Translate this page into:
Different drying techniques effect on the bioactive properties of rose petals
⁎Corresponding author. masifa@ksu.edu.sa (Mohammed Asif Ahmed)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
This study explored the effect of different drying methods (sun, shade, oven) on the total polyphenol content (TPC) using the Folin–Ciocalteu (FC) procedure, total flavonoid content (TFC) using the AlCl3 colorimetric method, volatile organic compounds using GC/MS and the antioxidant properties of the rose petals using the protocol of DPPH assy. The results demonstrated that the drying methods had a significant impact and the highest TPC (34.24 mg gallic acid equivalent (GAE)/ g fresh weight (FW)) and TFC (5.56 mg catechin equivalent (CE)/g FW) were obtained for the oven-dried sample. While, the fresh sample exhibited the lowest TPC (15.6 mg GAE/g FW) and TFC (3.83 mg CE/g FW), respectively. Similarly, the oven-dried sample showed the highest DPPH scavenging activity (60.30 %) and reducing power (absorbance 1.138) among all the samples. Fresh rose sample GC–MS analysis revealed that the there are two major compounds heptacosane 64.56 % and citronellyl propionate 28.35 %. Pentadecyl 2-phenylethyl ester oxalic acid was the second dominant compound in sun and oven-dried rose samples, 18.5 % and 14.79 % respectively.
Keywords
Rose petals
Antioxidants
TPC
TFC
VOC
GC–MS
1 Introduction
Flowers, also known as blossoms or blooms are the reproductive structure of flowering plants, and their consumption has been recorded in ancient Rome, Greece, and China, both for their uses as alternative medicines or as part of traditional culinary, along with the consumption of fruits, seeds, leaves, and roots of vegetables (Takahashi et al., 2020). Despite popular belief, flowers add more than ornamentation to savory dishes and desserts, delivering a unique combination of flavors, as well as enhancing nutritional value. There are plants that are used as food for wild animals, as well as plants that have medicinal properties, produces oils and essences, which are used in perfumery and cosmetics, or in cooking (Barbieri and Stumpf, 2005). It is vital to distinguish between flower and edible flower because flowering plants may contain several toxic and antinutritional compounds such as oxalic acid, alkaloids, hemagglutinins, and cyanogenic glycosides (Lara-Cortés et al., 2013). However, various researchers have reported that until now, there is no official list dispensed by any International body, such as the Food and Drugs Administration (FDA), United Nations Food and Agriculture Organization (FAO), or European Food Safety Authority (EFSA), on edible flowers (Fernandes et al., 2017a, 2017b).
Edible parts of flowers vary from one flower to another. Generally, the petals of plants are edible, but the pollen, nectar, and other parts of some plants are also consumed (Purohit et al., 2021). Although edible flowers have been consumed for centuries, they are not as widely used as other foods. Their use in gastronomic preparations is rather restricted to special events, gourmet cuisine, or the recommendations of certain chefs. Fernandes et al., (2017a,2017b) in their review reported the benefits of edible flower for human health. It is a good source of moisture, carbohydrates, and protein but low in lipids. Also, it has several minerals, such as calcium, magnesium, potassium, iron, phosphorous zinc. Numerous studies have demonstrated that flowers contain bioactive compounds. Compounds are responsible for color is anthocyanin, not phenols. This activity is attributed to the presence of potential bioactive compounds such as flavonoids, which include flavonols, flavones, carotenes, and anthocyanins (Purohit et al., 2021). In response to various pathologies, these chemicals are crucial in lessening oxidative stress (Benvenuti and Mazzoncini, 2021). A study on 65 flowers obtained from commercial sources and parks in China aimed to determine the antioxidant capacity, total flavonoids, and total phenolic content, which exhibited a high correlation between TPC and antioxidative values (Zheng et al., 2018). Compared to pharmaceutical drugs, these metabolites are relatively weak as bioactive compounds, however, when consumed regularly and in significant quantities as part of a healthy diet, they may have a powerful physiological impact. Edible flowers offer the potential for the development of functional foods due to their chemical composition, biological properties, and the availability of innovative analytical tools to identify their chemical components (Piovesana et al., 2019; Gostin and Waisundara, 2019).
