Translate this page into:
Diverse mitochondrial effects, antiplasmodial and anti-inflammatory potentials of Costus afer (Ker Gawl), Nauclea latifolia (Sm) and Sphenocentrum jollyanum (Pierre) in mice infected with Plasmodium berghei
⁎Corresponding author. jodel72000@yahoo.com (John Oludele Olanlokun)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Objectives
This study reported the antiplasmodial, anti-inflammatory and mito-protrective effects of Costus afer (CA), Nauclea latifolia (NA) and Sphenocentrum jollyanum (SJ) methanol extracts in Plasmodium berghei-infected mice.
Methods
Air-dried CA, NA and SJ were extracted with methanol. Antiplasmodial activity of these extracts were monitored using chloroquine-sensitive and resistant strains of Plasmodium berghei. Heme and hemozoin contents, interleukins and C-reactive protein as well as mitochondrial permeability transition (mPT) pore opening, lipid peroxidation (mLPO) and F0F1 ATPase activity were determined by spectrophotometry. Phytochemical constituents were determined using UPLC-QTOF-MS and NMR spectroscopy.
Results and Conclusions
CA, NL and SJ decreased percentage parasitemia to 0.25 ± 0.07; 0.30 ± 0.14 and 0.25 ± 0.07% relative to control (8.60 ± 0.15%) in the chloroquine-sensitive model and to 0.40 ± 0.14; 0.30 ± 0.14 and 0.45 ± 0.07, respectively as against 10.88 ± 0.26% of the infected control in the chloroquine-resistant model. In chloroquine-resistant model, NL decreased mLPO (0.41 ± 0.04) F0F1 ATPase (0.15 ± 0.02 mmol pi/mg protein /min) while CA enhanced mPT pore opening at 100 mg/kg,and SJ (50 mg/kg) reversed parasite-induced mPT pore opening (1.66 vs 9.4 folds). The NL increased heme, decreased hemozoin, IL-6, CRP, TNF-α, while SJ dose-dependently increased IL-10. UPLC-QTOF-MS analysis showed that coumaric acid, divaricatinic acid, diocin and aferiosides A and C were present in CA, 3-caffeoylquinic acid, 18, 19-dihydroangustine, jatrorrhizine, 17-epinaucleidinal, strictosamide and quinovic acid 3-O-rhamnoside in NL and quinic acid, jatrorrhizine and mabioside B in SJ. While the three medicinal plants have varying antimalarial effects, their decoction will be better for a synergistic purpose.
Keywords
Cytokines
Heme
Hemozoin
Malaria
Mitochondria
Natural Products
1 Introduction
Malaria has become a difficult disease to treat because of resistance to artemisinin-combinative therapy. It has also been difficult to design vaccine for the malaria because of the complex stages of the parasites’ life cycle. The hope for drugs from medicinal plants is evident with the discovery of artemisinin from Artemisia annua (Menan et al., 2006). In ethno-medicine, Costus afer’s rhizome, Nauclea latifolia and Sphenocentrum jollyanum parts have been used in the treatment of malaria either as single plant or in composite preparations (Omokhua, 2011). C. afer belongs to the Costaceae family formerly known as Zingiberaceae (Aweke, 2007). They have inflammatory, and antiplasmodial potentials (Soladoye and Oyesika, 2008). Herbal remedies of N. latifolia (Smith) classified as Rubiaceae is used to treat malaria (Elujoba, 1995). Folklorically, parts of Sphenocentrum jollyanum can be prepared to treat malaria (Olorunnisola and Afolayan, 2011). Most plants with antimalarial properties equally have inflammatory potentials. Oxidative stress can affect mitochondrion in the host leading to its dysfunction via the irreversible permeabilization of its membranes (Kent et al., 2021). Malaria infection causes oxidative damage, affecting host and parasites. Malaria treatment, therefore, will prevent inflammation, bioenergetics stress and mitochondrial dysfunction. The folkloric treatment of uncomplicated malaria using C. afer, N. latifolia and S. jollyanum has been well documented but their protective mechanism on host mitochondria has not been reported and information on the bioenergetics status of the infected host is lacking. This study reported the antiplasmodial, anti-inflammatory and mito-protective potentials of C. afer, N. latifolia and S. jollyanum.
2 Materials and methods
2.1 Plant sources and their extraction
Air-dried aerial parts of C. afer, stem bark of Nauclea latifolia, and root bark of Sphenocentrum jollyanum (1 kg each) were milled and separately soaked in 100% methanol,decanted, filtered and the filtrate was concentrated to solvent-free methanol extract.
2.2 Experimental animals and Research design
This study was divided into two phases. In Phase 1, mice were intraperitonealy infected with chloroquine-sensitive P. berghei (NK 65 strain) while in phase 2, chloroquine-resistant (ANKA strain) P. berghei was used. In Phase 1, fifty-five male Swiss mice were passaged with infected erythrocytes from a donor mouse. Five uninfected mice were classified as normal control. Parasitemia was confirmed after 72 h via microscopy. Similar procedure was repeated in phase 2 using resistant Plasmodium berghei (ANKA strain).
2.3 The design of the experiment
Mice in both phases received 10 mg/kg of Artemether-Lumefantrine in 5% DMSO as vehicle. The vehicle only was administered to the normal and infected control groups. Other test groups were treated with 50, 100 and 200 in mg/kg of the methanol extracts of the plant.for seven and five days in chloroquine-sensitive and resistant experiments, respectively.
2.4 Antimalarial effects of Costus afer, Nauclea latifolia, and Sphenocentrum jollyanum parts extracted with methanol
The antimalarial potentials of C. afer, N. latifolia and S. jollyanum for P. berghei (chloroquine sensitive and resistant strains) were carried out using the modified established infection method described by Ryley and Peters (1970). Briefly, after the confirmation of parasitemia, mice were treated orally for seven days (chloroquine-sensitive model) and five consecutive days (choloroquine-resistant model). At forty-eight hours interval, blood was collected from the mouse tail for slide preparation, allowed to dry and fixed in methanol, dried and stained with Giemsa stain. The slides were rinsed in buffered water and air-dried. The slides were viewed on microscope using oil immersion objective. Parasitemia and chemo-suppression, were then calculated.
2.5 Heme and hemozoin determination
Blood sample (10 μL) was lysed in SDS and dissolved in 1 M NaOH followed by sonication for 10 min. The samples were then incubated for 2 h in a water bath. Heme content was determined by reading the absorbance of the samples at 404 nm and the total heme calculated using its molar absorption coefficient (9.08 104/M/cm) (Asakura et al., 1977). The hemozoin content was estimated from 10 μL of blood, lysed in 0.08% saponin and centrifuged at 18,000 rpm. The sediment was washed repeatedly using 250 µL of 25% SDS buffered with 25 mM Tris-HCl (pH 7.4), incubated at 37 °C overnight and washed at 18,000 rpm. The pellets were then dissolved in 1 M NaOH and the absorbance was read at 404 nm (Orjih and Fitch, 1993).
