomopathogenic fungi and the fungal population (Klingen et al., 2002; Uzman et al., 2019). Pinruan et al. (2007) found 147 fungal species in rotting palm material, with 79 ascomycetes in 50 genera (53%), 65 anamorphic taxa in 53 genera (45%), and 3 basidiomycete species in 3 genera (2%) being new to science.
Insect cadavers or infected insects are one good source to isolate the entomopathogenic fungus. Similarly, opportunistic fungi also invade insect cadavers taking advantage of weaker conditions of insect near to death. Previously it has been reported that the occurrence of some EPF is more reliant on soil than plants, but some fungi are more associated with the vegetation (Zahid et al., 2020; Saeed et al., 2019; Steinwender et al., 2015; Nishi and Sato, 2019: Farooq et al., 2021). The EPF population is influenced by different cultivation (Tkaczuk et al., 2012; Kolczarek and Jankowski, 2014; Trizelia et al., 2015). M. anisopliae is prevailing in all habitats with cultivated plants, while B. bassiana prefers soil from orchards and natural sites (Jarmuł-Pietraszczyk et al., 2011; Medo and Cagan, 2011; Keyser et al., 2015).
Local soils and infect insects or the cadavers are the optimum media to harbor entomopathogenic fungi. The biggest advantage of using local resources is the greater adaptability to environmental conditions. Extreme weather conditions are the reason that leads to the failure of entomopathogenic fungi with its effect on spore health, germination, and virulence. The entomopathogens isolated from indigenous resources have the potential to be incorporated into an effective pest management system. Once formulated into ready to use the product, these entomopathogenic fungi will be able to successfully control the fruit fly infestations under all agricultural systems ranging from fruits, vegetable crops to forest systems.
Acknowledgement
The authors extend their appreciation to the Deanship of Scientific Research, King Khalid University for funding this work through research groups program under grant number R.G.P. 1/25/42.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Mi- crobial communities and enzyme activities in soils under alternative crop rotations compared to wheat−fallow for the Central Great Plains. Appl. Soil Ecol.. 2007;37:41-52.
- [Google Scholar]
- Barnett, H.L., Hunter, B.B., 1999. Illustrated Genera of Imperfect Fungi, 4th Edn. APS Press, The American Phytopathological Society, St. Paul, MN. p 218.
- Effect of heat stress and oil formulation on conidial germination of Metarhizium anisopliae ss on tick cuticle and artificial medium. J. Invertebr. Pathol.. 2016;138:94-103.
- [Google Scholar]
- Effect of Metarhizium anisopliae inoculation on the mating behavior of three species of African Tephritid fruit flies, Ceratitis capitata, Ceratitis cosyra and Ceratitis fasciventris. Biol. Control. 2009;50:111-116.
- [Google Scholar]
- Entomopathogenic fungi and their potential for the management of Aedes aegypti (Diptera: Culicidae) in the Americas. Mem. Inst. Oswaldo Cruz. 2018;113:206-214.
- [Google Scholar]
- Production suitability of date palm under changing climate in a semi-arid region predicted by CLIMEX model. Journal of King Saud University - Science 2021
- [CrossRef] [Google Scholar]
- Characterization of Metarhizium species and varieties based on molecular analysis, heat tolerance and cold activity. J. Appl. Microbiol.. 2010;108:115-128.
- [Google Scholar]
- Variability in tolerance to UV-B radiation among Beauveria spp. isolates. J. Invertebr. Pathol.. 2007;96:237-243.
- [Google Scholar]
- Occurrence of Entomopathogenic Fungi in Selected Parks and Urban Forests Of The Warsaw District Ursynow. Ecol. Chem. Eng. A. 2011;18:1571-1574.
- [Google Scholar]
- Micro- biological and chemical changes in two arable soils after long-term sludge amendments. Biol. Fertility Soils. 1999;30:160-167.