The roses are classified under the genus Rosa and the family Rosaceae. Around 100 species of genus Rosa are found throughout the Middle East, North America, Asia and Europe. They are beautifully shaped, fragrant and have different colors (Ercişli, 2005). A product's appearance usually is the deciding factor in whether it will be accepted or rejected. As a result, the first quality standard that customers examine in food is the color. Therefore, color has a high effect on the acceptance of the food product (Kramer, 1965). This plant is widely used in gardens and as cut flowers for ornamental purposes and also used to produce spices, candies, cakes, infusions, and functional foods due to their bright colors, aroma and nutritional power (Gostin & Waisundara, 2019). Furthermore, they have numerous medicinal properties. They are non-toxic, antioxidant, antimicrobial, stimulant, aseptic, antiviral, hypoglycemic, a tracheal chains relaxant, anti-coughing, hypnotizing, anti-HIV, and syrup for the stomach, heart, uterus, and liver (Kart and Çağındı, 2017; Sharma and Kumar, 2016).
Food and pharmaceutical industries consider preservation of raw materials to be their foremost concern, and drying is a widely used means to preserve these products, as removing a substantial amount of moisture reduces the possibility of microbial growth while reducing the rate of the biochemical reactions, thus extending the products shelf life at room temperature (Hincapie et al., 2014). Chemical, Nutraceutical, and organoleptic quality of dehydrated plant products is largely influenced by the duration and temperature of the drying process. It has also been reported earlier that the selection of drying method is also influenced by the raw material characteristics and the market price of the final product (Meng et al., 2018). Nevertheless, air drying is usually associated with exposure of product to high temperatures for a period of time, so few physical qualities like size, color, and texture and chemical properties for instance, taste and nutritional losses could happen. (Guiné, 2011). Some studies concerning the application of drying methods to edible flowers have been carried out on purple coneflower (Kim et al., 2000), roses, carnations (Dianthus caryophyllus L.) (Chen et al., 2000), daylilies (Tai and Chen, 2000). The cost of storing and transporting dried flowers is also lower since they require less space, weigh less, and do not need refrigeration.
Plants contain high concentrations of bioactive components with antioxidant activity. Among 30 medicinal plants, Vanderjagt et al. (2002) reported that rose had the second highest antioxidant levels and Choi et al. (2015) reported that roses possess a high level of antioxidant activity due to high level of phenolic compounds. Vinokur et al. (2006) evaluated the antioxidant properties of teas made from air-dried petals of twelve rose varieties for phenolic content, total anthocyanins, and total phenolics levels and concluded that teas made from rose petals might serve as caffeine-free drinks with high antioxidant capacities and may be combined with other herbal beverages. Generally, phenolic compounds are extracted from natural materials with solid–liquid extraction methods using various organic solvents, such as methanol, acetone, and ethanol, or a mixture of these with water (Will et al., 2008) and the extraction's efficiency depends on the extraction conditions, such as solvent, time, temperature, pressure, and so on (Franquin-Trinquier et al., 2014). Thus, the aim of this work was to investigate the effects of three different drying methods (hot-air oven drying, shade-drying, and sun drying) on the total polyphenol and flavonoid content, DPPH scavenging activity, reducing power and identification of different bioactive compounds extracted with 80 % ethanol in a solid to liquid ratio in rose.
2 Materials and methods
Four kg of rose petals were procured from a farm in Taif, Saudi Arabia. Petals were divided into 4 equal parts, the first part was dried in the sun at 38 ± 2 °C, the second part was dried in the shade, the third part was dried in a hot-air oven at 60 ± 2 °C (ED-56, Binder, Germany) and the fourth part was used fresh as control. The samples were dried to a constant weight (±0.05 g) using the related drying method.
2.1 Extraction
The rose petal samples were extracted with 80 % ethanol in a solid to liquid ratio of 1:20 on fresh weight basis using a shaker at 220 rpm for 1 h at 30 °C. The samples were then filtered using a Whatman filter paper No 2. The extracts were stored at 4 °C for further analysis.
2.2 Determination of total phenolic content (TPC)
The quantification of total phenolic contents was performed using the Folin–Ciocalteu (FC) procedure as previously reported by Alshammari et al., (2022). Methanol 4 mL was used to dissolve 1 g of sample using vortex, then the solution was filtered through Whatman No.1 filter paper. 200 µl of Folin–Ciocalteu reagent was mixed with 5 mL Milli-Q water and 100 µl sample solution, then 3 mL of sodium carbonate (Na2CO3) 20 % solution was added and incubated for 2 h at room temperature in the dark. The intensity of absorbance was recorded at 765 nm with methanol as a blank. Gallic acid standard (0–1.5 mg/mL) was used to obtain the standard curve for the calculation of total phenolic contents and results were reported as milligram gallic acid equivalent (GAE) per 100 g of sample.
