Translate this page into:
Distribution of Candida infections in patients and evaluation of the synergic interactions of some drugs against emerging fluconazole- and caspofungin-resistant C. auris
⁎Corresponding author. gkhaled@ksu.edu.sa (Jamal M. Khaled)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Pathogenic multidrug-resistant Candida species are considered some of the most important health risks. This work aimed to evaluate and monitor the prevalence of the human pathogenic Candida strains isolated from patients in King Fahd Medical City (KFMD), Riyadh, Saudi Arabia, and to evaluate the synergy of some antimicrobial agents against Candida species’ resistance to antifungal drugs. The retrospective analysis, identification using biochemical tests, minimal inhibitory concentrations using E-tests, determination of the fraction inhibitory concentration index value for synergic testing, and simulation of 100 experiments using Monte Carlo simulation methods were performed according to standard protocols. The findings showed that all age groups of males and females can be infected by Candida species; furthermore, human pathogenic Candida species can be isolated from several clinical samples and different human body sites. The minimal inhibitory concentration results showed that many multidrug-resistant Candida strains, such as C. albicans, emerged in 2020 compared to 2018. Candida albicans remains the most important pathogen among all Candia species, found in 51.7 % and 42.4 % of the isolates in 2018 and 2020, respectively. In 2018, many isolates of C albicans showed resistance to itraconazole, fluconazole, anidulafungin, amphotericin B, ketoconazole, voriconazole, caspofungin, and flucytosine. In 2018, all C. auris isolates (N = 94) were resistant to fluconazole, and more than 85 % (N = 76) of C. albicans isolates were resistant to itraconazole, while only 5.9 % (N = 2) were resistant in 2018. The study concluded that the resistance to antifungal drugs among pathogenic yeasts is increasing and constantly changing and that surveillance of these pathogens must continue. Also, the synergy between drugs remains an appropriate option for confronting this risk, especially between natural extracts and drugs. Despite the lack of evidence for any antifungal and antibacterial drug's ability to synergistically suppress the fluconazole- and caspofungin-resistant C. auris strains diagnosed in this study, the surveillance and synergic tactics continue to be viable options for dealing with these human pathogenic yeasts.
Keywords
Candida
Infection
Pathogens
Synergy
MIC
C. auris
1 Introduction
Candida species are eukaryotic microbes that belong to the fungi kingdom. These fungi (yeasts) live naturally on the skin and inside the human body and have the ability to occupy various natural habitats. Candida species can also cause serious diseases if they find a way to penetrate the internal parts of the human body, such as the kidney, heart brain, and bloodstream (Lynch 1994, Jabra-Rizk et al., 2016).
There are only three types of drugs (azoles, polyenes, and 5-fluorocytosine) currently used to fight fungal infection, and they use different mechanisms to kill or inhibit pathogenic fungi, including inhibition of ergosterol biosynthesis (azole antifungal drugs), disruption of the cell membrane (polyene antifungal drugs), and inhibition of DNA and RNA biosynthesis (5-fluorocytosine) (Carrillo-Munoz et al., 2006, Whaley et al., 2017, Van Daele et al., 2019). As available options are limited, the emergence of drug-resistant pathogenic fungi is a highly dangerous global risk for public health. Some strains of Aspergillus and Candida species have the ability to resist numerous standard antifungal drugs (Dismukes 2000, Houšť et al., 2020), and some Candida species are able to resist antifungal drugs via several mechanisms. For example, azole resistance mechanisms include inactivation of C-5 sterol desaturase, uptake of the sterols from out-environments, efflux pumps, inhibition of the main transporters to reduce the accumulation of azoles inside the cell, and reducing azole binding to lanosterol 14-alpha-demethylase (Ryley et al., 1984, Whaley et al., 2017). Polyene-resistant Candida strains can defeat polyene antifungals through genetic mutations in genes that encode enzymes for ergosterol biosynthesis or modification of the sterols and fatty acid of the cytoplasmic membrane and of the ratio of sterols to phospholipids (O'Shaughnessy et al., 2009, McCarthy et al., 2017). Mechanisms of resistance to 5-fluorocytosine in Candida species need further investigation. A genetic study on 5-fluorocytosine-resistant Candida glabrata showed genetic mutations in genes that encode cytosine deaminase, uridine monophosphate, and pyrophosphorylase. Thymidylate synthase or a cytosine permease may be responsible for the resistance to 5-fluorocytosine (Vandeputte et al., 2011). The mode of biological action of antifungal agents against human pathogenic yeast and mold is represented in Fig. 1.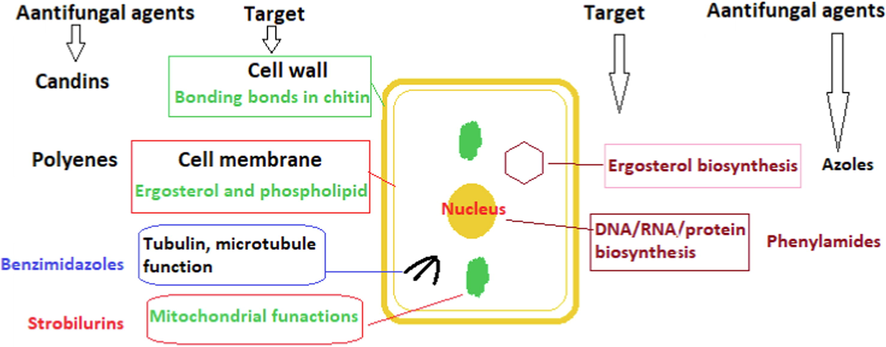
Mode of biological action of antifungal agents.
