Translate this page into:
Distribution and ultrastructure of sensillae on legs and anal cerci in earwig Anisolabis maritima (Dermaptera: Carcinophoridae)
*Tel.: +966 15445114; fax: +966 15494794 wisdom1425@yahoo.com (Mona Mohammed Al-Dosry)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Chemoreceptors play an important role for insects behavior in detecting their defined targets for feeding or mating. This study revealed the presence of different forms of receptors sensillae occurred on the legs and anal cerci of Anisolabis maritima using SEM. Leg surface contains three types of trichoid, one type basiconica sensillae. Generally, sensillae distribution is fewer on the femur than tibia and tarsus. Anal cerci also contained three types of trichoid, three types of basiconic, one type of coeloconica sensillae, and campaniform sensillae on the terminal end of females only.
Keywords
Earwig
Anisolabis maritima
Sensillae
Legs
Anal cerci
Scanning electron microscopy
1 Introduction
Anisolabis maritima is a cosmopolitan insects world wide in distribution (Arnell, 1993). Most stages have been found through out the year, males seemed more prevalent from late spring through autumn. Eggs were found during the warmer months June, July, and were guarded by the females (Nishida, 2002). Zimmerman (2001) stated that A. maritima is found only along the seashore, It is endemic species in order Dermaptera that represents an adaptive radiation from a marine littoral ancestor which is indigenous to Hawaii, A. maritima frequent in winter beneath piles of seaweed, boards, and debris just above high tide of Jacksonville for key west on east coast (Brindle, 1981). Receptors are the main tools of insect chemical communications and are mainly located on the legs. Receptors tune feeding preferences (De Boer, 2006), recognize host plant odors (Skiri et al., 2005), and play important roles in insect survival and environmental adaptation. Multiple receptors offer many functional advantages to an insects ability to perceive and respond to environmental signals by facilitating the detection of sensory stimuli (Debry and Steullet, 2001). This research is the first to describe the legs and anal cerci sensillae in female and male A. maritima using scanning electron microscopy (SEM). Our objectives were to establish the theoretic foundation to describe the functions of receptors, explore the relationships between structure and behavior. A. maritima may be used as a beneficial predator for the eggs of Rhynchophorus ferrugineus in future as a Toole in integrated pest management program (IPM), because it was recorded in KSA as a predator for the egg of the red palm weevil (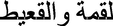 , 2002).
, 2002).
2 Materials and methods
Experimental adults males and females of A. maritima were obtained from the Ministry of Agriculture and Water in El-Kharj, Riyadh, KSA. Structure and location of different sensillae distributed on the legs and anal cerci were identified using the scanning electron microscopy. The insect soaked in 70% ethanolic for 24 h, then the legs and anal cerci were separated from the insect body. The specimens were rinsed thoroughly in distilled water and fixed in 4% glutraldehyde for 24 h at 5 °C, these were then dehydrated in conceding series concentration of acetone, for each case 1 h, and air dried. The specimens were individually mounted on stubs then coated with gold and examination was made under scanning electron microscopy (SEM) (JEOL-JSM b36 OLV), observation were obtained and recorded from four males and four females.
3 Results
3.1 The legs
The three pairs of legs for male and female are ambulatory type and their cuticle is characterized by irregular lines (Fig. 1A). Each leg consists linear series of segments as follows, (1) short coxa by which the leg is articulated with the thorax, (2) a small trochanter fused to a stout (3) femur which is the strongest part of the leg, (4) a slender tibia with a length nearly equal to the femur and (5) finally the tarsus, consisted of short three segment of movable tarsomeres. The length of 1st and 3rd tarsomeres are the same and the 2nd tarsomeres is a very small, the terminal end has claws (Fig. 1B–D). Each leg is Z-shaped. All parts of the three pairs of legs bear numerous number of different sensillae detected as follows.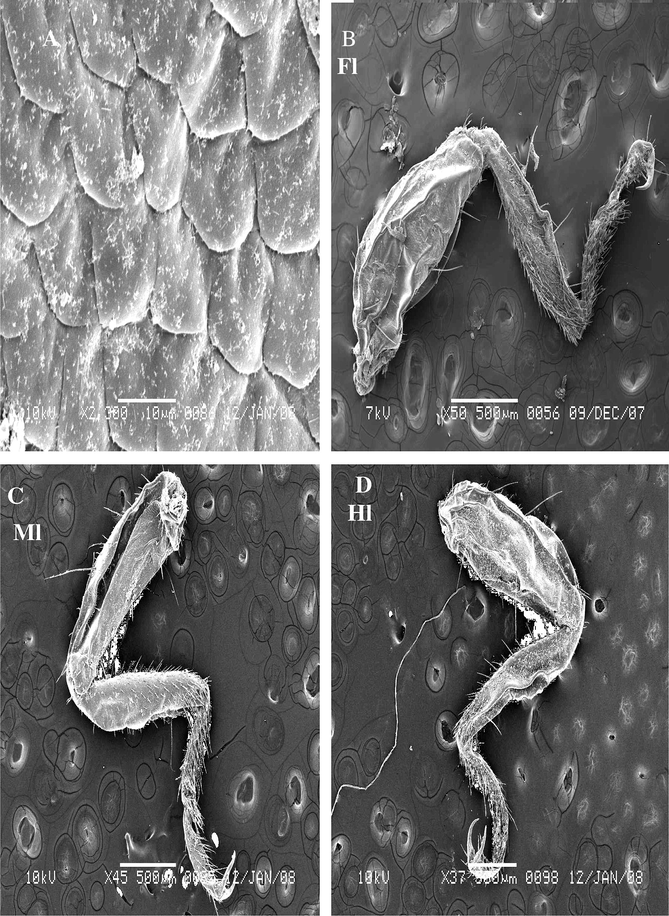
Steroscan micrograph showing legs of A. maritima. (A) Cuticle surface of legs. (B) Fore leg (Fl), (C) Med leg (Ml), (D) Hind leg (Hl).