2.6 Preparation of serum
Withdrawn blood was allowed to clot and centrifuged at 3.500 rpm for 5 min to obtain the serum. Serum was aspirated into clean sample bottle and kept in the refrigerator.
2.7 Mitochondria isolation
The liver was removed, rinsed in isolation buffer (sucrose (70 mM), Hepes-KOH (5 mM, pH 7.4), mannitol (210 mM) and EGTA (1 mM)), weighed, chopped and a 10% in isolation buffer was homogenized. The homogenate was then spun twice at 2,500 rpm for 5 min each. The supernatant was further centrifuged at 13,000 rpm for 10 min to obtain mitochondria that was washed in washing buffer (Hepes-KOH (5 mM, pH 7.4), sucrose (70 mM), mannitol (210 mM) and BSA (0.5%)) twice at 12000 rpm for 10 min each time. Mitochondria were then suspended in buffer (Mannitol (210 mM), Sucrose (70 mM) and Hepes-KOH (5 mM, pH 7.4)), dispensed in Eppendorf vials and kept on ice (Johnson and Lardy,1967).
2.8 Determination of total protein in mitochondria
Mitochondria (10 µL) were made up to 1 mL and 3 mL of a 100:1:1 mixture of Na2CO3 (2%), Na + -K + tartarate (1%) and CuSO4·5H2O (0.5%) in 100 mL of 0.1 M sodium hydroxide was added. After 10 min, 0.3 mL of Folin reagent was added and incubated for 30 min. The absorbance was read at 750 nm and mitochondria protein was quantified by using protein standard curve (Lowry et al., 1951).
2.9 Determination of mitochondrial membrane permeabilisation by P. berghei infection and extract intervention
Mitochondria (0.4 mg/mL protein) were incubated in the suspension buffer and rotenone (8 µM) for three and half min. Succinate (5 mM) was added and the absorbance read at 540 nm for 12 min at 30 s interval to ascertain mitochondrial quality. The same quantity of mitochondria was added to the suspension buffer, rotenone and the mixture was incubated for 3 min and then 3 µM calcium was added while succinate was added 30 s later and the absorbance read. Mitochondria with little decrease in absorbance in the absence of calcium but with decrease in absorbance in the presence of calcium are considered intact. Similar mitochondrial protein from the treated groups were subjected to the same mPT assay. The mPT opening effects of calcium, Plasmodium infection and the modulatory effect of C. afer, N. latifolia and S. jollyanum were then compared (Lapidus and Sokolove, 1993).
2.10 Assay of mitochondrial F0F1 ATPase activity
Sucrose, potassium chloride (25 and 0.5 mM, respectively), and Tris-HCl (65 mM) were added into the test tubes and made up to 1 mL. Mitochondrial protein (0.5 mg/mL) was added to their respective test tubes. ATP (1 mM) was added and the mixture was incubated for 30 min after which 10% SDS was added. The 25 µM of 2, 4-dinitrophenol was used as a standard uncoupler. One mL volume was taken and diluted with 4 mL of distilled water. Ammonium molybdate (1 mL) and ascorbic acid (1 mL) were sequentially added and the absorbance was read at 660 nm after 30 min of incubation. Inorganic phosphate concentration was estimated from phosphate standard curve (Lardy and Wellman, 1953).
2.11 Assay of mitochondrial membrane lipids peroxidation
Mitochondria (0.4 mL) were added to 1.6 mL of Tris-KCl (0.15 M) Later, 0.5 mL of trichloroacetic acid (30%) was added, together with 0.5 mL of thiobarbituric acid (0.75%). The mixture was heated in a water bath for 45 min, cooled, spun at 3000 rpm for 10 min and the absorbance was read at 532 nm. TBARS was quantified by using its extinction coefficient (0.156 µM−1cm−1.) (Varshney and Kale, 1990; Adam-Vizi and Seregi (1982).
2.12 Measurement of inflammatory markers interferon gamma, C-reactive protein and interleukins
Several serum interleukins such as IFN-γ, CRP, TNF-α and IL-1β,-6 and10 levels were determined using their corresponding ELISA assay kits manufactured by Elabscience, United States of America.
2.13 Chemical analysis of plant phytochemicals by ultra performance liquid chromatography coupled with-Quadrupole time of Flight-Mass spectrometry (UPLC-QTOF-MS)
Compounds separation and detection from the extracts was carried out on a Waters UPLC hyphenated with a Waters Synapt G2 QTOF instrument. An Acquity UPLC BEH C18 1.7dµm (2.1 × 100 mm column), running at 0.30 mL/min flow rate. Extracts were dissolved in 50% LC grade methanol, sonicated for 15 min before centrifuging at 15000 rpm and resulting supernatants injected for analysis. The mobile phase consisted 0.1% formic acid in LC grade water (A) and methanol + 0.1% formic acid (B). The MS source was Electrospray Ionization (ESI). It operated in both negative and positive ion modes with capillary and endplate voltages set at 2600 V and 2000 V, respectively. Nitrogen served as nebulizer gas, at 10 L/h, while m/z range was set from 50 to 1200 amu. Gradient elution was initiated with 97% A and 3% B which remain linear until 14 min. From 14 to 16 min, elution was kept constant with 0% A and 100% B. A linear gradient of 97% A and 3% B was then used to reach completion until 20 min. The MS data were processed through MassLynx 4.1 software, providing elemental formulae of possible compounds. Structures of compounds were further determined through library search and matching of high-resolution masses alongside MS/MS fragment ions with relevant databases.
2.14 Chemical analysis by nuclear magnetic resonance (NMR) spectroscopy
To validate the presence of compounds identified, the extracts were subjected to NMR analysis. The NMR spectra were recorded using deuterated methanol at room temperature on a Bruker Avance III, 400 MHz spectrometer. Raw NMR data were processed using Bruker Top Spin (version 3.6.4) software.
2.15 Statistical analysis of data
Representative profile was used for mPT assays. Other assays were analyzed by using descriptive statistics and multiple comparison between the means of the groups was carried out by using Tukey’s post-hoc multiple comparison test in a GraphPad prism (9.0 version).