In recent years, monitoring and screening studies have shown a global prevalence of antifungal-resistant strains in hospital-acquired infections caused by Candida species It has been reported that 7 % of the Candida strains isolated from blood samples collected by the Centers for Disease Control and Prevention (CDC) were pathogenic isolates that have the ability to resist antifungal drugs (https://www.cdc.gov/fungal/diseases/candidiasis/antifungal-resistant.html). Globally, it has been reported that more than 2.5 % and 9 % of human pathogenic Candida species can resist fluconazole and itraconazole, respectively (Pfaller et al., 2000). Multidrug-resistant C. auris strains have been isolated and diagnosed in ear, blood, and invasive infections in many countries around the world (Chowdhary et al., 2016). In Saudi Arabia, numerous studies have confirmed the presence of strains of resistance in C. auris to several antifungal drug categories (Lone and Ahmad 2019, AlJindan et al., 2021). The importance of the present research is in monitoring this highly dangerous pathogenic yeast, and the ongoing need to update data on its resistance to standard drugs, especially, with the rare studies on synergistic methods in Saudi Arabia as an option that can be applied in the treatment of this pathogenic yeast. This work aimed to evaluate and monitor the prevalence of the human pathogenic Candida strains isolated from patients in King Fahd Medical City (KFMD), Riyadh, Saudi Arabia, and to evaluate the synergy of some antifungal drugs against Candida species’ resistance to antifungal drugs.
2 Methodology
2.1 Retrospective analysis
From January 1, 2018, to August 30, 2018, and January 1, 2020, to December 30, 2020, the data of antifungal-resistant Candida species isolated from patients in KFMC were analyzed to evaluate the biological differences between microbial species and strains regarding biochemical characteristics and responses to standard antifungal agents. The data were analyzed using chi-square test, Ward linkage method, and one-way ANOVA (IBM SPSS Statistics 26 and OriginPro 2018). The work was part of an investigation approved by the Ethics Committee, KFMC, Saudi Arabia, IRB: 21-466E.
2.2 Testing standard antibiotics against antifungal-resistant Candida strains
Minimal inhibitory concentrations (MICs) (Moore et al., 1987) were determined to screen the biological activity of the standard antibacterial drugs against Candida strains using E-tests of itraconazole, fluconazole, anidulafungin, amphotericin B, ketoconazole, voriconazole, caspofungin, and flucytosine (Biomerieux, France).
2.3 Antifungal-resistant Candida strains collection
The Candida strains that showed resistance to standard antifungal agents were cultivated on blood agar (Oxoid, UK). The Candida strains were preserved in a sterile glycerol solution (30 % V/V) at −80 °C, and the subcultivation was done at least three times before the further tests.