3.1.1 Sensillae trichoidea
Sensilla trichoidea were the most abundant sensilla type found on the legs of A. maritima. These are densely distributed on the all parts of legs except the femur consists a fewer number of sensillae. Three types of trichoidea were identified.
3.1.1.1 Trichoid sensillae I (ST1)
These are few number on the femur of the fore leg and located between other hairs. This sensilla is short with swelling apex. Multi-porous can be seen on the walls of ST1 occurring at a density (Fig. 2A).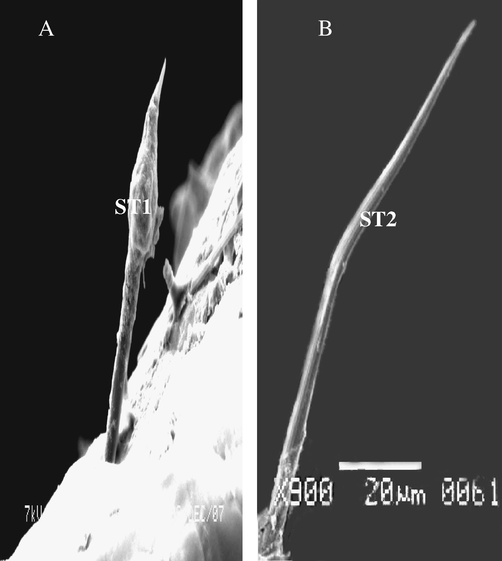
Steroscan micrograph showing: trichoid sensillae on the femur of males and females of A. maritima. (A) ST1 swelling. (B) ST2 curved.
3.1.1.2 Trichoid sensillae II (ST2)
These are sharply pointed structures are inclined and slightly curved toward its outer until apex. ST2 inserts in a flexible socket, which is slightly elevated above the cuticle (Fig. 2B). Sensilla exhibits longitudinal grooves that spiral slightly around its surface. The cuticle of this sensillum exhibits a thick, non-porous wall, and tapering edge. It occurs on external terminal of femur and there are also fewer (Fig. 2B).
3.1.1.3 Trichoid sensillae III (ST3)
It was distribution on each parts of legs with different length. ST3 inserts in a flexible socket, which is slightly elevated above the cuticle. Some other, tapering tip with apical while some other curved apex. This sensilla is planted and found on ventral view of tarsi of males and females (Fig. 3A–C). The cuticle of this sensillae exhibits a thick and multi-porous wall.
Steroscan micrograph showing: (A) General view of tibia (T) and part of the 1st tarsomere of A. maritima. (B) Magnification part of tibia show ST3 different length and tip. (C) Magnification part of ST3 showing pores and planner of surface (arrow).
3.1.2 Basiconic sensillae (BS)
The density of BS is greater on the ventral side of the tarsomeres than on the dorsal side, it was short, thick, grooved surface, blunt tipped shafts. The whole cuticular wall of BS sensillae is penetrated by non-porous, and it goes out from pit of cuticle (Fig. 4C).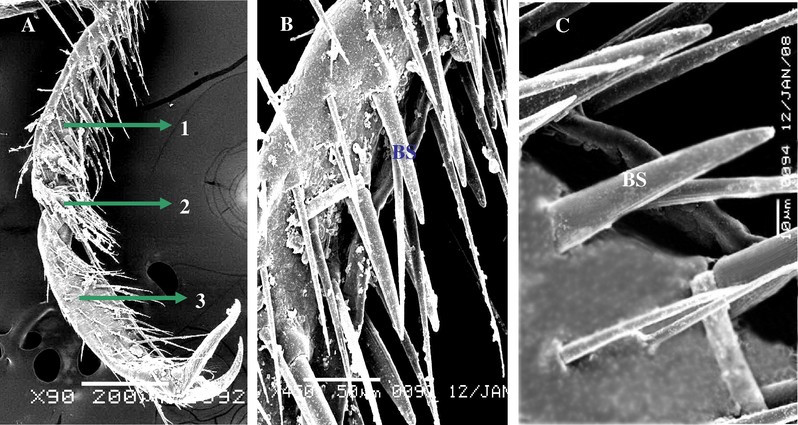
Steroscan micrograph showing: (A) Ventral view of tarsi of A. maritima. (B) Magnification part of the 1st tarsomere show many number of BS. (C) Magnification of B show BS.