3 Results
3.1 Methanol extracts of C. afer, N. Latifolia and S. Jollyanum decrease parasite burden in P. berghei-infected mice (chloroquine-sensitive model)
Relative to the infected control (0.84 ± 0.09), 200 mg/kg dose of C. afer reduced the parasite burden to 0.42 ± 0.16, N. latifolia (0.35 ± 0.05) and 200 mg/kg S. jollyanum 0.38 ± 0.04 (Fig. 1a). Methanol extracts of C. afer, N. latifolia and S. jollyanum significantly decreased the parasite burden in mice infected with P. berghei (chloroquine-sensitive strain) compared with the infected control (Fig. 1b).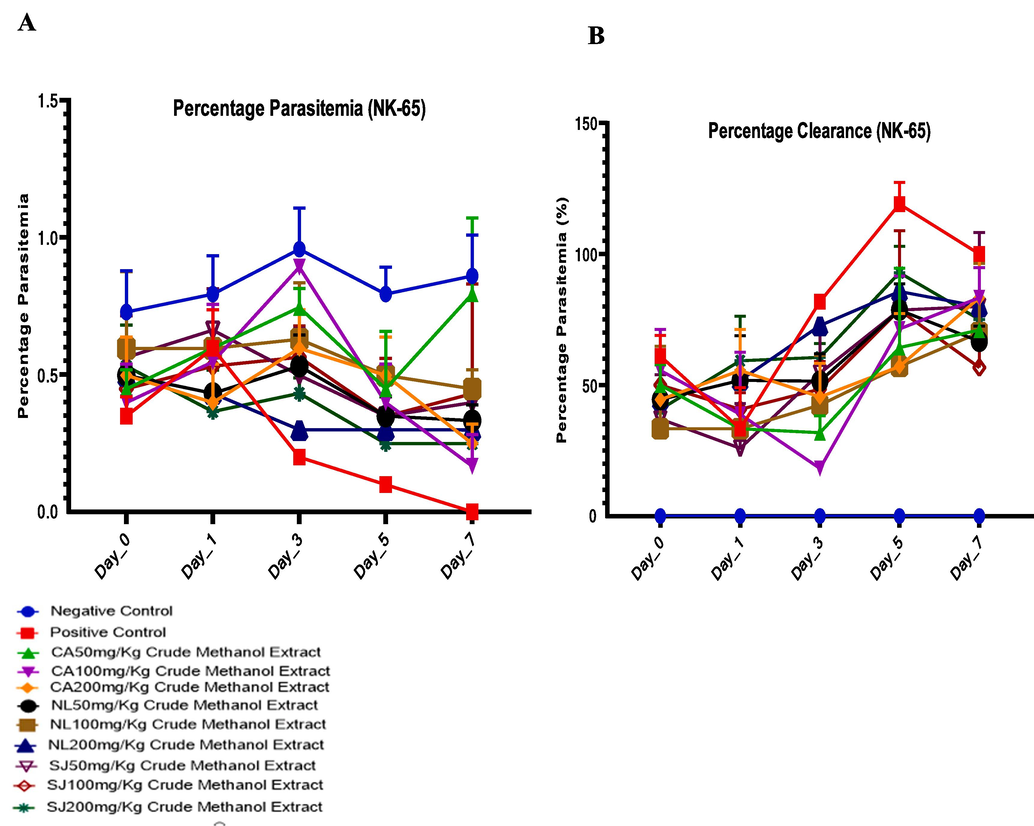
Antiplasmodial potentials (A) and chermosuppression (parasite clearance) (B) of C. afer, N. latifolia and S. jollyanum methanol extracts in mice infected with chloroquine-sensitive P. berghei.
3.2 Therapeutic potentials of C. afer, N. Latifolia and S. Jollyanum against resistant (ANKA strain) P. Berghei infection in mice
Compared with the parasitemia of the infected control (1.01 ± 0.13), N. latifolia decreased the parasite burden (0.48 ± 0.13), followed by the S. jollyanum (0.52 ± 0.04) and lastly C. afer (0.57 ± 0.06) (Fig. 2a). The percentage clearance was corresponded with the decrease in the parasitemia (Fig. 2b).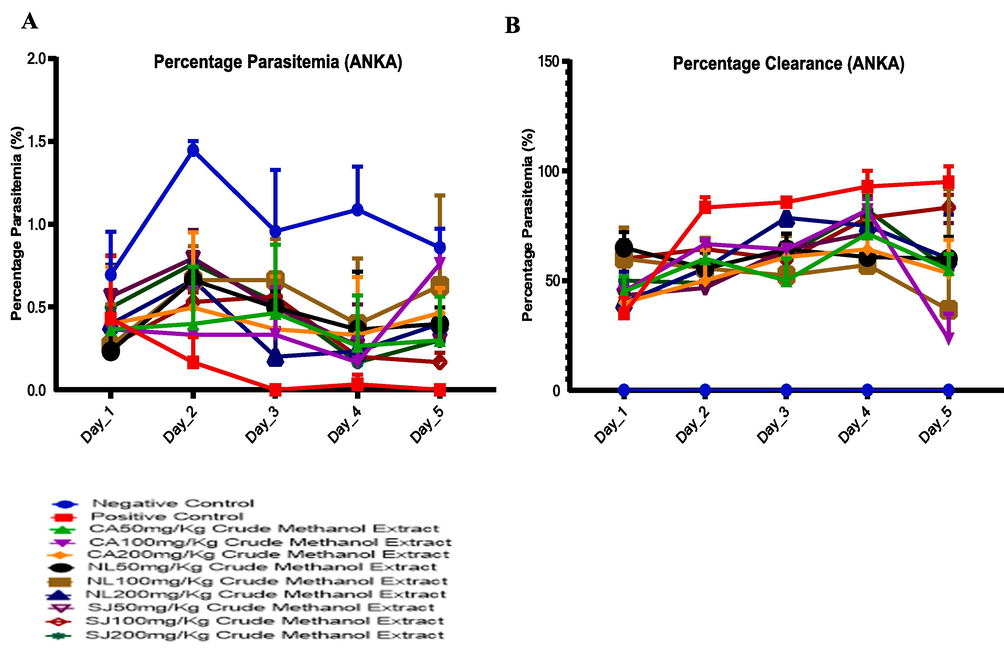
Assessment of the percentage parasitemia (A) of resistant P. berghei-infected mice treated with methanol extracts of C. afer, N. latifolia and S. jollyanum and the corresponding parasite clearance (B).
3.3 Inverse relationship between heme and hemozoin contents in infected mice treated with methanol extracts of C. afer, N. Latifolia and S. Jollyanum
In Fig. 3a, it was only the concentration of the bound heme of the test groups treated with N. latifolia that favourably compared with that of the normal and drug control groups. The heme content of mice treated with N. latifolia (200 mg/kg dose) in chloroquine-sensitive model was higher (P < 0.0001) when compared with methanol extracts of C. afer and S. jollyanum. In the chloroquine-resistant groups, heme content of all the treated groups increased than that of the infected control (Fig. 3b). Hemozoin content of infected control increased while it was decreased by C. afer, N. latifolia and S. jollyanum, (200 mg/kg) (Fig. 3c).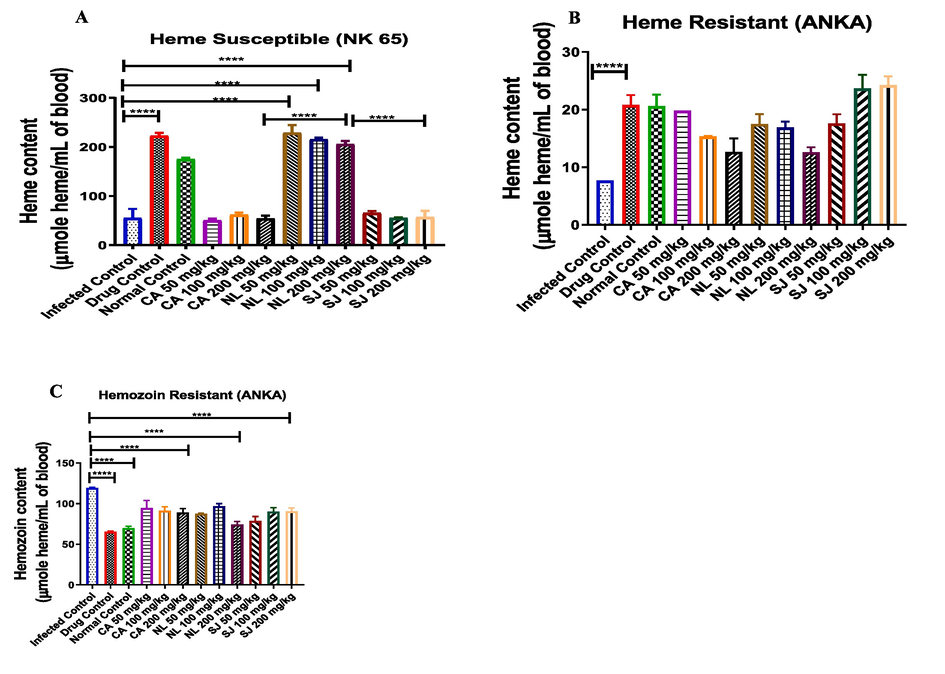
The impact of Plasmodium berghei infection and treatment with methanol extracts of C. afer, N. latifolia and S. jollyanum on bound heme in chloroquine-sensitive (A), chloroquine-resistant (B) as well as the hemozoin content. **** = P < 0.001.