2.4 Synergic test of antifungal and antibacterial agents
The synergy between some standard antibiotics and antifungal drugs against fluconazole- and caspofungin-resistant C. auris strains was evaluated using an E-test assay according to (Orhan et al., 2005). The fractional inhibitory concentration (FIC) index (ΣFIC) of the tested drugs was calculated using the following equation (Mehta et al., 2022): ΣFIC = FICd1 + FICd2 FICFlu = (MIC of combination d1 and d2 / MIC of d1)FICD2 = (MIC of combination d1 and d2 / MIC of d2)
The simulation of 100 experiments on FIC (N = 2) used in this investigation was performed using Monte Carlo simulation methods according to (Meletiadis et al., 2010) with some modifications. Log2 of the FICs were calculated, and then the mean and standard division were determined. The normal distribution was tested using the Norm.dist(FIC,mean, standard division, false) function in Excel 2016. The first simulation was calculated using the norm.inv(rand.()mean, standard division) function in Excel 2016. Two hundred tests of expected FICs were done using a data table and calculating new functions. The percentage of simulated FICs that showed synergy was calculated using the countif(FICs,“<=1″/100) function.
3 Results
In 2018, 63.5 % of patients infected by pathogenic yeasts were females, but in 2020, 52 % of the infected patients were male. Pearson chi-square analysis reported a significant (at the 0.05 level) association between males and females in both 2018 and 2020.
The results shows that all age groups can be infected by pathogenic yeast. The prevalence of pathogenic yeast infection for the age group 61–80 years was 28.3 % in 2018 and 35.48 % in 2020. The lowest prevalence of infection was in babies younger than one year of age. In both 2018 and 2020, 11.5 % of males and 8.31 % of females older than 80 years of age were infected. In 2020, more than 50 % of the patients infected by pathogenic yeasts were 15–80 years old.
The mean monthly rates of pathogenic yeast isolates (Candida and non-Candida species) diagnosed were 12.5 % during 2018 and 8.3 % during 2019. The statistical analysis showed a significant difference between 2018 and 2020 (Fig. 2).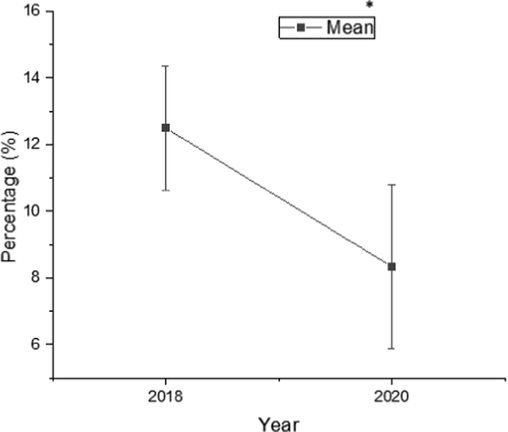
Means (%) of Candida spp. and other pathogenic yeasts isolates identified during 2018 and 2020. *At the 0.05 level, the means (%) are significantly different (P < 0.05).
In 2018, 39.2 % of the pathogenic yeasts isolated from patients were Candida species; C. albicans strains exceeded 50 % of the isolates, and ten species of Candida, including C. parapsilosis (10.8 %), C. glabrata (9.8 %), and C. tropicalis (9.02 %), were isolated and identified. In 2020, Candida species were 98.8 % of isolates; C. albicans strains were 42.4 %, C. parapsilosis were 2.2 %, C. glabrata were 12.8 %, and C. tropicalis were 9.7 %. Many species emerged, such as C. auris (5.5 %) and C. famata, bringing the total number of species to 16 (Fig. 3).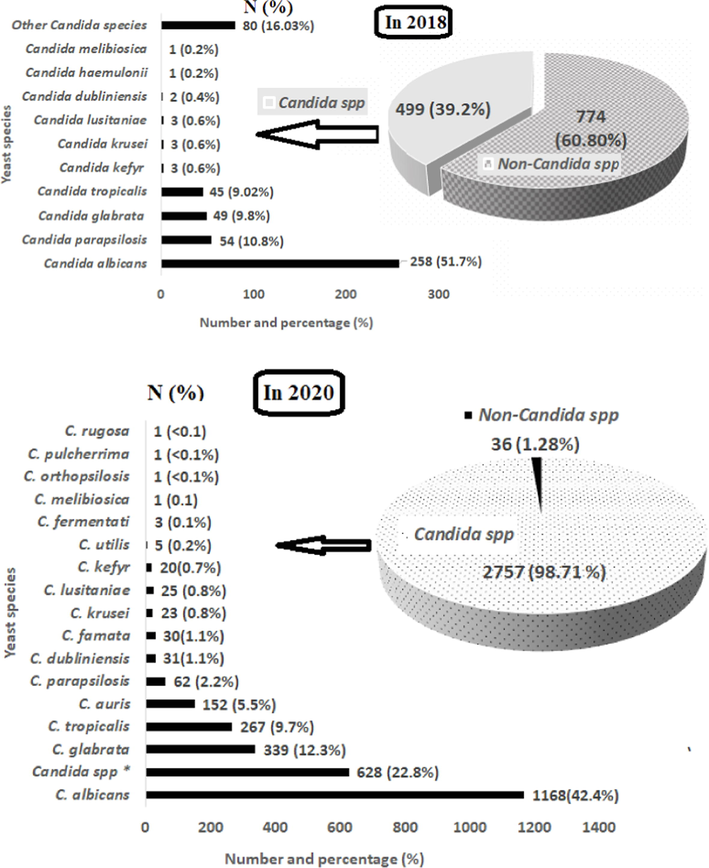
Number (N) and percentage (%) of Candida and non-Candida spp. isolated and diagnosed in patients during 2018 and 2020.