3.2 Description of anal cerci of A. maritima
Anal cerci are non-reproductive appendages while connected with the lateral abdominal segment (10th abdominal segment in male and 8th abdominal segment in female (Fig. 5A and B). In earwig the cerci form powerful forceps which are usually straight and unarmed in the female, but incurved and toothed in the male (Chapman, 1998). Cerci are articulated on sexes many sensillae which functional olfaction, gestation or mechanosensitive, and in male may be used in catching with female copulation. This study showing many sensillae distribution on anal cerci, there are follow.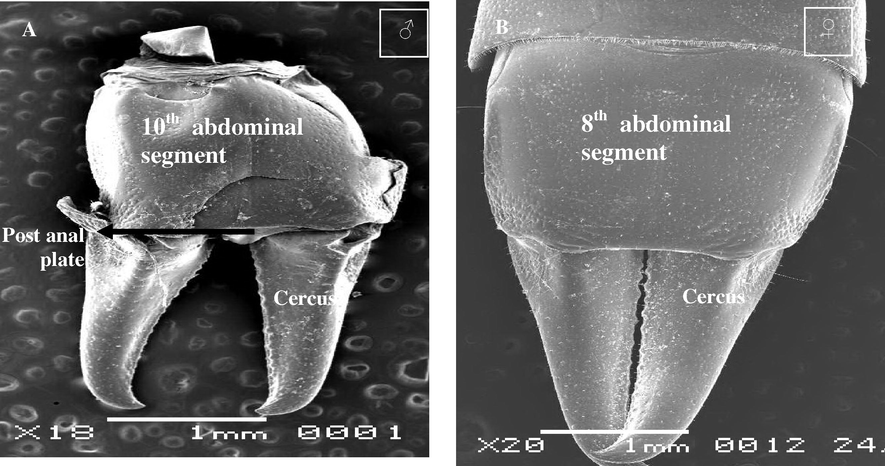
Steroscan micrograph showing anal cerci of A. maritima. (A) Male and (B) Female.
3.2.1 Cuticle
Dorsal surface of the cuticle of anal cerci is irregular spines (Fig. 6A), and side surface is like swelling (Fig. 6B and C).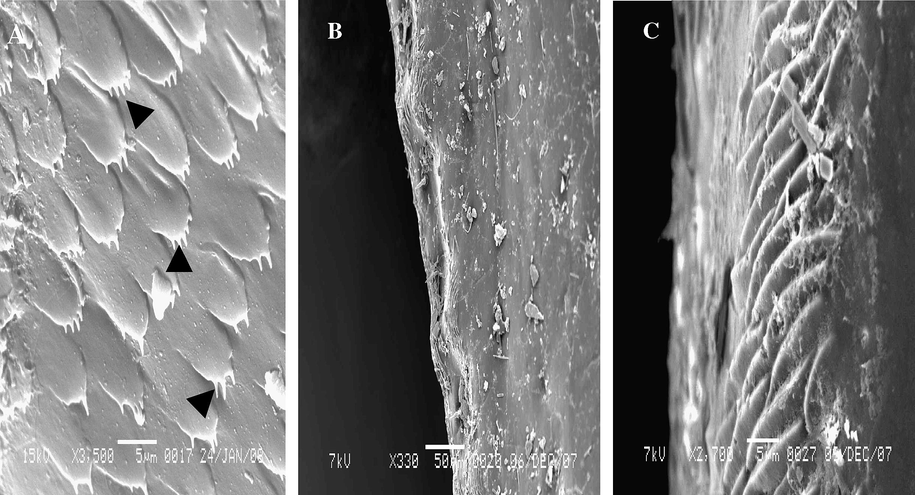
Steroscan micrograph showing cuticular surface of anal cerci of A. maritima. (A) Dorsal view (spine arrow), (B) Side view, (C) Magnification part of B.