3.4 Extracts of C. afer, N. Latifolia and S. Jollyanum reverse parasite-mediated mitochondrial pore opening, FoF1 ATPase and lipid peroxidation
Plasmodium berghei infection caused large amplitude swelling of host mitochondria (Fig. 4a). This occurs both in the chloroquine-sensitive (6 folds) and chloroquine-resistant models (9 folds) (Fig. 4b). In the susceptible model, C. afer, reversed the pore opening from 5 folds (50 mg/kg) through 4.7 folds (100 mg/kg) to 2.6 folds (200 mg/kg). N. latifolia decreased absorbance from 7 folds (50 mg/kg) through 3 folds (100 mg/kg) to 3.8 folds (200 mg/kg). The 50 mg/kg of S. jollyanum decreased the pore opening at 100 mg/kg to 4.6 folds. In the resistant model, C. afer reversed the pore opening at 200 mg/kg to 3 folds. Sphenocentrum jollyanum reversed the pore opening at 50 mg/kg. N. latifolia and S. jollyanum (200 mg/kg) reversed F0F1 ATPase activity significantly at P < 0.0001. N. latifolia and S. jollyanum (200 mg/kg) significantly (P < 0.0001) decreased ATP hydrolysis compared to same dose of C. afer. N. latifolia (200 mg/kg) further decreased ATP hydrolysis (P < 0.05) compared with the drug control (Fig. 4c). In Fig. 4d, the extracts decreased the ATP hydrolysis. C. afer (200 mg/kg) decreased (P < 0.0001) peroxidation compared with the drug control. S. jollyanum (200 mg/kg) decreased lipid peroxidation (P < 0.0001) more than N. latifolia (200 mg/kg) and is also preferred to C. afer at the same dose (Fig. 4e). In the resistant model, 200 mg/kg dose decreased (P < 0.0001) lipid peroxidation relative to the infected control (Fig. 4f).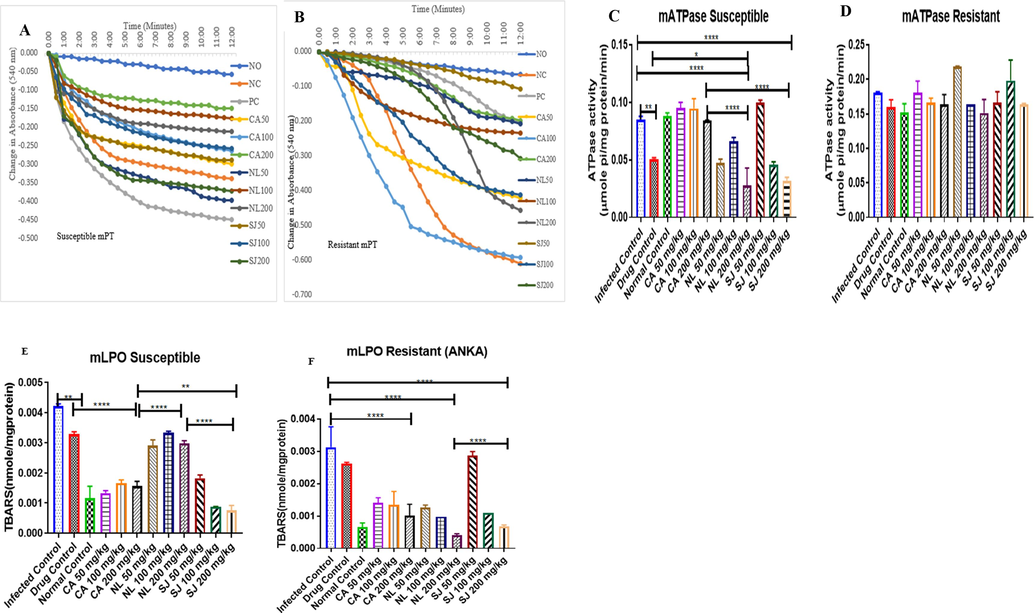
Modulatory effects of Plasmodium berghei infection on some mitochondrial parameters: reversal effects of C. afer, N. latifolia and S. jollyanum extracts on mitochondrial permeabilisation in susceptible (A) and resistant (B) models. These extracts modulated ATPase activity in susceptible (C) and resistant (D) models. They also inhibited lipid peroxidation in susceptible (E) and and resistant (F) models. * = P < 0.05; ** = P < 0.01; **** = P < 0.0001.