The results showed that the urine, respiratory, and blood samples were the major sources of Candida and non-Candida isolates. In 2018, more than 50 % of Candida and non-Candida species were isolated from urine samples, and in 2018 and 2020, respectively, 83.8 % and 67.6 % of the isolates were obtained from urine, respiratory, and blood samples.
The finding shows the number and percentage of Candida species that have the ability to resist one or more of the standard antifungal drugs tested in this investigation: 16.6 % of Candida isolates were resistant to fluconazole, 13.4 % to itraconazole, 11.9 % to amphotericin B, 11.1 % to voriconazole, 7.1 % to caspofungin, 4.7 % to anidulafungin, 4.0 % to flucytosine, and 1.2 % to ketoconazole (Fig. 4). Itraconazole was resisted by C. glabrata (79 %), C. tropicalis (15 %), and C. albicans (5.9 %). No isolate of C. haemulonii, C. parapsilosis, C. krusei, and C. lusitaniae resisted itraconazole. More than 30 % of the C. albicans isolates were resistant to amphotericin B and ketoconazole. Although most Candida strains that resisted itraconazole, fluconazole, anidulafungin, ketoconazole, ketoconazole, and caspofungin were isolates of C. glabrata, no C. glabrata strain isolated in this work had the ability to resist flucytosine.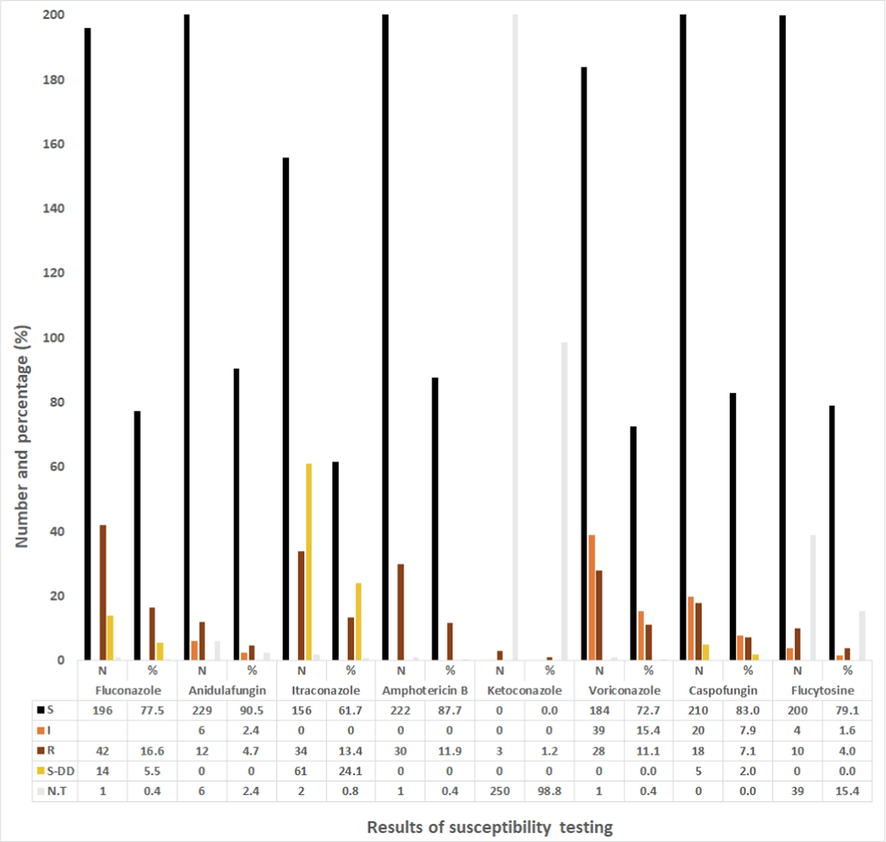
Number (N) and percentage (%) of Candida spp. (N = 253) that have the ability to resist the standard antifungal drugs in 2018. *All MICs values were interpreted according to CLSI and EUCAST.