3.2.2 Sensillae tichoidea
Sensilla trichoidea were the most numerous type found of sensillae on the anal cerci of A. maritima. These are densely distributed over the lateral surface than the dorsal surface, but rarely found on the proximal part of the ventral surface. Three sub types of sensilla trichoidea were recorded on the anal cerci of both sexes: parallel of cuticle (ST1), vertically on the cuticle (ST2), and thin sensilla trichoidea (ST3). However, only the parallel sub types occurred on the dorsal surface of both sexes, but the vertically and thin sub types occurred on the side surface of anal cerci. ST1 has grooved surface, tapering and multi-porous (Fig. 7A–C), as for vertically trichoidea is similar to parallel trichoidea but it is different bearing on the cuticle (Fig. 7D and E). The length is 65.38 μm On the other hand, thin trichoides is shorter than ST1 and ST2, more straight and tapering to the tip, their mean length 43.75 μm, they are distributed between the ST2. The cuticular wall of the ST3 is thinner than the ST1 and ST2. The whole cuticular wall of ST3 is non-porous, and it is articulated vertically on the surface of anal cerci (Fig. 7F).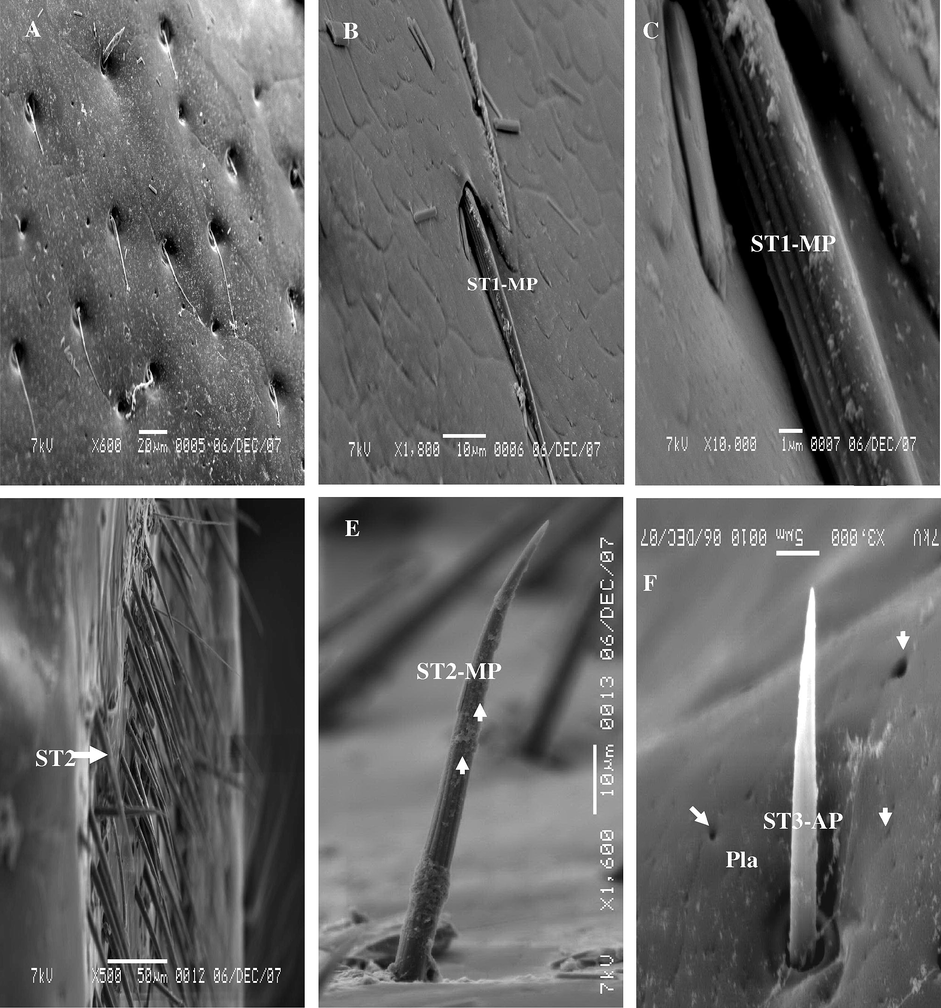
Steroscan micrograph showing: (A) Dorsal view of external surface of anal cerci of A. maritima show distribution ST1. (B) Magnification part of A show ST1-MP. (C) Magnification part of B show ST1-MP. (D) Side view of anal cerci show many hairs of ST2-MP. (E) Magnification part of D show ST2-MP. F: ST3-AP and Pla (arrow).
