Translate this page into:
Discussion on bone healing rate and expression of cytokines and growth factors in rat models with simple fracture and brain injury
⁎Corresponding author. Emergency Truma Surgery, South Court of East Hospital, 1800 Yuntai Road, Pudong New Area, Shanghai 200120, China. chenchunhuasceh@yeah.net (Chunhua Chen)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
This research aims to analyze the differences in bone healing rate, cytokines content, and growth factor content in rats with fracture and traumatic brain injury. In this study, rat models of tibial fracture (fracture group) and tibial fracture combined with brain injury (combined group) were first constructed. Callus and brain tissue sections of the two groups of rat models were made, and the pathological changes were analyzed by hematoxylin – eosin staining. Tissue sections were made to compare the differences in callus volume, trabecular width, and trabecular bone ratio between the two groups of rats. Taking normal rats as controls, serum samples were collected from rats in the control group, fracture group, and combined group. The differences of levels in nerve growth factor (NGF), insulin-like growth factor 1 (IGF1), bone morphogenetic protein 7 (BMP7), and transforming growth factor β1 (TGFβ1) in serum of different groups of rats were detected by enzyme-linked immunosorbent assay, and the expression of NGF, IGF 1, BMPR7, and TGFβR1 in rat callus was detected by Western-Blot. It was found that the rate of bone healing in the combined group was faster than that in the fracture group. The bone callus volume, trabecular width, and trabecular proportion of the rats in the combined group were significantly higher than those in the fracture group (P < 0.05). The content of NGF, IGF1, BMP7, and TGFβ1 in the serum of the fracture group and the combined group and the expression of their receptor proteins were significantly higher than that of the control group (P < 0.05), but the content of NGF, IGF1, BMP7, and TGF 1 in the serum and the expression of their receptor proteins of the combined group were significantly higher than that of the fracture group (P < 0.05). Accordingly, tibial fractures combined with brain injury can accelerate bone healing, which may be caused by regulating the expression of NGF, IGF1, BMP7, and TGFβ1 and accelerating the binding of its receptors.
Keywords
Fracture of tibia
Fracture with brain injury
Callus
NGF
BMP7
1 Introduction
With the rapid development of the transportation industry and the wide application of mechanical equipment, the incidence of injuries and fractures caused by external factors such as traffic and machinery is also increasing. The healing cycle of fracture is long, and the probability of fracture non-healing is high in severe cases. This condition may lead to the prolonged healing time of patients and also cause great economic burden to patients (Ono and Takayanagi, 2017). Therefore, it is of great significance to explore ways to accelerate the rate of bone healing and repair in patients with fracture to reduce the probability of disability caused by fracture in our clinic, which is also the key issue that we need to study. In clinical diagnosis and treatment, patients with fracture are often accompanied by traumatic brain injury. However, compared with patients with simple fracture, patients with brain injury have faster fracture healing speed and ectopic ossification, while the incidence of ectopic ossification in patients with brain injury is as high as 25% (Huang et al., 2018).
Fracture healing refers to the process of repair after the physiological results and functions of the bone are damaged, which is jointly affected by nerve regulation and humoral regulation (Pietsch et al., 2018). A large number of cytokines and nerve factors are expressed in the process of bone healing, and previous studies have shown that NGF and igf-1 play an important role in accelerating the rate of bone healing in patients with brain injury combined with fracture (Chisalita et al., 2017). BMP7 and TGF β1, respectively, are of great significance for the proliferation and differentiation of cartilage and osteoblasts, the formation of new bone, tissue repair and other processes (Sreekumar et al., 2017). When the concentration of cytokines and growth factors secreted by the relevant body increases, the efficiency of these factors to bind to the receptors on the cell surface of the fracture or damaged site increases, thus accelerating the process of fracture repair.