In the hierarchical cluster analysis of Candida strains, C. albicans and C. parapsilosis isolates were grouped in the cluster with the isolates resistant to fluconazole, amphotericin B, voriconazole, caspofungin, and flucytosine (Fig. 5 and Fig. 7). C. glabrata isolates were grouped in an independent cluster; more than 30 % of C. glabrata isolates were resistant to itraconazole, anidulafungin, ketoconazole, voriconazole, and caspofungin, and all isolates were susceptible to flucytosine.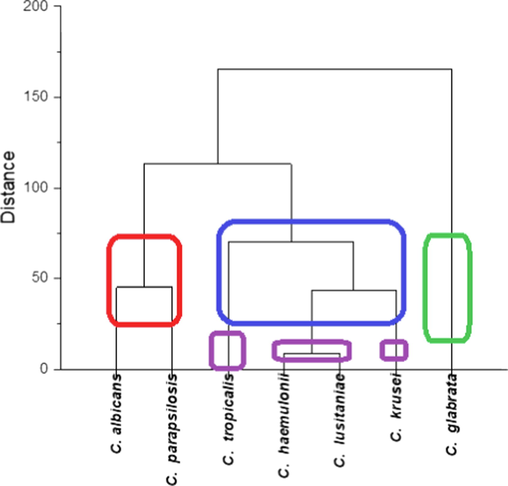
Hierarchical clusters of Candida strains isolated in 2018 from patients that have resistance to itraconazole, fluconazole, anidulafungin, amphotericin B, ketoconazole, voriconazole, caspofungin and flucytosine. Percentage (%) analyzed using Ward method in OriginPro software.
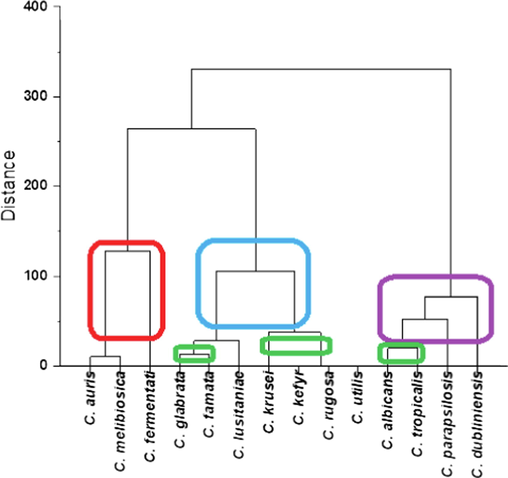
Hierarchical clusters (Ward method) of Candida strains isolated in 2020 from patients that have resistance to fluconazole, itraconazole, amphotericin B and ketoconazole.
Fig. 6 shows that more than 50 % of the isolates were resistant to fluconazole and ketoconazole, and some isolates showed resistance to anidulafungin, itraconazole, amphotericin B, voriconazole, and caspofungin. (Only 4.3 % of the isolates were resistant to caspofungin.) All isolates of C. auris were resistant to fluconazole. In addition, more than 75 % of isolates of C. albicans, C. tropicalis, and C. parapsilosis were resistant to itraconazole.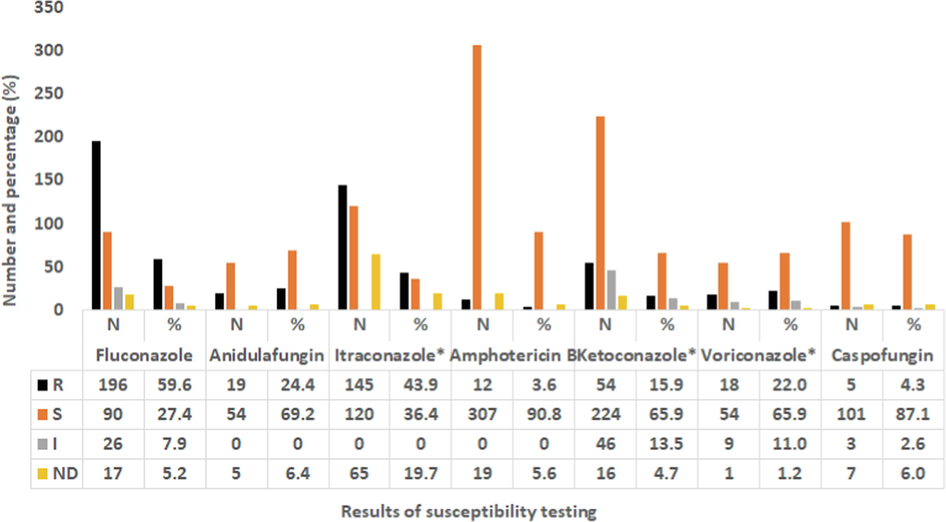
Number (N) and percentage (%) of Candida spp. (N = 370) that have the ability to resist the standard antifungal drugs in 2020. *All MICs values were interpreted according to CLSI and EUCAST.