3.5 C. afer, N. Latifolia and S. Jollyanum modulate inflammatory cytokines in resistant (ANKA) P. berghei-infected mice
The methanol extracts decreased the levels of IL-1β but 200 mg/kg of S. jollyanum had the highest inhibitory effects (Fig. 5a). In Fig. 5b, there was a significant (P < 0.0001) increase in IL-6 in infected mice. Similarly, C. afer and N. latifolia at 200 mg/kg significantly (P < 0.0001) decreased serum level of IL-6 in infected mice. Although, the serum level of IL-6 remains significantly (P < 0.0001) high in mice treated with 200 mg/kg of S. jollyanum, C. afer decreased (P < 0.0001) the serum IL-6 level relative to S. jollyanum. Serum level of interleukin-10 (IL-10) is higher (P < 0.0001) in normal controlmice and similar effect was observed in infected mice treated with 200 mg/kg S. jollyanum (Fig. 5c). Treatment of Plasmodium infection with higher doses of the extracts decreased serum CRP level (Fig. 5d). Serum level of TNF-α was significantly (P < 0.0001) decreased by N. latifolia (200 mg/kg) relative to the infected control (Fig. 5e),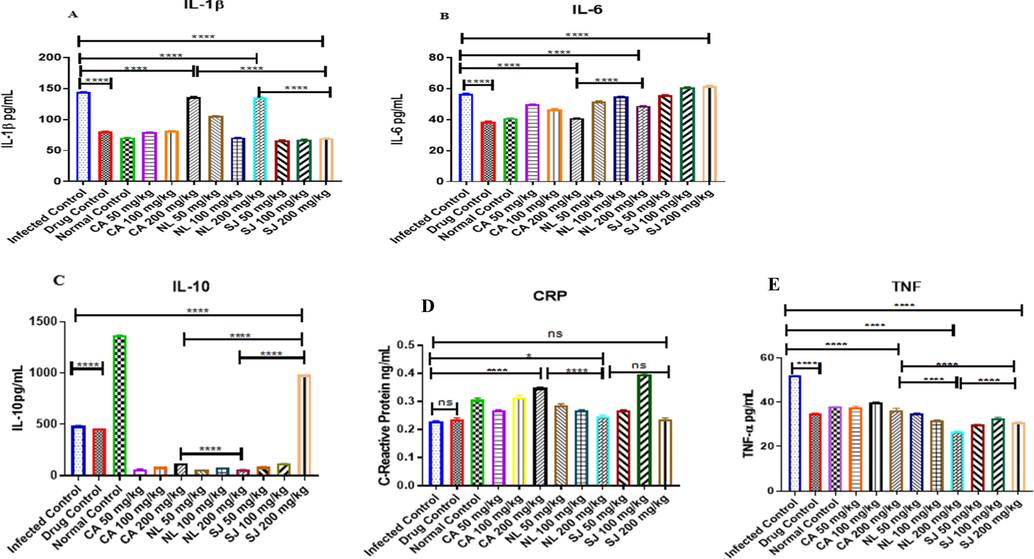
Anti-inflammatory potentials of the methanol extracts of C. afer, N. latifolia and S. jollyanum via the determination of serum interleukins 1β, (A), interleukin 6 (B) interleukin 10 (C), C-reactive protein (D) and Tumour Necrosis Factor alpha (E) in mice infected with resistant (ANKA strain) Plasmodium berghei. Ns = not significant, * = P < 0.05; **** = P < 0.0001.
3.6 Chemical profile of the extracts of C. afer, N. Latifolia and S.jollyanum
3.6.1 Compounds profile by UPLC-QTOF-MS analysis
Identification of compounds (Fig. 6) present in the methanol extracts of C. afer, N. latifolia and S. jollyanum was achieved in both positive and negative ionization modes. The MS data features such as quasimolecular ion, chemical formula and MS/MS fragment ions were generated from MassLynx and used for tentative compounds identification. C. afer was found to contain as part of its constituents; two p-coumaroyl compounds, coumaric acid and coniferyl aldehyde, two phenolic acids, divaricatinic acid and 3-hydroxy-4-(2-hydroxy-6-methylheptan-2-yl)benzoic acid, two hydroxylated unsaturated fatty acids, (Z)-9,12,13-trihydroxyoctadec-15-enoic acid, and 9-hydroxy-10,12-octadecadienoic acid, unsaturated fatty alcohol, icos-19-ene-1,2,4-triol and three steroidal saponins, dioscin, aferoside A and C. Methanol extract of N. latifolia reveals the presence of the quinic acid derivative, 3-O-caffeoylquinic acid, isoquinoline alkaloids, tetrahydropalmatine, palmatine, jatrorrhizine, indole alkaloids, 18,19-dihydroangustine, 17-epinaucleidinal and strictosamide with a saponin, quinovic acid 3-O-rhamnoside. Lastly, S. jollyanum methanol extract revealed the presence of a phenolic acid, protocatechuic acid, quinic acid and its derivatives, 3-O-caffeoylquinic acid and 3,4-dicaffeoylquinic acid, a saponin, mabioside B while the only identified alkaloid was jatrorrhizine which was also detected in N. latifolia.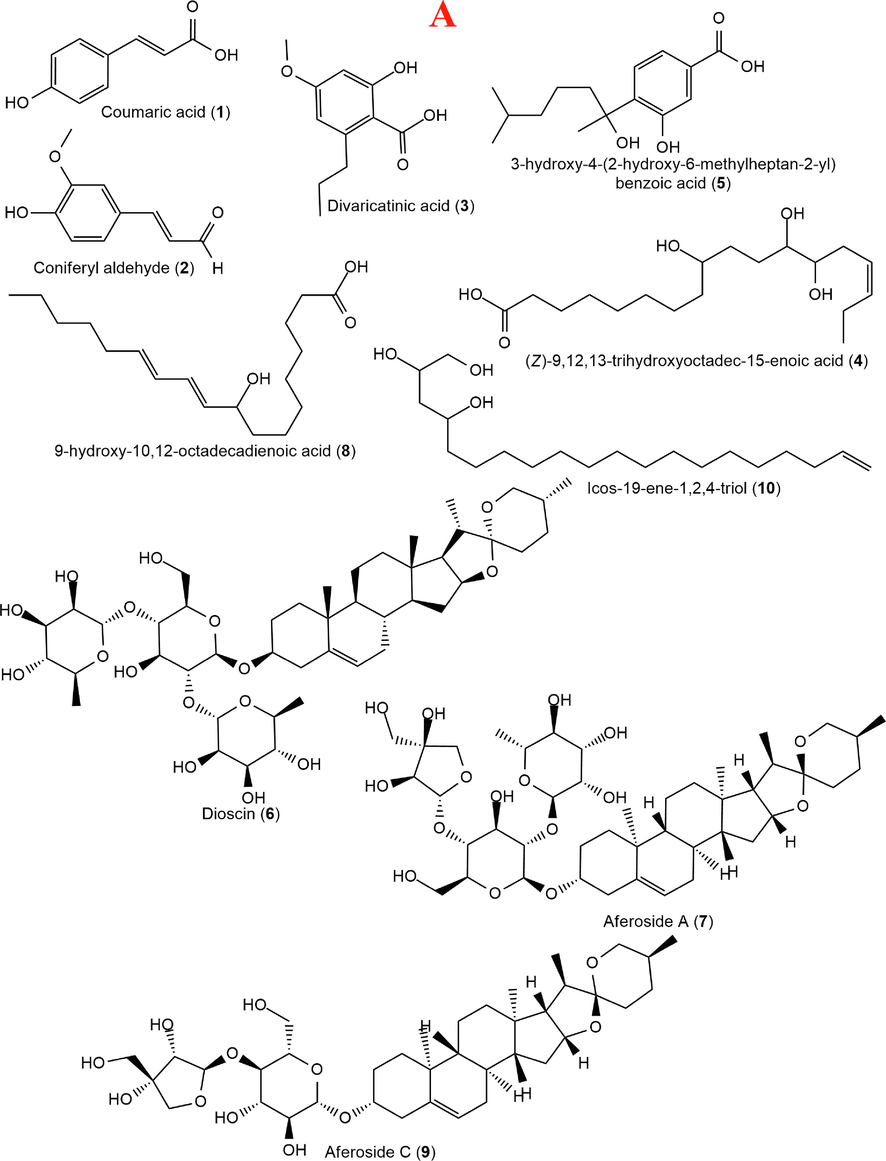
Structures of compounds identified in Costus afer (A), Nauclea latifolia (B) and Sphenocentrum jollyanum (C) contd. Structures of compounds identified in Costus afer (A), Nauclea latifolia (B) and Sphenocentrum jollyanum (C).