Therefore, in order to study the reasons for the accelerated bone healing rate of fracture combined with traumatic brain injury, the rat models of simple fracture and fracture combined with brain injury were constructed in the research, respectively, and the callus tissue sections and brain tissue sections of the two groups of models were made for observation and analysis. The differences of callus volume, trabecular width and trabecular proportion between the two groups were compared. The serum concentrations of NGF, IGF1, BMP7 and TGF β1 in normal rats and the two groups of model rats were also detected. The results provide a theoretical basis for the involvement of cytokines and nerve factors in the accelerated process of bone healing in fractures complicated with traumatic brain injury.
2 Materials and methods
2.1 Experimental animal
A total of 40 clean grade 8-week-old male SD rats with an average weight of 205 ± 20 g were selected for this study. All the rats were purchased from Shanghai slack experimental animal Co., LTD. The rats were kept separately in a clean animal feeding laboratory where the temperature was maintained at 22 ± 2 °C and 65 ± 5% relative humidity. The rats were kept under continuous lighting for 12 h every day and free to eat and drink. All the rats were fed basic feed. Moreover, the procedure and feeding of this study are in accordance with the relevant regulations formulated by the ethics committee of our hospital.
2.2 Construction of tibial fracture model in rats
In this study, 15 healthy rats were randomly selected as experimental models for the tibial fracture group alone. After weighing the weight of the modeling rats, 1% pentobarbital sodium was injected intraperitoneally at a dose of 40 mg/kg to anesthetize the rats. After the rats were completely anesthetized, the rats were fixed on the operating table, the hair on the left leg of the rats was shaved off, and the surgical area was sterilized with iodine. The anterior lateral leg of the rat was dissected and exposed along the muscle line of the calf to the tibia of the rat. In the middle position of tibial disruption in rats, the transverse fracture of tibia was caused by using orthopaedic wire saw. Then, the 0.8 mm kirschner wire after sterilizing treatment was reversely inserted into the bone marrow of the rats with an electric drill, and the fracture position was fixed and the stability was checked. After completion, saline was used to irrigate the wound site, and 3 drops of 8 × 104U gentamicin were added to the incision. After the incision was sutured with full layer, erythromycin ointment was evenly applied. After the operation, the mice were put back into the cage to continue feeding and observe whether the wound infection occurred in the rats.
2.3 A model of tibial fracture with traumatic brain injury in rats
In this study, 15 healthy rats were randomly selected as the experimental model group of tibial fracture with brain injury, and the remaining 10 healthy rats were the control group. The rats were given intravenous injection of 1% pentobarbital sodium at a dose of 40 mg/kg. After the rats were completely anesthetized, the rats were fixed on the operating table, the hair on the top of the head was shaved off, the central part of the head was cut open, and the periosteum was stripped off to reveal the parietal bone. After avoiding the important vessels, the parietal bone was cut with a dental drill about 5 mm, but the integrity of the dural membrane should not be destroyed. Free fall with 20 g weight was carried out to cause moderate brain damage in rats. At the incision, 5 drops of 8 × 104U gentamycin were added, and the parietal orifice was closed with bone wax. Apply erythromycin ointment evenly after full - thickness suture of the incision. After the operation, the mice were put back into the cage and the rats were observed whether the symptoms of activity inhibition, slow movement and brain contusion were observed. The model of tibial fracture in rats was constructed according to the steps 2.2, and the rats were put back into the cage after awake to continue feeding. The model of tibial fracture combined with cerebral trauma in rats was obtained. The severity scale of craniocerebral trauma (NSS) was used to evaluate the degree of nerve injury in rats. The scoring criteria included degree of autonomous activity, hemiplegia of the left forelimb, inability to extend the left forelimb when lifting the tail, ability to resist lateral push, inclination to the left, and torus to the left. 0 point indicated normal neural function; 1 point indicated mild neurological injury (left forelimb flexes when lifting tail); 2 points indicated moderate neurological impairment (turning to the left while walking); 3 points indicated that the nerve function of the rats was severely impaired (tilting to the left); 4 points showed no spontaneous walking and decreased consciousness; and 5 points were ischemia-related deaths.