The results show no synergy between fluconazole and caspofungin; furthermore, none of the antibiotics tested in this work, which included cefepime, ceftazidime, and moxifloxacin, showed any effect. Additive action was only recorded between cycloheximide and caspofungin, for which the FIC index was ≥ 0.5 (Fig. 8). Simulation of 100 tests using Monte Carlo simulation methods showed that 61 % of the experiments would probably show the same results.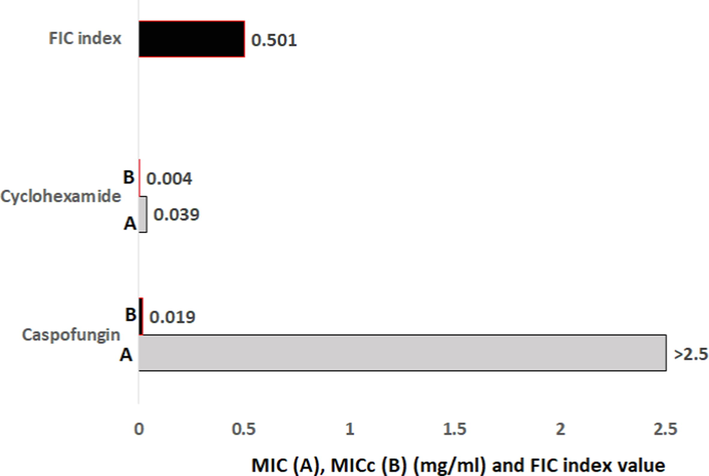
Minimal inhibitory concentration (MIC) (mg/mL) and fractional inhibitory concentration (FIC) index that showed activity against C. auris strain isolated from the patients. *MIC (A) for each drug alone, MICc (B) for formulation of cycloheximide and caspofungin (20:2.5 mg/ml).
4 Discussion
The results of this work confirmed that males and females of all age groups, children, adults, and elders, are at risk of infection. In 2018, we found a female:male infection ratio of 1.7:1, which agrees with the results of (Loster et al., 2016), who found more infections in females than in males (1.9:1 female:male). In 2020, our results were completely different: the infection ratio was 0.9:1 (female:male), different from that in Loster his colleagues but in agreement with (Al Thaqafi et al., 2014) (0.87:1, female:male). The female-to-male infection rate varies notably from year to year according to our results and the results obtained from previous studies, for example, Loster and his colleagues (2016); Al Thaqafi and his colleagues (2014). There are many psychosocial and medical factors that can answer the question whether the Candida and non-Candida infection is dependent on gender, but the answer will differ by case and country.
The Candida infections are caused by many species of opportunistic pathogens (Vázquez-González et al., 2013), so it is a logical impossibility that elderly individuals (more than 60 years) are most susceptible to infections, as confirmed by our results. Lack of correlation between age and average number of isolates is common in many previous studies; for example, as in our study, Kim et al. (Kim et al., 2016) showed that the average number of isolates varies according to many factors.
We found many antifungal drug-resistant Candida strains that emerged in 2020. Emerging drug-resistant Candida strains have been reported in several countries. For this reason, improving current strategies and monitoring processes are urgently needed to fight these fungi. Notwithstanding the isolation of three isolates of fluconazole-resistant C. haemulonii and voriconazole-resistant C. haemulonii in 2018, not one strain of C. haemulonii was isolated in 2020. It has been reported that C. haemulonii is a rare opportunistic pathogen, and the accuracy of the identification for this yeast is complex due to its high similarity to other Candida strains (Coles et al., 2020).