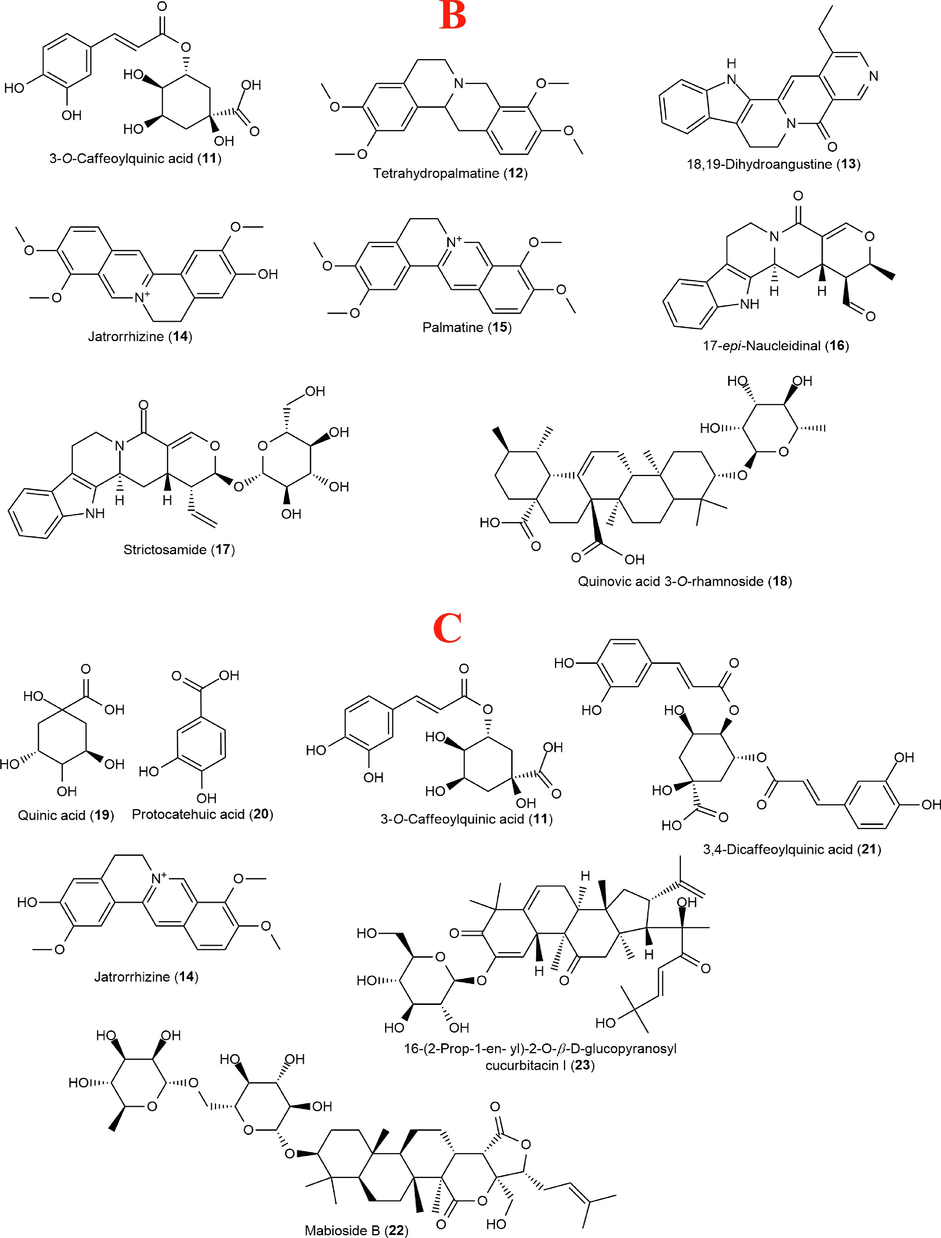
Structures of compounds identified in Costus afer (A), Nauclea latifolia (B) and Sphenocentrum jollyanum (C) contd. Structures of compounds identified in Costus afer (A), Nauclea latifolia (B) and Sphenocentrum jollyanum (C).
4 Compounds profile by NMR analysis
The 1H NMR fingerprints of the three extracts (Fig. 2) revealed common chemical shifts in most regions of the spectra.
The 1H NMR spectrum of C. afer had a unique doublet at δH 6.36 with a coupling constant (J value) of 15.8 Hz confirms the presence of coumaric acid (1). This peak was supported with the immediate neighbouring proton at δH 7.37 observed with overlaps of other compounds’ signals. The δH 6.36 peak was unambiguously absent or insignificant in the other two extracts indicating that coumaric acid may be a unique signature molecule for C. afer in comparison to other species in this study. A similar pattern was observed for coniferyl aldehyde (2) although with more deshielded protons around δH 6.76 and 7.78, which were further supported by the aldehydic singlet proton at δH 9.72.
The 1H spectrum of N. latifolia showed some similarities with that of C. afer in regions belonging to caffeoylquinic acid which has a common p-coumaric acid moiety as 1 and 2 in addition to the quinic acid’s polyol and saponins’ sugar chemical shifts between δH 3.15 and 4.50. However, additional peaks of higher deshielded protons at δH 7.64 and 9.76 which are characteristic of unique aromatic skeletons of indole and isoquinoline alkaloids with 17-epi-naucleidinal claiming one of the aldehydic singlets between δH 9.73 and 9.76. The peaks at δH 8.52 and 8.79 could be assigned to the two singlets of the pyridinyl moiety of 18,19-dihydroangustine. The rhamnosyl methyl resonance of quinovic acid 3-O-rhamnoside was observed as a doublet at δH 1.22 (J = 5.1 Hz).
Most of the chemical environments of S. jollyanum were observed in the other two extracts. However, chemical shift above δH 7.45 were very insignificant indicating that alkaloids with highly deshielded aromatic protons were either not present or in trace amounts in S. jollyanum, compared to N. latifolia, thus validating the result of the UPLC-QTOF-MS (Table 1). The intensity of the anomeric protons at δH 4.50 and 5.13 with additional anomeric peaks at δH 4.60 and 4.72 suggest extended glycosylation when compared to N. latifolia (Fig. 7).