2.4 Collection of rat samples
After the rats were anesthetized with the same method, the abdominal vein blood of the rats was collected at modeling 0d, 3d, 1w, 2w and 4w. After the anticoagulant treatment with heparin sodium, the serum was centrifuged at 4500 rpm for 5 min and then placed in a new 1.5 ml centrifuge tube treated with autoclaved sterilization and stored in the refrigerator at −20 °C. After blood collection, the rats were sacrificed. After X-ray observation of the fracture healing of the rats, the bone callus tissues and the whole brain tissues of the 4w rats were dissected and soaked in formalin solution for subsequent hematoxylin - eosin (HE) staining analysis.
2.5 HE staining of rat tissue specimens
The bone callus and brain tissues were modeled by paraffin immersion in the rats. The paraffin wrapped tissues were cut into 3 μL thick sections with a slicer. Xylene solution was used to soak the tissue sections for 2 times ×10 min to dewax the sections. The tissue was soaked in 100%, 95%, 80% and 70% alcohol twice for 5 min, and then soaked in distilled water for 1 min for gradient rehydration. The tissue was soaked in hematoxylin dye for 5 min and then washed. Then it was immersed in eosin dye for 5 min and washed with water. Soak the tissue with 85%, 90%, 95% and 100% alcohol to dehydrate the tissue. The xylene was soaked until the tissue was transparent, and then the tablet was sealed. Finally, the pathological changes of callus and brain tissue were observed and analysed under optical microscope.
2.6 The expression of related factors and their receptors in rat serum
The contents of NGF, IGF1, BMP7 and TGF β1 genes in serum of rats were detected by ELISA, which were divided into blank group, control group and experimental group. The control group was added 100 μL 1:1 mixture of standard substance and streptavidin, while the experimental group was added 100 mu L 1:1 mixture of serum and streptavidin. 10-µm NGF, IGF1, BMP7 and TGF β1 antibodies were added to the mixture and sealed at 37 °C for 1 h. After dilution of washing solution at 1:20, add it into the sample hole and let it stand for 5 times ×30 s. After that, the coloring agent was added at 37 °C to avoid light for 10 min. After the termination solution was added to terminate the reaction, the OD value of the sample was detected with an enzyme marker. Western blotting was used to detect the protein expressions of NGF, IGF1, BMP7 and TGF β1 gene receptors in the callus tissues of 4w different rat models, and the gray value of protein expression was calculated.
2.7 Statistical treatment
In this research, SPSS19.0 software was used for statistical analysis, and all the data obtained from the experiment were expressed as mean ± standard deviation. The expression of different genes detected by ELISA was expressed as percentage. Univariate analysis of variance (ANOVA) was used for statistical treatment among different groups. The comparison between the two groups was statistically processed by independent sample t-test. The difference was statistically significant when P < 0.05.
3 Results
3.1 Effects of brain injury on nerves in rats
The nerve injury of rats with tibial fracture combined with brain injury was examined at 0d, 3d, 1w, 2w and 4w after the establishment of the model, and the NSS score scale was used to evaluate the degree of nerve injury in the control group and tibial fracture with brain injury model group, as shown in Fig. 1. It indicated that there was no significant difference in brain NSS score between the control group and the modeling group on modeling day 0d (P > 0.05). However, at 3d, 1w, 2w and 4w after modeling, NSS scores of rats in the modeling group were significantly lower than those in the control group (P < 0.05), and the degree of nerve damage in the brain injury model of rats had become stable after modeling 3d, indicating that the previous model of brain injury of rats was successful.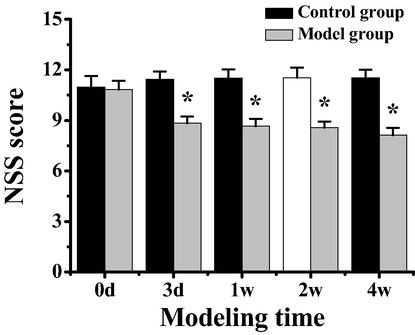
NSS score of rats in the control group and the model group of tibial fracture combined with brain injury. Note: *indicated significant difference between the two groups (P < 0.05).