2.3 Total flavonoid content (TFC)
The TFC was analyzed according to the method reported by (Salamatullah et al., 2021). In total, 250 µl of rose petal extract was mixed with 1000 µl of water and then 75 µl each of 5 % NaNO2 (w/v) and 10 % AlCl3 (w/v) was added. The prepared mixture was allowed to stand for 5 min at room temperature. After that, 600 µl of water and 500 µl of 1 M NaOH were added. Blank was prepared without extract. The absorbance was measured at 510 nm (Jasco V-630 UV–Vis spectrophotometer, Easton, MD, USA). TFC was expressed as catechin equivalent per gram dry weight of the sample (mg CE/g DW) against a catechin standard curve prepared at a concentration of 0.05–0.6 mg/ml.
2.4 DPPH radical scavenging assay (RSA)
The DPPH (1,1-diphenyl-2-picrylhydrazil, SIGMA, St. Louis, MO, USA) radical scavenging activity was determined in this study using the method of Alshammari et al., (2022). Briefly, 0.75 mL of the sample solution (0.1 g/mL) in warm water was mixed with 1.5 mL of DPPH (0.09 mg/mL) in methanol. The mixture was then incubated at 25 °C in a water bath for 5 min. The absorbance was measured at 517 nm against a blank sample consisting of sample solution with distilled water. The absorbance of a radical blank was also measured using 0.75 mL of distilled water.
The radical scavenging activity (RSA) of sample extract was expressed in terms of percentage inhibition of DPPH radical by rose petal and was calculated as follows:
Where AB = Absorbance of radical blank (DPPH, without sample).
AT = Absorbance of test sample (DPPH, with sample).
2.5 Antibacterial and anticandidal activity
Antimicrobial activity of rose extracts using different methods was determined with agar well diffusion assay against Staphylococcus aureus, Listeria monocytogenes, Pseudomonas aeruginosa, Escherichia coli, and Candida albicans (Salamatullah et al., 2021).
2.6 Determination of minimum inhibitory concentration (MIC)
The MIC of rose extracts was obtained using the previously published microbroth dilution method. (Hussein and Joo, 2018; Alyousef, 2021).
2.7 Gas chromatography-mass spectrometry (GC–MS) analysis
Analysis was carried out using gas chromatograph coupled with mass spectrometer (Turbomass) Perkin-Elmer, Auto system XL using Elite 5MS capillary column (30 mts × 0.25 mm ID). The oven temperature program was 80 °C for 5 min, rising to 310 °C at a rate of 10 °C/min. The injector and interface temperatures were kept at 260 and 250 °C, respectively. The flow rate of the carrier gas, helium, was fixed to 1.0 mL/min. In ionization mode, mass spectral scanning was performed at 40–600 (m/z). The temperatures of the source and intake lines were set to 180 and 250 °C, respectively. To identify the compounds, the spectra were compared to the National Institute of Standards and Technology (NIST, 2005 v2.1) collection.
2.8 Statistical analysis
Data was statistically analyzed using SAS (Version 9.2, 2000–2008; SAS Institute Inc., Cary, NC, USA). The experiments were independently replicated 3 times (n = 3). The data were presented as mean standard deviation (SD). If the differences were found to be significant between the groups, a post-hoc analysis using Duncan's multiple range testing was carried out. The differences between the treatment groups were examined using one-way analysis of variance (ANOVA) at a significance threshold of p ≤ 0.05.