C. albicans strains remain major pathogens despite the changes in the species and numbers of Candida isolates taken from patients in 2018 and 2020. Reports published in previous years confirmed the predominance of C. albicans over other Candida species (Talapko et al., 2021). Grouping of the isolates based on susceptibility test patterns showed a difference between 2018 and 2020. For example, in 2018, all C. glabrata, C. tropicalis, C. haemulonii, C. krusei, and C. lusitaniae isolates were susceptible to flucytosine. In 2018, all tested Candida isolates except 10.6 % of the isolates of C. auris were susceptible to caspofungin. Caspofungin and anidulafungin (echinocandin drugs) can damage yeast cell walls by inhibiting the enzymatic systems that form glucan, but the present work confirmed emerging isolates of C. auris that have the ability to resist caspofungin and anidulafungin. Also, in this work, anidulafungin-resistant C. albicans, C. tropicalis, C. glabrata, and C. krusei strains were isolated from the patients.
In medical strategies, synergistic interactions are frequently recommended to maximize biological activity to remove infectious agents; however, the relationship between these interactions and the development of multi-drug resistance is not yet clear (Nyilasi et al., 2010). Our results confirm that synergistic interaction strategies using tested standard drugs have limited effectiveness in fighting fluconazole- and caspofungin-resistant C. auris strains isolated from patients in KFMC. The synergistic interaction strategies between natural compounds and standard drugs remain good options that need to be investigated further in the future. A good result has been obtained from the formulation of the fungicide cycloheximide (By interfering with the translocation stage, the activity of two tRNA molecules and an mRNA relative to the ribosome, it inhibits translation extension and hence blocks the production of proteins (Schneider-Poetsch et al., 2010)), and caspofungin (it prevents the biosynthesis of beta-(1,3)-d-glucan (Letscher-Bru and Herbrecht 2003)), but the toxicity of cycloheximide limits this option. In the present work, the Candida species were isolated from many types of samples, including medical materials; for this reason, cycloheximide can be used for some medical media and materials such as transport media. These findings agree with the conclusion of (Dal Pizzol et al., 2021), who reported that cycloheximide led to decreasing fungal growth in an optisol-GS medium without any toxicity to the endothelium of the corneal material transported in it.
5 Conclusion
The results of our work show that every age group is susceptible to infection by a human pathogenic Candida species at any time of the year. Major infections were identified in male and females aged 61–80 years. Candida strains can be isolated from clinical samples from many sources, including the human body (including the respiratory system, the axilla, the groin, superficial wounds, body tissues, eye, ear, under the skin), body fluids (urine, blood, peritoneal drain, abscess, cerebrospinal fluid, aspirated fluid, surgical drain fluid, oropharyngeal samples, oral swabs, vaginal swabs), and medical tools (catheter tools, stent devices, nephrostomy tubes). In 2018 and 2020, more than 65 % of Candida species were isolated from urine, respiratory, and blood samples; C. albicans strains were the major pathogens, and C. glabrata and C. tropicalis strains are still the main pathogens among all patients. The C. auris strain was not isolated in 2018 but emerged in 2020 as one of the most resistant to antifungal drugs. Drug resistance was shown in several Candida species, including the major pathogenic Candida species. There is an increasing and constant change in the number and types of yeast strains that can cause disease and develop resistance to drugs. Although there is no evidence of synergy between any of the antifungal drugs tested that would inhibit the fluconazole- and caspofungin-resistant C. auris strain isolated in this work, surveillance and synergic strategies remain suitable choices to confront these human pathogenic yeasts.
Acknowledgment
The authors extend their appreciation to the Researchers Supporting Project number (RSP2023R70), King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Predictors and outcomes of Candida bloodstream infection: eight-year surveillance, western Saudi Arabia. International Journal of Infectious Diseases.. 2014;21:5-9.
- [Google Scholar]
- Drug Resistance-Associated Mutations in ERG11 of Multidrug-Resistant Candida auris in a Tertiary Care Hospital of Eastern Saudi Arabia. Journal of Fungi.. 2021;7(1):18.
- [Google Scholar]
- Antifungal agents: mode of action in yeast cells. Rev Esp Quimioter.. 2006;19(2):130-139.