3.2 Results of HE staining of callus tissue in rats
Paraffin sections of callus tissues of rats in the tibial fracture group and the tibial fracture group combined with brain injury were made and stained with HE. After staining, observation sections were made to analyse the histopathological differences of callus tissues of rats in different groups, and the results were shown in Fig. 2. Fig. 2A shows the callus section of rats in the tibial fracture group after 3d modeling. It indicated that there was no tissue proliferation in the callus tissues of rats in the fracture group and no mature bone trabecular structure was formed in the fracture site of inflammatory cells, and the distribution of osteoblasts was relatively disorderly. Fig. 2B shows the 3d callus sections of rats in the tibial fracture group with brain injury after modeling, and it showed that compared with the rats in the fracture group, a small part of bone trabecular transformation has occurred in the callus tissues of rats in the combined group. Fig. 2C shows the callus section of 4w after modeling in rats in the tibial fracture group. It shows that the trabecular bone has been transformed into osteoblastic tissue, but the lamellar bone tissue is not mature yet. Fig. 2D shows the 4 W callus sections of rats in the tibial fracture group with brain injury after modeling, and it shows that compared with the rats in the fracture group, mature lamellar bone tissue has appeared in the rats in the combined group.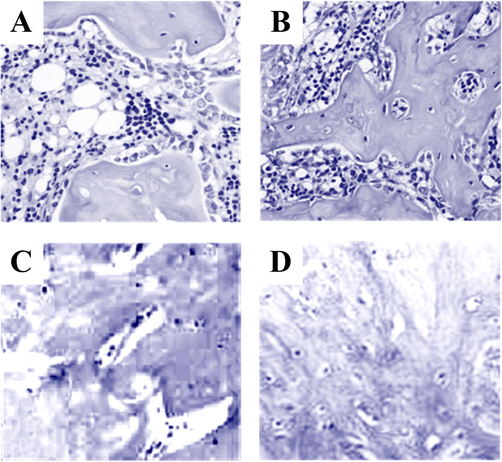
HE staining results of callus tissues in different rat models Note: A was the callus staining result of tibial fracture group in 3d modeling; B was the callus staining result of tibial fracture combined with brain injury in 3d modeling; C was the callus staining result of the tibial fracture group at modeling 4 W. D was the callus staining results of the group with tibial fracture combined with brain injury at the modeling time of 4 W.
3.3 Results of bone callus related parameters in rats
The changes of bone callus volume (BV), average trabecular width (TW), and bone trabecular area ratio (TV%) in rats after constructing the model of tibial fracture and tibial fracture combined with brain injury were detected. According to Fig. 3, the ratio of callus volume to trabecular area in the rat model of tibial fracture combined with brain injury was significantly higher than that in the rat model of tibial fracture combined with brain injury (P < 0.01), and the average width of trabecular bone in the rat model of tibial fracture combined with brain injury was significantly higher than that in the rat model of tibial fracture combined with brain injury (P < 0.05).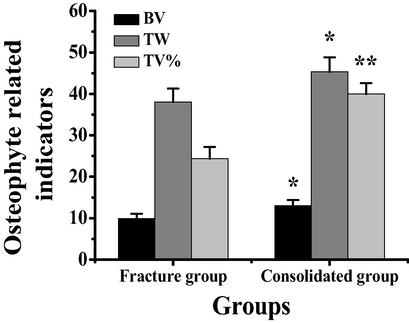
Comparison of callus parameters between tibial fracture and fracture with brain injury in rats. Note: *indicated significant difference between the two groups (P < 0.05); ** meant the difference was very significant (P < 0.01).