3.2.3 Basiconic sensillae
Three sub types of BS were recorded on the anal cerci of male and female A. maritima: first type of BS1 was blunt-tip but the BS2 and BS3 were sharp-tip (Fig. 8A–D). The blunt-tip (BS1) bears stem-like, blunt tipped shafts, whereas the sharp-tip (BS2 and BS3) gradually tapers to a sharp-tip distally. Generally, most pegs of these sensilla do not curve. All sub types are evenly distributed over the dorsal side. The cuticular wall of the BS3 sharp-tip is slightly thicker than that of the BS1 and BS2. The pores are uniformly distributed toward the base of the sensillum in the blunt-tip (SB1), besides the edge sensilla consists of the pore (Fig. 8A and B). The BS2 of sharp-tip is non-porous on the surface (Fig. 8C), but the BS3 is multi-porous all the surface and seemed form thick (Fig. 8D).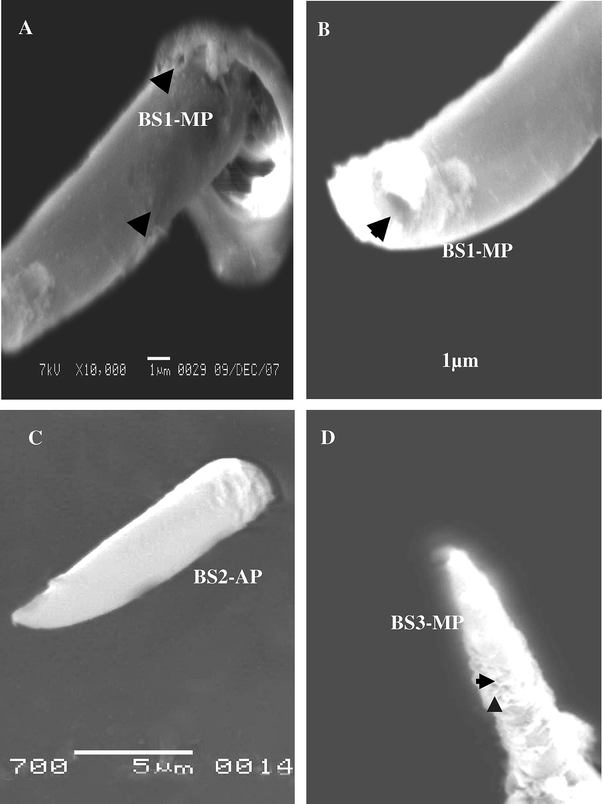
Steroscan micrograph showing sensilla basiconic: (A) BS1-MP (arrow). (B) Magnifiction part of B show terminal pore of tip BS1-MP. C: BS2-AP. D: BS3-MP.
3.2.4 Coeloconic sensillae (Coe)
Coeloconic sensillae were recorded in deep pits. These are stumps like pegs with on grooved trunk, nearly as long as wide with blunt ridged tip (Fig. 9A–C). It was the shortest kind found, they are scattered irregularly on the anal cerci. The shaft sensilla coeloconicum is longitudinally grooved on appears to be mad up of closely apposed cuticle linger like projection.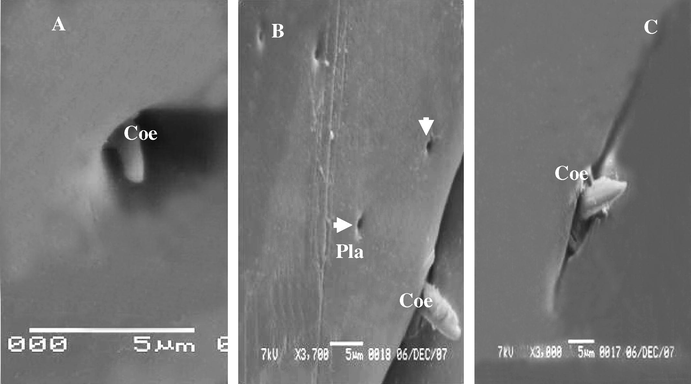
(A–C) Stereoscan micrograph showing sensilla coeloconica on the anal cerci A. maritima different longs. Arrow detected Placoid sensilla (Pla).