Rt (min)
Compound name
Molecular formula
Theoretical mass (m/z)
Found mass (m/z)
Adduct
DBE count
Fragment ions
Costus afer
3.68
Coumaric acid (1)
C9H8O3
164.04734
163.0374
[M−H]−
6
119.1
6.12
Coniferyl aldehyde (2)
C10H10O3
178.06299
177.0565
[M−H]−
6
177.1; 145.1
6.76
Divaricatinic acid (3)
C11H14O4
210.08921
209.0842
[M−H]−
5
209.1; 97.0
7.40
(Z)-9,12,13-trihydroxyoctadec-15-enoic acid (4)
C18H34O5
330.24062
329.2308
[M−H]−
2
329.2; 229.1; 211.1; 171.1
10.42
3-hydroxy-4-(2-hydroxy-6-methylheptan-2-yl)benzoic acid (5)
C15H22O4
266.15181
265.1440
[M−H]−
5
265.1; 183.0
10.75
Dioscin (6)
C45H72O16
868.48204
867.4802
[M−H]−
10
N/D
10.90
Aferoside A (7)
C44H70O16
854.46639
853.4674
[M−H]−
10
N/D
11.50
9-hydroxy-10,12-octadecadienoic acid (8)
C18H32O3
296.23514
295.2271
[M−H]−
3
295.2; 277.2; 171.1
11.80
Aferoside C (9)
C38H60O12
708.40848
707.3923
[M−H]−
9
N/D
16.03
Icos-19-ene-1,2,4-triol (10)
C20H40O3
328.29775
327.2925
[M−H]−
1
327.3; 281.3
Nauclea latifolia
7.12
3-Caffeoylquinic acid (11)
C16H18O9
354.09508
354.1356
[M]+
7.54
Tetrahydropalmatine (12)
C21H25NO4
355.17836
356.1819
[M + H]+
10
340.1; 309.1; 294.1; 192.1
8.54
18,19-Dihydroangustine (13)
C20H17N3O
315.13716
338.1354
[M + Na]+
14
N/D
8.64
Jatrorrhizine (14)
C20H20NO4
338.13923
338.1375
[M]+
12
322.1; 307.1; 294.1; 279.1
9.37
Palmatine (15)
C21H22NO4
352.15488
352.1554
[M]+
12
336.1; 320.1; 308.1; 294.1
10.14
17-epi-Naucleidinal (16)
C20H20N2O3
336.14739
337.1557
[M + H]+
12
N/D
10.15
Strictosamide (17)
C26H30N2O8
498.20022
497.1906
[M−H]−
13
N/D
12.14
Quinovic acid 3-O-rhamnoside (18)
C36H56O9
632.39243
631.3870
[M−H]−
9
587.4
Sphenocentrum jollyanum
0.86
Quinic acid (19)
C7H12O6
192.06339
191.0532
[M−H]−
2
N/D
1.72
Protocatechuic acid (20)
C7H6O4
154.02661
153.0189
[M−H]−
5
109.0
2.10
3-Caffeoylquinic acid (11)
C16H18O9
354.09508
353.0911
[M−H]−
8
191.1 179.0 173.0
4.49
3,4-Dicaffeoylquinic acid (21)
C25H24O12
516.12678
515.1179
[M−H]−
14
353.1
191.1 179.0 173.0
4.76
Jatrorrhizine (14)
C20H21NO4+
338.13923
338.1346
[M]+
12
322.1 307.1 294.1 279.1
5.09
Mabioside B (22)
C42H66O15
810.44017
809.4332
[M−H]−
10
765.4
645.4 603.4
8.44
16-(2-Prop-1-en- yl)-2-O-β-D-glucopyranosyl cucurbitacin I (23)
C39H56O11
700.38226
699.3698
[M−H]−
12
587.4
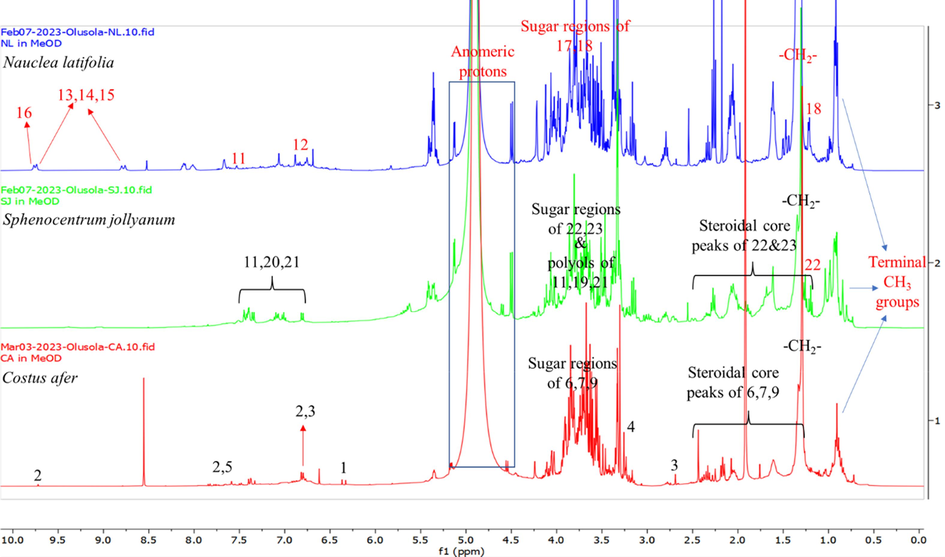
1H NMR spectra of the methanol extracts of Costus afer (red fingerprint), Nauclea latifolia (blue fingerprint) and Sphenocentrum jollyanum (green fingerprint).
5 Discussion
This study investigated the potentials of C. afer, N. latifolia and S. jollyanum as sources of drug candidates for malarial chemotherapy. The methanol extracts of these plants decreased parasite load and suppresses parasite growth, thus substantiating the indigenous clams (Iyamah and Idu, 2015). The three extracts supported increase in bound heme content of erythrocyte and prevented its degradation and polymerization to hemozoin in order to decrease parasite survival. Heme content decreased with a corresponding increase in hemozoin content in infected control, thus acting as one of their mechanisms of action (Kotepui et al., 2015; White, 2018).
The reversal of parasite-induced mitochondrial permeabilisation by methanol extracts of C. afer, N. latifolia and S. jollyanum will increase ATP synthesis and prevents cell death. Our previous studies on Diospyros mespiliformis (David et al., 2021), Mondia whitei (Olanlokun et al., 2018, Olanlokun et al., 2019), Phyllanthus amarus (Olanlokun et al., 2020) and Alstonia boonei (Olanlokun et al., 2013) showed that the antimalarial potency of medicinal plants coupled with their mito-protective effects will improve host survival (Olanlokun et al., 2021). Stimulation of ATPase activity by the extracts showed that the effects of these medicinal plants may be bidirectional (Baker et al., 1986). Also, C. afer and S. jollyanum significantly decreased the total peroxidative products, thus preventing oxidative damage (Chicco and Sporagna, 2007; Vähäheikkilä et al., 2018).