3.4 Results of HE staining in rat brain tissue
The brain tissues of rats in the tibial fracture group and the tibial fracture group combined with brain injury were sectioned with paraffin and stained with HE, as shown in Fig. 4. According to Fig. 4A, the brain tissues of the rats in the tibial fracture group were normal, and the neurons showed triangular shape after staining, the nuclei were nearly round, and the glial cells were darker in colour. According to Fig. 4B, the brain cells of rats with tibial fracture combined with brain injury showed swelling and inflammatory cell infiltration.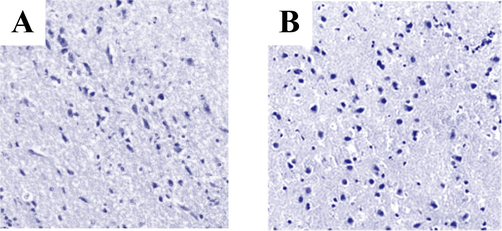
HE staining results of rat brain tissue. Note: figure A showed brain sections of rats in tibial fracture group. Figure B showed brain sections of rats with tibial fracture and brain injury.
3.5 Detection results of different factors in rat serum
The serum concentrations of NGF, IGF1, BMP7 and TGF β1 were detected at 0d, 3d, 1w, 2w and 4w in the control group, tibial fracture group and tibial fracture combined with brain injury group. According to Fig. 5, with the advancement of the modeling time, NGF, IGF1, BMP7, and TGF β1 levels tended to rise before falling in the serum of rat models of the 3 groups. According to Fig. 5A, NGF concentration in fracture group was significantly higher than that in control group at 3d, 1w and 2w after modeling (P < 0.01), and that at 4w after modeling was significantly higher than that in control group (P < 0.05). After modeling, the NGF concentration in the fracture group with brain injury was significantly higher than that in the control group at 3d, 1w, 2w and 4w (P < 0.01). Moreover, the NGF concentration in the fracture group with brain injury was significantly higher than that in the fracture group at 3d, 1w and 4w after modeling (P < 0.05), and the NGF concentration in the fracture group with brain injury was significantly higher than that in the fracture group after modeling (P < 0.01).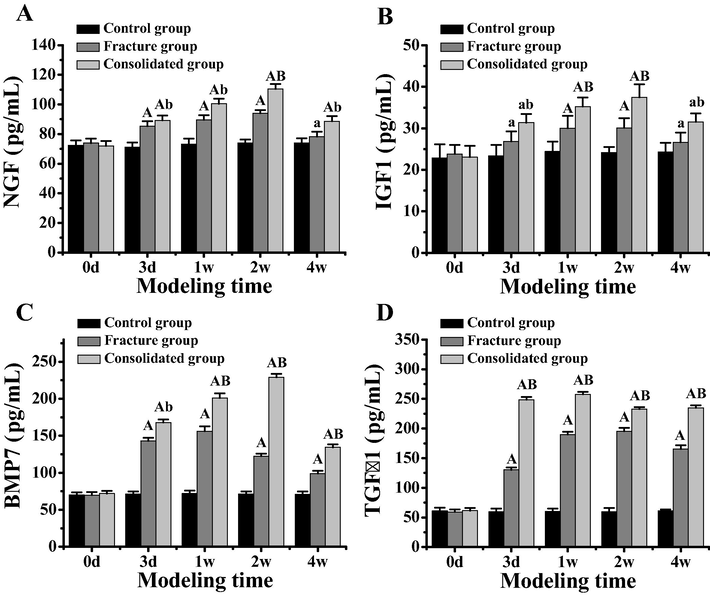
Comparison of serum concentration of related factors in different rat models. Note: figure A showed the difference of NGF concentration in serum of different rat models. Figure B showed the difference of serum IGF1 concentration in different rat models. Figure C showed the difference of serum BMP7 concentration in different rat models. Figure D showed the difference of TGF β1 concentration in serum of different rat models. In the figure A, the difference was extremely significant compared with the control group (P < 0.01). In the figure C, the difference was significant compared with the control group (P < 0.05). In the figure B, the difference between the tibial fracture group and the tibial fracture group is extremely significant (P < 0.01). In the figure D, there was a significant difference between the tibial fracture group and the tibial fracture group (P < 0.05).