3.2.5 Campaniform sensillae (Cam) (Fig. 10A and B)
Campaniform sensilla were the most numerous found on the terminal ending of anal cerci of females only. Sensillae are shallow round or oval pits (Fig. 10A), and these are distributed between the coeloconic sensillae, scattered irregularly on the terminal ending of the anal cerci. Campaniform sensillae were absent in males (Fig. 10B).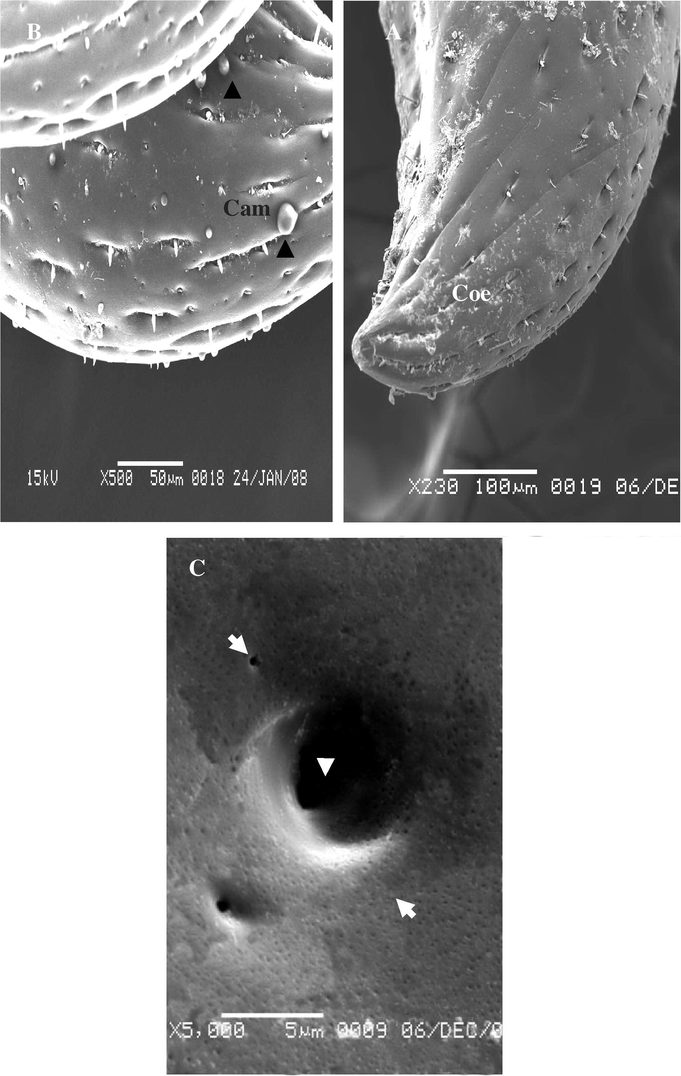
Steroscan micrograph showing: (A) Lateral end of anal cerci of female show campaniform sensillae (Cam). (B) Lateral end of anal cerci of A. maritima (male). (C) Placoid sensilla (arrow).
3.2.6 Placoid sensilae (Pla)
Placoid sensilla are plate like structures made up of a round or oval cuticular plate surrounded by a narrow membranous. Placoid sensillum or pore plate they are a flattened plate of fairly thick cuticle surrounded by a very delicate membrane (pore-plates or sensilla placoidea), there are numerous number of small or big round disk generally flat or slightly sunken in the cuticle, with a central orifice, it distributed all over the outer surface of the anal cerci between other hairs (Figs. 9B and 10C).
4 Discussion
The results of the present study, which is the first attempt to characterize the sensillae on the legs and anal cerci of earwig A. maritima an important predator on the eggs of R. ferrugineus. The legs consists typically five segments and anal cerci has one segments, many sensillae were found on these parts in males and females. Most insects mechanosensitive sensilla on the abdominal segments, In addition, the appendages of segments 11 often form a pair of structures called cerci which usually function as sense organs. Cerci are present and well-developed in the Apterygota and the hemimetabolous orders other than the Hemipteroids. In Holometabolous insects, cerci are present in the adults of Mecoptera and some Deptera; they are not present in holometabolous larvae. Cerci are present some different between other orders insects, and sometimes it differ in two sexes of a species, and they may play a role in copulation (Chapman, 1998). Campaniform sensillae were found on the lateral ending of anal cerci for females only. Three main types of sensilla trichoidea were recorded on the legs and three types of trichoidea on anal cerci. A total of six sub types of morphologically different sensillae were identified. Sensilla trichoidea were the most abundant type observed on the legs and anal cerci in both of the sexes due to their assumed mechanoreceptors function.
Many types of trichoid sensillae are multi-porous ST-MP on these organs, which may have olfaction or contact function according to (Bleeker et al., 2004; Steinbrecht, 1987, 1997; Onagbola et al., 2008). Electrophysiological studies have confirmed sex pheromone receptor function for the trichoid sensilla of Neodiprion sertifer (Hansson et al., 1991). The great abundance of the ST-MP on the legs of males and female A. maritima may indicate a probable role in mate location, possibly for detection of female sex pheromones, as reported for some other parasitoid (Barline et al., 1981; Bleeker et al., 2004). Also, in this study we observed sensilla trichoidea swelling on the legs earwig A. maritima which appear multi-porous on surface. These sensillae found in many insects species specific on external genitalia and antennae (Alm and Hall, 1986; Bland, 1981; Sharaby and Al-Dossary, 2007).
The type sensilla trichoidea a porous structure described in the current study are similar to trichoidea sensilla described on the other species (Schmidt and Smith, 1987; Amornska et al., 1998). These authors proposed that this sensilla type may play a role proprioceptors (Chapman, 1998).
The basiconic type of sensillae were present on legs and anal cerci were present in both sexes of A. maritima. These types of basiconic were previously described as fluted basiconic sensilla (Norton and Vinson, 1974; Bleeker et al., 2004). These types of sensilla are similar to which described in the previous studies were with pores on the tip or on the wall. The basiconic sensilla on legs were with grooved in the surface and the inner lumen was surround by a thick non-porous wall (Fig. 4C), which were similar to that discovered in some other insects (Ochieng et al., 2000). The wall of BS on anal cerci of A. maritima has pores than that of BS on legs. Sensilla basiconic types should be a gustative or olfactory function. This results is consistent with that demonstrated in the previous studies (Steinbrecht, 1984; van Baaren et al., 1999; Ochieng et al., 2000).