- [Google Scholar]
- Hemipterous bug (dysdercus koenigii) (hemiptera: pyrrhocoridae) and boll rot disease of cotton (gossypium hirsutum) grown in the field. Saudi J. Biol. Sci. 2021
- [CrossRef] [Google Scholar]
- Successful management strategies for Agonoscelis sp. (Heteroptera: Pentatomidae) in Alfalfa. Saudi J. Biol. Sci. 2021
- [CrossRef] [Google Scholar]
- Diversity within the entomopathogenic fungal species Metarhizium flavoviride associated with agricultural crops in Denmark. BMC Microbiol.. 2015;15:249.
- [Google Scholar]
- Effects of farming system, field margins and bait insect on the occurrence of insect pathogenic fungi in soils. Agric. Ecosyst. Environ.. 2002;91:191-198.
- [Google Scholar]
- Occurrence of entomopathogenic fungi in soils from Festuca pratensis Huds. Crop. J. Ecol. Eng.. 2014;15
- [Google Scholar]
- Factors affecting the occurrence of entomopathogenic fungi in soils of Slovakia as revealed using two methods. Biol. Control. 2011;59:200-208.
- [Google Scholar]
- Occurrence and distribution of soil borne entomopathogenic fungi within a single organic agroecosystem. Agric. Ecosyst. Environ.. 2006;113:336-341.
- [Google Scholar]
- Ecology of the entomopathogenic fungi Beauveria bassiana and Metarhizium anisopliae in temperate agroecosystems: potential for conservation biological control. Biol. Control. 2007;43(2):145-155.
- [Google Scholar]
- Isolation of Metarhizium spp. from rhizosphere soils of wild plants reflects fungal diversity in soil but not plant specificity. Mycology. 2019;10:22-31.
- [Google Scholar]
- Factors affecting the occurrence and distribution of entomopathogenic fungi in natural and cultivated soils. Mycol. Res.. 2007;111:947-966.
- [Google Scholar]
- Saeed. S., M.A., Bashir, Khan., K.A., Sajjad. A., Alvi. A.M., Atta. S. and Ansar.J. 2019. Assemblage of pollinator communities in four widely isolated natural reserves of Southern Punjab, Pakistan. Saudi Journal of Biological Sciences. Volume 26, Issue 4, May 2019, Pages 860-865.
- Root isolations of Metarhizium spp. from crops reflect diversity in the soil and indicate no plant specificity. J. Invertebr. Pathol.. 2015;132:142-148.
- [Google Scholar]
- Population biology of entomopathogenic nematodes: concepts, issues and models. Biological Con- trol. 2006;38:80-102.
- [Google Scholar]
- Occurrence and diversity of insect-associated fungi in natural soils in China. Appl. soil Ecol.. 2008;39:100-108.
- [Google Scholar]
- The occurrence of entomopathogenic fungi in soils from fields cultivated in a conventional and organic system. Eng: J. Ecol; 2014. p. :15.
- The occurrence of entomopathogenic fungi in soils from mid-field woodlots and adjacent small-scale arable fields. Acta Mycologica. 2012;47(2)
- [Google Scholar]
- The diversity of entomopathogenic fungi on rhizosphere of various vegetable crops. Prosiding Seminar Nasional Masyarakat Biodiversitas Indonesia. 2015:998-1004.
- [Google Scholar]
- Drivers of entomopathogenic fungi presence in organic and conventional vineyard soils. Appl. Soil Ecol.. 2019;133:89-97.
- [Google Scholar]
- Zahid A, Fozia, M. Ramzan, M. Amjad Bashir, M. Ahsan Khatana, M. Tahir Akram, S. Nadeem, M. Saad Qureshi, W. Iqbal, M. Umar, S. Walli, R. Muhammad Sabir Tariq, S. Atta, D.A. Al Farraj, M.T. Yassin, Effect of humic acid enriched cotton waste on growth, nutritional and chemical composition ofoyster mushrooms (Pluerotus ostreatus and Lentinus sajor-caju), Journal of King Saud University – Science (2020), Volume 32, Issue 8, December 2020, Pages 3249-3257 10.1016/j.jksus.2020.08.016