3 Results and discussion
3.1 Effect of drying method on the total polyphenol content of rose
Fig. 1 shows the effect of drying methods on the total polyphenol content (TPC) of rose petals. The drying methods showed a significant effect on the TPC of rose samples (P < 0.05). Fresh sample showed the lowest TPC (15.6 mg gallic acid equivalent (GAE)/g fresh weight (FW)), while the highest TPC (34.24 mg GAE/g FW) was exhibited by the oven-dried sample. Statistically, there was no differences (P ≥ 0.05) between the TPC of shade-dried and sun-dried samples. The results of our study are in contrary to the findings of Barimah et al. (2017) who reported that the fresh leaves of Taraxacum officinale exhibited the highest TPC (7.78 mg GAE/g) as compared to the solar dried (4.19 mg GAE/g), hot-air oven dried (2.95 mg GAE/g), and freeze dried (4.31 mg GAE/g). In another study, the total polyphenol contents of Magnolia liliflora extracts were high after shade drying, microwave drying, and hot air drying. While the sun drying caused damage and showed a lower content (Feng-Ying et al., 2015). The total polyphenol content of rose petals ranged from 7.61−9.65 g GAE/100 g dry weight collected from 7 industrial scale plantations from Bulgaria for two consecutive growing seasons (Ginova et al., 2013). The TPC of the methanolic extract of fresh Rosa damascena flower from Turkey was reported as 276.02 mg GAE/g. Alizadeh and Fattahi (2021) studied 24 accessions of cultivated Damask rose and found that the TPC of rose petals ranged from 64.92 to 165.16 mg GAE/g dry weight. In previous work, the amount of TPC (GAE/g DW) for Damask rose petals was reported as 211.92–268.71 mg (Baydar and Baydar, 2013). The divergence in results can be attributed to the location, variety, and the extraction method (Özkan et al., 2004). Convection and vacuum-microwave drying method was studied by Matłok et al. (2020). Polyphenol content are high (208gm 100 g-1 dry matter) in the samples of pink rose. Stępień et al. (2019) reported that the convection drying at 40 °C and vacuum-microwave drying results in highest bioactive total polyphenol content and antioxidant activity.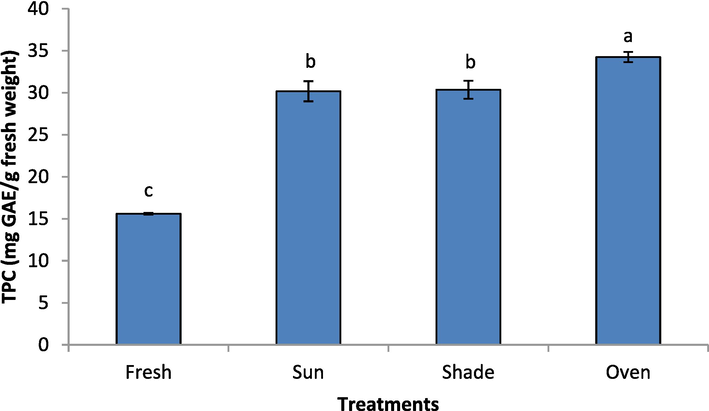
Drying methods effect on the total polyphenol content of rose.
3.2 Effect of drying methods on the total flavonoid content of rose
The effect of drying methods on the total flavonoid content (TFC) is shown in Fig. 2. The drying methods almost exhibited a similar trend for TFC as it was shown for the TPC of the rose samples. The TFC of the samples were in the following order: fresh < sun-dried < shade-dried < oven-dried, which were significantly different from each other (P < 0.05). In a recent study, the TFC of 24 accessions of cultivated Damask rose petals was reported between 28.59 and 81.35 mg Qu/g dry weight (Alizadeh and Fattahi, 2021). In another study, the TFC of Damask rose petals was reported as 28.1–98 (mg catechin equivalents/ g dry weight) (Baydar and Baydar, 2013). The total flavonoid content of the mint leaves was increased on drying compared to the fresh one (Hayat, 2020). The vacuum drying method of Bletilla striata flowers exhibited a higher TFC content than the hot-air drying, microwave drying, infrared