Coeloconic sensillae is the least occurring sensilla type on A. maritima, and it has been previously described as pit organs because these are recessed in to deep pits (Wcislo, 1995), and as coeloconic sensilla type II (Bleeker et al., 2004). However, this sensillum has been considered to have thermo-hygroreceptive functions in several non-parasitic species (Altner et al., 1983). The absence of wall pores on the sensilla coeloconica of male and female A. maritima suggests that these are unlikely to function as a chemoreceptors. Using electrophysiologically bioassays, Schneider and Steinbrecht (1968) and Cuperus (1983) demonstrated response of sensilla coeloconica on antennae of several insect species to CO2, temperature, and humidity. Our data also suggest the a porous sensilla coeloconica of A. maritima possibly may function as thermo-hygroreceptors.
The campaniform organ play a role as sound and mechanoreceptor (Romoser and Stoffolano, 1998). These were found in both sexes. This organ has been observed in many insects in different parts as a mechanoreceptors between the 9th and 10th segment of flagellum of Conotrachlus nenuphar as proprioception (Alm and Hall, 1986). Also Whitehead (1981) recorded campaniform organ on the antennae of female of Dendroctonus ponderosae. Merivee et al. (2003) mentioned this organ as temperature receptor in Pterostichus aethiops, these are located at the terminal segment of the antennae of both males and females. Maher and Thiery (2004) mentioned that campaniform sensilla at the 5th segment of the tarsus of Lobesia botrana moth for chemoreception for semiochemicals emanating from plants.
Placoid sensillae has been found in various sizes on the antennae of nearly all parasitic Hymenoptera (Ochieng et al., 2000; Roux et al., 2005). These were found in numerous number and size of both sexes of A. maritima. The function of sensilla placodea is assumed to be olfactory because they posses a multiple cuticular pore system, (Stutcliffer and Mitchell, 1980; Symondson and Williams, 1997) found similar these sensillae on the maxillary palps of Pterostichus melanarius with a raised orifice and Pterostichus niger as a flat or sunken orifice, these are probable chemoreceptors, and may indicate their role in host location, possibly in the detection of host-related semiochemicals (Onagbola and Fadamiro, 2007; Yang et al., 2009).
References
- Antennal sensory structures of Conotrachelus nenuphar (Coleoptera: Curculionidae) Ann. Entomol. Soc. Am.. 1986;79:324-333.
- [Google Scholar]
- Poreless sensilla with inflexible sockets: a comparative study of a fundamental type of sensilla probably comprising thermo- and hygroreceptors. Cell Tissue Res.. 1983;234:279-307.
- [Google Scholar]
- External morphology of antennal sensilla of Trichogramma australicum Girault (Hymenoptera: Trichogrammatidae) Int. J. Insect Morphol. Embryol.. 1998;27(2):67-82.
- [Google Scholar]
- American Insects. A Handbook of Insects of America North of Mexico. Gaines: The Sandhill Crane Press Inc.; 1993. p. 850
- Ultrastructure of the antennal sensilla of the cockroach-egg parasitoid. Tetrastichus hagenowii (Hymenoptera: Eulophidae) J. Morphol.. 1981;168:97-108.
- [Google Scholar]
- Antennal sensilla of the adult alfalfa weevil Hypera postica (Gtyllenhal) (Coleoptera: Curculionidae) Int. J. Insect Morphol. Embryol.. 1981;10(3):265-274.
- [Google Scholar]
- Antennal sensilla of two parasitoid wasps: a comparative scanning electron microscopy study. Microsc. Res. Techniq.. 2004;63:266-273.
- [Google Scholar]
- The type of Dermaptera described by Fabricius. Entomol. Record J. Var.. 1981;93(1):14-16.
- [Google Scholar]
- The Insects Structure and Function (fourth ed.). Cambridge University Press; 1998. p. 770
- Distribution of antennal sense organs in male and female ermine moth Yponomeuta vigintipunctatus (Retzius) (Lepidoptera: Yponomeutidae) Int. J. Insect Morphol. Embryol.. 1983;12:59-66.
- [Google Scholar]
- The role of the antennae and maxillary palps in mediating food preference by larvae of the tobacco hornworm Manduca sexta. Entomol. Exp. Appl.. 2006;119:29-38.
- [Google Scholar]
- Why do animals have many receptors? The role of multiple chemosensors in anima perception. Biol. Bull.. 2001;200:211-215.
- [Google Scholar]
- Sex some parasitoid hymenoptera with hypothesis on their role sex and host recognition. J. Hymen. Res.. 1991;5:206-239.