- [Google Scholar]
- Multidrug-resistant Candida auris:‘new kid on the block’in hospital-associated infections? Journal of Hospital Infection.. 2016;94(3):209-212.
- [Google Scholar]
- Candida haemulonii: An emerging opportunistic pathogen in the United States? IDCases.. 2020;21:e00900.
- [Google Scholar]
- Dal Pizzol, M., E. C. Freitas, C. Locatelli, et al., 2021. Antifungal efficacy and safety of cycloheximide as a supplement in optisol-GS. Drug Design, Development and Therapy. 2091-2098.
- Antifungal drugs. Metabolites.. 2020;10(3):106.
- Candida albicans pathogenesis: fitting within the host-microbe damage response framework. Infection and immunity.. 2016;84(10):2724-2739.
- [Google Scholar]
- Isolation frequency characteristics of Candida species from clinical specimens. Mycobiology.. 2016;44(2):99-104.
- [Google Scholar]
- Caspofungin: the first representative of a new antifungal class. Journal of Antimicrobial Chemotherapy.. 2003;51(3):513-521.
- [Google Scholar]
- Correlation between age and gender in Candida species infections of complete denture wearers: a retrospective analysis. Clinical interventions in aging.. 2016;11:1707.
- [Google Scholar]
- Oral candidiasis: history, classification, and clinical presentation. Oral surgery, oral medicine, oral pathology.. 1994;78(2):189-193.
- [Google Scholar]
- McCarthy, M., E. M. O’Shaughnessy and T. J. Walsh, 2017. Amphotericin B: Polyene resistance mechanisms. Antimicrobial Drug Resistance, Springer: 387-395.
- Role of medicinal plants from North Western Himalayas as an efflux pump inhibitor against MDR AcrAB-TolC Salmonella enterica serovar typhimurium: In vitro and In silico studies. Journal of Ethnopharmacology.. 2022;282:114589
- [Google Scholar]
- Defining fractional inhibitory concentration index cutoffs for additive interactions based on self-drug additive combinations, Monte Carlo simulation analysis, and in vitro-in vivo correlation data for antifungal drug combinations against Aspergillus fumigatus. Antimicrobial agents and chemotherapy.. 2010;54(2):602-609.
- [Google Scholar]
- Clinical response to aminoglycoside therapy: importance of the ratio of peak concentration to minimal inhibitory concentration. Journal of Infectious Diseases.. 1987;155(1):93-99.
- [Google Scholar]
- In vitro synergistic interactions of the effects of various statins and azoles against some clinically important fungi. FEMS microbiology letters.. 2010;307(2):175-184.
- [Google Scholar]
- Synergy tests by E test and checkerboard methods of antimicrobial combinations against Brucella melitensis. Journal of clinical microbiology.. 2005;43(1):140-143.
- [Google Scholar]
- O'Shaughnessy, E. M., C. A. Lyman and T. J. Walsh, 2009. Amphotericin B: Polyene resistance mechanisms. Antimicrobial drug resistance, Springer: 295-305.
- Bloodstream infections due to Candida species: SENTRY antimicrobial surveillance program in North America and Latin America, 1997–1998. Antimicrobial Agents and Chemotherapy.. 2000;44(3):747-751.
- [Google Scholar]
- Azole resistance in Candida albicans. Sabouraudia: Journal of Medical and Veterinary. Mycology.. 1984;22(1):53-63.
- [Google Scholar]
- Inhibition of eukaryotic translation elongation by cycloheximide and lactimidomycin. Nature chemical biology.. 2010;6(3):209-217.
- [Google Scholar]
- Candida albicans—The Virulence Factors and Clinical Manifestations of Infection. Journal of Fungi.. 2021;7(2):79.
- [Google Scholar]
- Antifungal drugs: what brings the future? Medical mycology.. 2019;57(Supplement_3):S328-S343.
- [Google Scholar]
- Molecular mechanisms of resistance to 5-fluorocytosine in laboratory mutants of Candida glabrata. Mycopathologia.. 2011;171(1):11-21.
- [Google Scholar]
- Opportunistic yeast infections: candidiasis, cryptococcosis, trichosporonosis and geotrichosis. JDDG. Journal der Deutschen Dermatologischen Gesellschaft.. 2013;11(5):381-394.
- [Google Scholar]
- Azole antifungal resistance in Candida albicans and emerging non-albicans Candida species. Frontiers in microbiology.. 2017;7:2173.
- [Google Scholar]