drying natural shade drying, and freeze-drying methods (Lu et al., 2021). The hot-air drying, microwave drying, infrared drying, natural shade drying, and freeze-drying methods (Lu et al., 2021). In an earlier study it was found that the total flavonoid contents of Magnolia liliflora extracts were high after shade drying, microwave drying, and hot air drying, while the sun drying showed a lower flavonoid content (Feng-Ying et al., 2015). There are contradicting reports in the literature about the drying methods impact on plant samples. On one hand, the cell wall of plant materials may rupture during the drying process to facilitate the release of bioactive compounds into the extracting solvent (Hayat et al., 2010; Bernard et al., 2014; Hayat et al., 2019; Hayat, 2020). On the other hand, drying could cause the changes in the chemical structure of the phenolic compounds or cause them to adhere to each other components like proteins, which makes their extraction difficult (Youssef and Mokhtar, 2014). Moreover, the degradation enzymes such as polyphenol oxidase could play an important role in certain moisture content of plant materials (Orphanides et al., 2013). However, the plant species and cell wall stability have been reported to influence the effect of drying on the phenolic content of plants (Youssef and Mokhtar, 2014). Italian apple Mela Rosa dei Monti Sibillini was subjected to dehydrated by drying and freez-lyohilization methods (López et al., 2020). Flavan-3-ols, flavonol glycosides and dihyrochalcones are high in the extracts obtained by freez-lyophilization.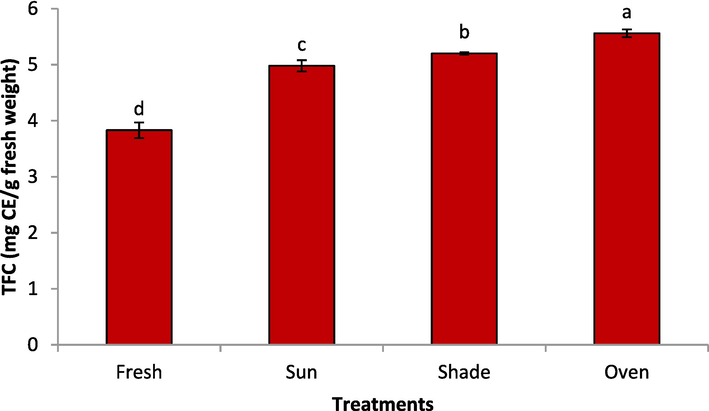
Drying methods on the total flavonoid content of rose.
3.3 Effect of drying methods on the DPPH scavenging of rose
The 2,2-diphenyl-1-picrylhydazyl scavenging activity of the rose samples under different drying methods is depicted in Fig. 3. The oven dried sample displayed the high DPPH scavenging activity, reflecting the results of TPC and TFC. (60.30 %) followed by the sample dried under shade (52.24 %). While, the lowest activity (26.66 %) was obtained for the fresh rose sample. Özkan et al. (2004) reported the DPPH antiradical activity of 74.51 % for the fresh Rosa damascena flower collected from Turkey. In a previous study, which assessed the effect of drying methods on Taraxacum officinale leaves, it was reported that fresh leaves had DPPH scavenging effect, which was significantly higher 83.71 % (freeze), 74.34 % (solar), and 69.51 % for oven dried samples (Barimah et al., 2017). Lu et al. (2021) recently studied the vacuum drying and shade drying showed higher DPPH scavenging for the Bletilla striata flowers as compared to the hot-air drying, microwave drying, infrared drying, and freeze-drying methods. The bioactive components of the fresh bitter water rose flower were better preserved by freeze-drying treatment than the hot air drying, and the resulting DPPH scavenging capacity was strong (Gao et al., 2014). Hendrysiak et al. (2023) reported the significant influence on the antioxidant capacity of rosehip (Rosa canina L.) with the addition of carrier substances.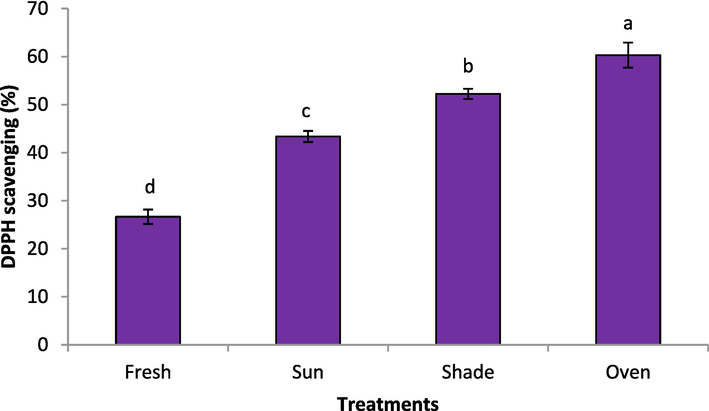
Drying methods effect on the DPPH scavenging of rose.