According to Fig. 5B, the IGF1 concentration of the fracture group and fracture brain injury group was significantly higher than that of the control group at 3d and 4w after modeling (P < 0.05), and the IGF1 concentration of the fracture group and fracture brain injury group was significantly higher than that of the control group at 1w and 2w (P < 0.01). However, IGF1 concentration in the fracture group with brain injury was significantly higher than that in the fracture group at 3d and 4w (P < 0.05), and IGF1 concentration in the fracture group with brain injury at 1w and 2w was significantly higher than that in the fracture group (P < 0.01).
According to Fig. 5C, BMP7 concentration in the fracture group and the fracture group combined with brain injury was significantly higher than that in the control group at 3d, 1w, 2w and 4w after modeling (P < 0.01). The concentration of BMP7 in the fracture group with brain injury was significantly higher than that in the fracture group (P < 0.05). At 1w, 2w and 4w, IGF1 concentration in the fracture group with brain injury was significantly higher than that in the fracture group (P < 0.01).
According to Fig. 5D, TGF β1 concentration in the fracture group and the fracture combined with brain injury group was significantly higher than that in the control group at 3d, 1w, 2w and 4w after modeling (P < 0.01). Moreover, at 3d, 1w, 2w and 4w after modeling, TGF β1 concentration in the fracture group with brain injury was significantly higher than that in the fracture group (P < 0.01).
3.6 Protein detection results of different factor receptors in rats
The receptors of NGF, IGF1, BMP7 and TGF β1 genes in the bone callus of rats in the control group, the tibial fracture group alone and the fracture group combined with brain injury were detected, and the results were shown in Fig. 6. Compared with the control group, the protein expressions of NGFR, BMPR7 and TGF β R1 in the model group significantly were increased (P < 0.01), while the protein expressions of IGFR1 were significantly increased (P < 0.05). Compared with the rats in the tibial fracture group alone, the protein expressions of NGFR and TGF β R1 in the combined group were significantly increased (P < 0.01), while the protein expressions of BMPR7 and IGFR1 were significantly increased (P < 0.05).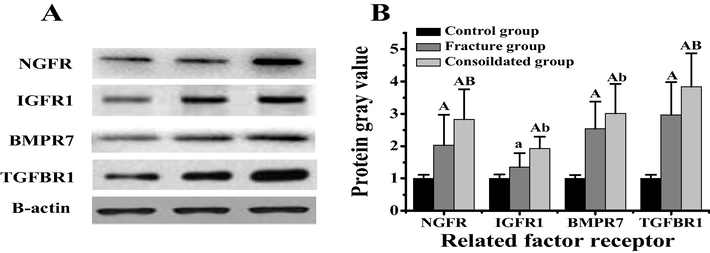
Protein expression differences of related factor receptors in callus tissues of different rats. Note: figure A was the Western bolt diagram of related factor receptors. Figure B showed the difference in the gray value of related factor receptors. In the figure A, the difference was extremely significant compared with the control group (P < 0.01). In the figure B, the difference was significant compared with the control group (P < 0.05).
4 Discussion
The probability of traumatic brain injury combined with limb fracture accounts for about 10% of current traumatic patients. It is also found that patients with traumatic brain injury combined with fracture show a faster growth trend of callus at the fracture site compared with patients with simple fracture, and finally the callus volume is larger, resulting in slower recovery of injury (Suto et al., 2018). Some experts and scholars believe that after brain injury, it will be regulated by changes in the concentration of endocrine hormones or certain cells and nerve factors (Dehghan et al., 2017). To study and compare the pure fractures and traumatic brain injury combined fracture between the readjustment of the difference of bone healing and involved factors, the model of rat tibia fracture merger with tibial fractures in rats brain damage model were constructed and analysed. Through different model rats nerve injury score, brain tissue biopsy and callus tissue staining analysis, various models of rats were successfully constructed, and the model has a good stability, which laid the foundation for follow-up study. After comparing the callus volume, trabecular width and trabecular proportion of the rats in the fracture group and the combined group, it was found that the callus volume, trabecular width and trabecular proportion of the rats in the fracture group and the combined group were significantly higher than the rats in the fracture group combined with brain injury (P < 0.05). It shows that fracture combined with brain injury can improve the recovery speed of fracture, which is consistent with Graef's study that fracture healing with traumatic brain injury has larger callus volume and faster bone healing speed (Graef et al., 2017).