- [Google Scholar]
- Distribution of chemo- and mechanoreceptors on the tarsi and ovipositor of female European grapevine moth Lobesia botrana. Entomol. Exp. Appl.. 2004;110(2):135-143.
- [Google Scholar]
- Electrophysiological identification of cold receptors on the antennae of the ground beetle Pterostichus aethiops. Physiol. Entomol.. 2003;28(2):88-96.
- [Google Scholar]
- Hawaiian Terrestrial Arthropod Checklist (fourth ed.). Honolulu (HI): Biological Survey, Bishop Museum; 2002.
- Antennal sensilla of three parasitic hymenoptera. Int. J. Insect Morphol. Embryol.. 1974;3:305-316.
- [Google Scholar]
- Functional morphology of antennal chemoreceptors of the parasitoid Microplitis croceipes (Hymenoptera: Braconidae) Arthropod. Struct. Dev.. 2000;29:231-240.
- [Google Scholar]
- Scanning electron microscopy studies of antennal sensilla of Pteromalus cerealellae (Hymenoptera: Pteromalidae) Micron. 2007;39(5):526-535.
- [Google Scholar]
- Morphological characterization of the antennal sensilla of the Asian citrus psyllid, Diaphorina citri kuwayama (Hemiptera: Psyllidae), with reference to their probable functions. Micron. 2008;39(8):1184-1191.
- [Google Scholar]
- The Science of Entomology. Boston, Massachusetts Burr Ridge, Illinois Dubuque, Iowa Madison, Wisconsin New York San Francisco, California St. Louis, Missouri: McGraw-Hill; 1998.
- Antennal structure and oviposition behaviour of the sprcialist parasitoid: Cotesia plutellae. Microsc. Res. Techniq.. 2005;68:36-44.
- [Google Scholar]
- The external sensory morphology of the legs and hairplate system of female Trichogramma minutum Riley (Hymenoptera: Trichogrammatidae) Proc. R. Soc. London, Ser. B Biol. Sci.. 1987;232:323-366.
- [Google Scholar]
- Ultra morphological characteristics of sensory sensillae on the legs and external genitalia of the red palm weevil Rhynchophorus ferrugineus (Oliv.) Saudi J. Bio. Sci.. 2007;14(1):29-36.
- [Google Scholar]
- Associative learning of plant odorants activating the same or different receptor neurones in the moth Heliothis virescens. J. Exp. Biol.. 2005;208:787-796.
- [Google Scholar]
- Arthropods: chemo–thermo, and hygro-receptors. In: Bereiter-Hahn J., Matolsty A.G., Richards K.S., eds. Biology of the Integument. In: Bereiter-Hahn J., Matolsty A.G., Richards K.S., eds. vol. 1. Berlin: Springer-Verlag; 1984. p. :523-553.
- [Google Scholar]
- Functional morphology of pheromone-sensitive sensilla. In: Prestwich G.D., Blomquist G.J., eds. Pheromone Biochemistry. Orlando: Academic Press; 1987. p. :353-383.
- [Google Scholar]
- Pore structures in insect olfactory sensilla: a review of data and concepts. Int. J. Insect Morphol. Embryol.. 1997;26:229-245.
- [Google Scholar]
- Structure of galleal sensory complex in adults of the red turnip beetle Entomocelis Americana Brown (Coleoptera: Chrysomelidae) Zoomorphology. 1980;96:63-76.
- [Google Scholar]
- Low-vacuum electron microscopy of carabid chemoreceptors: a new tool for the identification of live and valuable museum specimens. Entomol. Exp. Appl.. 1997;85:75-82.
- [Google Scholar]
- Comparison of antennal sensilla of Anaphes victus and A. listronoti (Hymenoptera: Mymaridae), egg parasitoids of pseudococcid mealybugs. Can. J. Zool. (74):710-720.
- [Google Scholar]
- Sensilla numbers and antennal morphology of parasitic and non-parasitic bees (Hymenoptera: Apoidea) Int. J. Insect Morphol. Embryol.. 1995;24:63-81.
- [Google Scholar]
- Ultrastructure of sensilla of the female mountain pine beetle Dendroctonus ponderosae Hopkins (Coleoptera: Scolytidae) Int. J. Insect Morphol. Embryol. 1981;10(1):19-28.
- [Google Scholar]
- Ultrastructural observations on antennal sensilla of Colephora obducta (Meyrick) (Lepidoptera: Coleophoridae) Micron. 2009;40:231-238.
- [Google Scholar]
- Insect of Hawaii Vol. 1: Introduction. Honolulu: University of Hawaii; 2001.
Further reading
- From sampling and baiting indicate European earwig (Forficula anricularia) foraging in chards. J. Appl. Entomol.. 2006;130(5):263-267.
- [Google Scholar]