3.4 Effect of drying methods on the reducing power of rose
Fig. 4 represent the influence of drying procedures on the reducing power of rose samples. The reducing power followed the same pattern as the TPC activity. The highest reducing power was found for the dried rose sample by oven (absorbance 1.138), while the lowest activity (abs 0.886) was noted for the fresh sample, respectively. There was no significant difference (P > 0.05) between the reducing power of shade- (1.032) and sun-dried (0.999) samples. These results showed that the reducing power was due to at least a part of the TPC and TFC of rose sample. The antioxidant capacity of shade dried, microwave dried, and hot air-dried Magnolia liliflora was good, while sun drying caused great damage to polyphenols and flavonoids, and the antioxidant activity was low (Feng-Ying et al., 2015). The roses retained strong antioxidant activity after oven drying, but low temperature refrigeration or frozen storage accelerated the decomposition and loss of antioxidant components.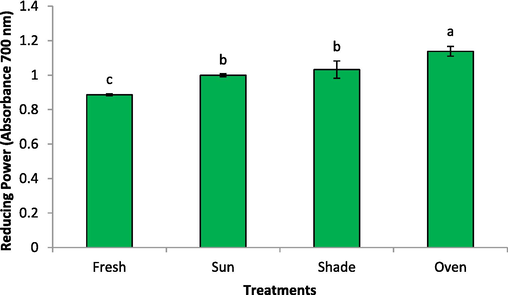
Drying methods effect on the reducing power of rose.
3.5 Antimicrobial activity of the rose extracts
Extracts of rose were also assessed for their antibacterial and antifungal activity against 4 bacterial strains and 1 Candida sp. We observed that highest antibacterial and anticandidal activity was demonstrated Fig. 5, by the extracts that were prepared from the oven dried sample followed by the extract of shade dried sample, sun dried and lowest activity was recorded for the fresh samples. After treatment with extract prepared from the oven dried sample as depicted in the figure, growth suppression zones of 16, 15, 17, 15, 14 mm were obtained against S. aureus, L. monocytogenes, E. coli, P. aeruginosa, and C. albicans, respectively. Fluconazole and doxycycline were employed as positive controls drugs, since they are both antifungal and antibacterial respectively. Microorganism are responsible for the spoilage of food leading to food-borne disease that cause severe health issues to the consumers and incur huge economic losses (Uthpala et al., 2021). Extract prepared from the oven dried rose samples demonstrated highest inhibition against the test bacteria and Candida sp. and thus could prove effective against food associated pathogens.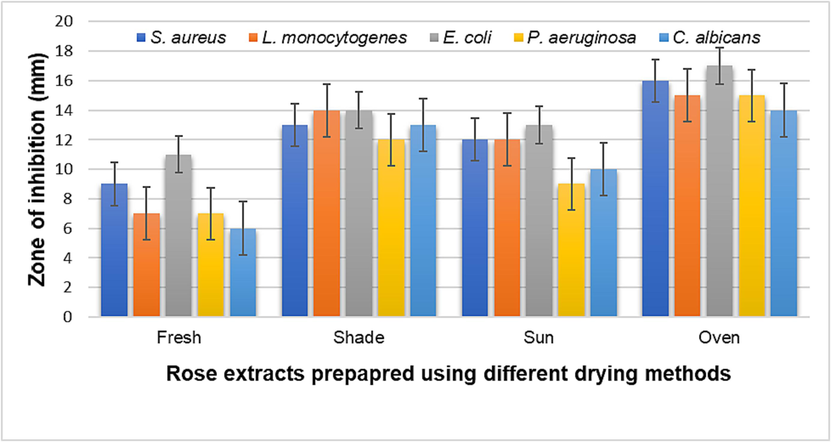
Antimicrobial activity of rose extracts subjected to different drying techniques.
The lowest concentration at which the extracts demonstrates complete growth inhibition of the bacteria is termed as its minimum inhibitory concentration (MIC) Fig. 6. We observed that lowest MICs were demonstrated by extract prepared from oven-dried sample against all test pathogens (Figure). MIC values were recorded to be 1 mg/mL against S. aureus and E. coli while 2 mg/ml against L. monocytogenes, P. aeruginosa and C. albicans. MICs ranged from 4 −16 mg/ml, 2–8 mg/ml and 4–8 mg/ml for the fresh, shade dried and sun-dried samples, respectively. Similar MIC values have been reported previously with the extract of Acmella flowers that were subjected to different drying techniques (Uthpala et al., 2021).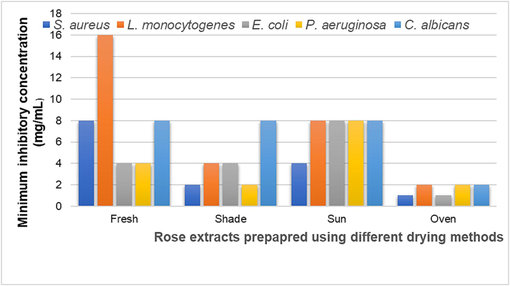
MIC of rose extracts subjected to different drying techniques against test pathogens.