After traumatic brain injury combined with fracture, there will be systemic or local regulation of nerves and body fluids, and multiple cytokines and nerve regulation participate in the process of bone healing. In order to study the nerve growth factor, IGF1, BMP7 and TGF β1 role in the fracture merge brain damage to accelerate bone healing, the normal group, the simple fracture merge brain injury in rats and fractures in serum of rats were collected, and by using enzyme-linked immunosorbent experiment tested the NGF plays in the three groups of rats serum, IGF1, BMP7 and TGF β1 and its receptor protein expression differences. The results showed that the serum concentrations of NGF, IGF1, BMP7 and TGF β1 in the combined group were significantly higher than those in the fracture group (P < 0.05). NGF, and as a nerve growth factor, NCF plays an important role in regulating the growth and differentiation of nerve fibres in damaged parts of the brain. In addition, previous studies have shown that high concentration of NGF can promote the growth of bone callus in fractures and improve the rate of fracture repair, which is basically consistent with the results of this study (Zhuang and Li, 2013). IGF1 can promote the growth of cartilage in the body, and when it is combined with IGFR1 of osteoblasts, it can accelerate the differentiation of osteoblasts. BMP7 plays an important role in induction and new bone formation. Previous studies have shown that the up-regulation of BMP7 gene expression is accompanied by the acceleration of bone healing rate in the fracture combined with brain injury model, which is consistent with the results of this study (Yan et al., 2018). TGF β1 binds to cell surface receptors and regulates cell proliferation and differentiation, playing an important role in bone healing after fracture.
In conclusion, the simple fracture and fracture merge brain injury model of rats was constructed and it indicated that the fracture merge brain injury model of bone heals faster than simple fracture group, and that the fracture merge brain injury model NGF plays in serum, IGF1, BMP7 and TGF β1 concentrations were higher than in simple fracture group, suggesting that the accelerated rate of bone healing associated with fracture combined with brain injury may be closely related to the process of increasing binding efficiency with cell receptors by up-regulating the expressions of NGF, IGF1, BMP7 and TGF β1. The results of this study can lay a foundation for the follow-up research on the process of bone healing of fracture combined with brain injury.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Association of insulin-like growth factor-1, bone mass and inflammation to low-energy distal radius fractures and fracture healing in elderly women attending emergency care. Orthopaedic Surg.. 2017;9(4):380.
- [Google Scholar]
- Does the administration of melatonin during post-traumatic brain injury affect cytokine levels? Inflammopharmacology. 2017;26(8):1-7.
- [Google Scholar]
- Impaired fracture healing with high non-union rates remains irreversible after traumatic brain injury in leptin-deficient mice. J. Musculoskelet. Neuronal Interact.. 2017;17(2):78-85.
- [Google Scholar]
- Relationship between heterotopic ossification and traumatic brain injury. J. Orthopaedic Trans.. 2018;12:16-25.
- [Google Scholar]
- Modelling the fracture-healing process as a moving-interface problem using an interface-capturing approach. Comput. Methods Biomech. Biomed. Eng.. 2018;21(8):512-520.
- [Google Scholar]
- BMP9 a possible alternative drug for the recently withdrawn BMP7? New perspectives for (re-)implementation by personalized medicine. Arch. Toxicol.. 2017;91(3):1-14.
- [Google Scholar]
- A concomitant bone fracture delays cognitive recovery from traumatic brain injury. J. Trauma Acute Care Surg.. 2018;85:1.
- [Google Scholar]
- BMP7-overexpressing bone marrow-derived mesenchymal stem cells (BMSCs) are more effective than wild-type BMSCs in healing fractures. Exp. Therapeutic Med.. 2018;16:1381-1388.
- [Google Scholar]
- Serum EGF and NGF levels of patients with brain injury and limb fracture. Asian Pacific J. Tropical Med.. 2013;6(5):383-386.
- [Google Scholar]