Increased IL-1β level in Plasmodium-infected patients is responsible for pathological changes and symptoms observed in malaria disease. Their elevated level in the peripheral blood is also linked to parasite clearance. This is one of the adaptive responses noticed in the infected individuals shortly before chemotherapy (Farrington et al., 2017). Pleiotropic IL-6 can perform defensive role in the host, showing that the increase in the levels of IL-6 in infected mice treated with N. latifolia and S. jollyanum may be because of the healing processes (Simpson et al., 1997). High levels of TNF-α in the infected control may indicate severity of hyper-parasitemia (as observed in this study), severe anemia and hypoglycemia (Shaffer et al., 1991). The C reactive protein performs pathological role in malaria disease. It binds to infected erythrocytes and assists in the clearance of same, indicating parasite burden. Increased serum IL-10 observed in S. jollyanum-treated mice is profitable because it prevents damage to the host cell and normal tissue homeostasis (Iyer and Cheng, 2012).
We identified diocin and divaricatinic acid in C. afer for the first time in addition to other phytochemicals earlier profiled. Previous study has shown that this compound has antiproliferative and antimalarial property (Lomchid et al., 2016). We also identified tetrahydropalmatine and jatrorrhizine in N. latifolia for the first time. Pharmacological usefulness of tetrahydropalmatine includes analgesic, neuro-protection and anti-inflammatory effects (Du et al., 2022). Mabioside B and a sugar derivative of cucurbitacin, 16-(2-Prop-1-en- yl)-2-O-β-D-glucopyranosyl cucurbitacin, were identified for the first time in S. jollyanum. Mabioside B has anti-inflammatory and immunomodulatory activity (Ruan et al., 2016).
In summary, different phytochemicals with varying pharmacological potentials are present in the medicinal plants and they occur as composite medicines in the form of polypharmacy for synergistic and combinative therapy purposes. This may be the reason why medicinal plants are prepared as decoctions and composite preparations. The purification and formulation of relevant phytochemicals from this plant for the treatment of diseases will have beneficial purposes for the treatment of malaria.
Ethical statement
The conduct of this study follows the ARRIVE guidelines. Moreover, this study was revised and approved by University of Ibadan Animal Care and Use Research Ethics Committee and approval number UI-ACUREC/073-0122/03 was assigned to this study.
Funding
This study was self-sponsored by all the authors.
Authors contributions
JOO conceived and designed the study, performed experiments and wrote the draft manuscript. SOO perfomed experiments, AO performed experiments, BO performed experiments, OB did the LC-MS and NMR study, VM corrected the manuscript, OOO corrected the draft manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Receptor Dependent Stimulatory Effect of Noradrenaline on Na+/K+ ATPase in Rat Brain Homogenate. Role of Lipid Peroxidation. Biochem. Pharmacol.. 1982;31:2231-2236.
- [Google Scholar]
- Aweke Costus afer Ker Gawl, G. 2007. In: Schmelzer GH, Gurib-Fakim, A. Prota. medicinal plants/plantes médicinales. Wageningen, Netherlands: PROTA.
- Selective toxicity of the antimalarial primaquine-evidence for both uncoupling and inhibitory effects of a metabolite on the energetics of mitochondria and its ATP synthase complex. Pharm. Res.. 1986;3:290-293.
- [Google Scholar]
- Role of cardiolipin alterations in mitochondrial dysfunction and disease. Am. J. Physiol-Cell. Physiology. 2007;292:C33-C44.
- [Google Scholar]
- Studies on the mitochondrial, immunological and inflammatory effects of solvent fractions of Diospyros mespiliformis Hochst in Plasmodium berghei infected mice. Sci. Reports. 2021;11:6941.
- [Google Scholar]
- A Comprehensive review on the chemical properties, plant sources, pharmacological activities, pharmacokinetic and toxicological characteristics of tetrahydropalmatine. Front. Pharmacol.. 2022;13:890078.
- [Google Scholar]
- Elujoba Female Infertility in the Hands of Traditional Birth Attendants in South-West Nigeria. Fitoterapia. 1995;66(1995):239-248.
- [Google Scholar]
- Both inflammatory and regulatory cytokine responses to malaria are blunted with increasing age in highly exposed children. Malar. J.. 2017;16:499.
- [Google Scholar]
- Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Crit. Rev. Immunol.. 2012;32:23-63.
- [Google Scholar]
- Ethnomedicinal survey of plants used in the treatment of malaria in Southern Nigeria. J. Ethnopharmacol.. 2015;173:287-302.
- [Google Scholar]
- Targeting the mitochondrial permeability transition pore to prevent age-associated cell damage and neurodegeneration. Oxid. Medi. Cellular. Long.. 2021;2021
- [Google Scholar]
- Spermine inhibition of the permeability transition of isolated rat liver mitochondria: an investigation of mechanism. Ach. Biochem. Biophy.. 1993;306:246-253.
- [Google Scholar]
- The catalytic effect of 2,4-dinitrophenol on adenosine triphosphate hydrolysis by cell particles and soluble enzymes. J. Biol. Chem.. 1953;201:357-370.
- [Google Scholar]
- Protein measurement with the Folin phenol reagent. J. Biol. Chem.. 1951;193:262-275.
- [Google Scholar]
- Antiplasmodial activity and cytotoxicity of plants used in West African traditional medicine for the treatment of malaria. J. Ethnopharmacol.. 2006;106:131-136.
- [Google Scholar]
- Mondia whitei, an African spice inhibits mitochondrial permeability transition in rat liver. Prev. Nutri. Food. Sci. 2018;23:206-213.
- [Google Scholar]
- In vivo antiplasmodial effects of Diospyros mespiliformis and Mondia whitei methanol extracts on Plasmodium berghei-induced malaria in mice. Interv. Medi. Applied. Sci. 2019;11:197-206.
- [Google Scholar]
- Antimalarial properties and preventive effects on mitochondrial dysfunction by extract and fractions of Phyllanthus amarus (Schum. And Thonn) in Plasmodium berghei-infected mice. J. Basic. Clin. Physiol. Pharmacol. 2020;32:255-266.
- [Google Scholar]
- Comparative antimalarial, toxicity and mito-protective effects of Diospyros mespiliformis Hochst. ex A. DC. and Mondia whitei (Hook.f.) Skeels on Plasmodium berghei infection in mice. J. Ethnopharmacol.. 2021;268:113585.
- [Google Scholar]
- In Vivo antimalaria of methanol leaf and root extracts Sphenocentrum jollyanum. Afr. J. Pharm. Pharmacol.. 2011;5:1669-1673.
- [Google Scholar]
- Hemozoin production by Plasmodium falciparum: variation with strain and exposure to chloroquine. Biochim. Biophys. Acta.. 1993;1157:270-274.
- [Google Scholar]
- Plant Resources, 13C-NMR Spectral Characteristic and Pharmacological Activities of Dammarane-Type Triterpenoids. Molecules. 2016;21:1047.
- [Google Scholar]
- The antimalarial activity of some quinolone esters. Am. Trop. Med. Parasitol.. 1970;84:209-222.
- [Google Scholar]
- How cardiolipin peroxidation alters the properties of the inner mitochondrial membrane? Chem. Phy. Lipids. 2018;214:15-23.
- [Google Scholar]
- Effects of calmodulin antagonists on radiation-induced lipid peroxidation in microsomes. Int. J. Radiat. Biol.. 1990;58:733-743.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2023.103065.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