3.6 Gas chromatography-mass spectrometry (GC–MS) analysis
Results of GC–MS analysis is reported in Table 1. Fresh sample shows the aliphatic hydrocarbons are the dominant compounds 64.73 %. Whereas aliphatic hydrocarbon for sun and oven dried samples are 11.69 and 8.93 %, respectively. Generally the representative aliphatic hydrocarbons for rose samples was tetracosane, heptacosane and 11-decyl-docosane in all rose samples. Major compound in sun and oven dried rose samples were Phenylethyl alcohol, 30.41 % and 40.21 % respectively.
Compound Name
Oven
Sun
Fresh
Phenylethyl alcohol
40.21
30.41
0.17
2,3-dihydro-3,5-dihydroxy-6-methyl 4H-pyran-4-one
13.91
8.30
0.00
Tetracosane
2.83
2.51
0.08
Hexadecanoic acid
3.36
4.80
0.08
Heptacosane
5.25
4.90
64.56
Octadecanoic acid
1.45
2.26
0.69
11-decyl-docosane
0.85
4.27
0.08
Citronellyl acetate
1.52
0.99
0.76
β-Sitosterol
1.04
0.75
4.91
Pentadecyl 2-phenylethyl ester oxalic acid
14.79
18.45
0.00
Citronellyl propionate
8.16
9.60
28.35
Neryl acetate
2.01
2.19
0.18
β-Phenylethyl n-decanoate
4.63
10.56
0.13
Fresh Rose sample GC–MS analysis revealed a high ratio of heptacosane 64.56 % and citronellyl propionate 28.35 %. Pentadecyl 2-phenylethyl ester oxalic acid was the second dominant compound in sun and oven dried rose samples, 18.5 % and 14.79 %, respectively. Fresh rose samples have three major compounds and other compounds are found as traces comparing with sun and oven dried samples. Higher monoterpene alcohols and lower quantity of alkanes indicate good quality of rose oil (Baser, 1992). Therefore, this agrees to our analysis for sun and oven dried rose samples, this corelates with the TPC, TFC, and DPPH scavenging activity results. However, extraction of fresh Rose samples can be considered as a good source of aliphatic hydrocarbon. Halawani (2014) reported citronellol (15.9–33.3 %) nonadecane (5.5–16.0 %) in rose oil. Ulusoy et al. (2009) reported that citronellol and geraniol are the major compounds in Turkish rose oils. El-Sharnouby et al. (2021) reported the concentrations of geraniol, phenyl ethyl alcohol, and citronellol were 8.67, 9.87, and 16.56 % respectively for rose samples grown under 500 PPM salinity water.
4 Conclusions
This study presents a significant effect of drying methods on the bioactive properties of the rose petals. The fresh sample exhibited the lowest TPC, TFC, and antioxidant activity, while the oven-dried sample demonstrated the high activity. The results suggested that the oven drying of rose petals not only could save the processing time of rose but also could enhance its bioactive properties. GC–MS analysis also revealed the same results, fresh rose has the high content of aliphatic hydrocarbons whereas sun and oven dried samples have the high amount of monoterpene alcohols. This knowledge might be useful in the preparation of rose for food and pharmacological uses.
Acknowledgment
The authors would like to extend their sincere appreciation to the Researchers Supporting Project, King Saud University, Riyadh, Saudi Arabia for funding this work through the project number (RSP-2023R437).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Essential oil, total phenolic, flavonoids, anthocyanins, carotenoids and antioxidant activity of cultivated Damask Rose (Rosa damascena) from Iran: With chemotyping approach concerning morphology and composition. Sci. Hortic.. 2021;288:110341
- [Google Scholar]
- Phenolic compounds, antioxidant activity, ascorbic acid, and sugars in honey from ingenious hail province of Saudi Arabia. Appl. Sci.. 2022;12(16):8334.
- [Google Scholar]
- Antifungal activity and mechanism of action of different parts of Myrtus communis growing in Saudi Arabia against Candida Spp. J. Nanomater.. 2021;2021:1-10.
- [Google Scholar]
- Origin, evolution and history of cultivated roses. Rev. Bras. Agroc. 2005;11(3):267-271.
- [Google Scholar]
- Effect of drying methods on phytochemicals, antioxidant activity and total phenolic content of dandelion leaves. Am. J. Food Nutr. 2017;5(4):136-141.
- [Google Scholar]
- Phenolic compounds, antiradical activity and antioxidant capacity of oil-bearing rose (Rosa damascena Mill.) extracts. Ind. Crop. Prod.. 2013;41:375-380.
- [Google Scholar]
- The biodiversity of edible flowers: discovering new tastes and new health benefits. Front. Plant Sci.. 2021;11:569499
- [Google Scholar]
- The effect of different drying methods on the phytochemicals and radical scavenging activity of Ceylon cinnamon (Cinnamomum zeylanicum) plant parts. Eur. J. Med. Plants. 2014;4(11):1324.
- [Google Scholar]
- The effects of different freeze-drying processes on the moisture content, color, and physical strength of roses and carnations. Sci. Hortic.. 2000;84(3–4):321-332.
- [Google Scholar]
- Extraction conditions for phenolic compounds with antioxidant activities from white rose petals. J. Appl. Biol. Chem.. 2015;58(2):117-124.
- [Google Scholar]
- Oil and flower production in Rosa damascena trigintipetala Dieck under salinity stress in Taif region, Saudi Arabia. Sustainability. 2021;13(8):4547.
- [Google Scholar]
- Rose (Rosa spp.) germplasm resources of Turkey. Genet. Resour. Crop Evol.. 2005;52:787-795.
- [Google Scholar]
- Effects of drying methods on chemical composition and antioxidant activity of the flowers of Magnolia liliflora. Acta Horticulturae Sinica. 2015;42(6):1150.
- [Google Scholar]
- Effect of high hydrostatic pressure (HHP) treatment on edible flowers’ properties. Food Bioproc. Tech.. 2017;10(5):799-807.
- [Google Scholar]
- Edible flowers: a review of the nutritional, antioxidant, antimicrobial properties and effects on human health. J. Food Compos. Anal.. 2017;60:38-50.
- [Google Scholar]
- Optimization of the extraction of apple monomeric phenolics based on response surface methodology: comparison of pressurized liquid–solid extraction and manual-liquid extraction. J. Food Compos. Anal.. 2014;34(1):56-67.
- [Google Scholar]
- Analysis of active components in kushui roses. Flavour Fragrance Cosmetics. 2014;2:22-26.
- [Google Scholar]
- Antioxidant capacity of petals and leaves from different rose (Rosa damascena Mill.) plantations in Bulgaria. Int. J. Pure Appl. Biosci.. 2013;1:38-43.
- [Google Scholar]
- Edible flowers as functional food: A review on artichoke (Cynara cardunculus L.) Trends Food Sci. Technol.. 2019;86:381-391.
- [Google Scholar]
- Influence of drying method on some physical and chemical properties of pears. Int. J. Fruit Sci.. 2011;11(3):245-255.
- [Google Scholar]
- Antimicrobial activity of Rosa damascena petals extracts and chemical composition by gas chromatography-mass spectrometry (GC/MS) analysis. Afr. J. Microbiol. Res. 2014;8(24):2359-2367.
- [Google Scholar]
- Impact of drying methods on the functional properties of peppermint (Mentha piperita L.) leaves. Sci. Lett.. 2020;8:36-42.
- [Google Scholar]
- Liberation and separation of phenolic compounds from citrus mandarin peels by microwave heating and its effect on antioxidant activity. Sep. Purif. Technol.. 2010;73:371-376.
- [Google Scholar]
- Effect of microwave and conventional oven heating on phenolic constituents, fatty acids, minerals and antioxidant potential of fennel seed. Ind. Crop. Prod.. 2019;140:111610
- [Google Scholar]
- Juice powders from rosehip (Rosa canina L.): physical, chemical, and antiglycation properties. Molecules. 2023;28(4):1674.
- [Google Scholar]
- Technological and functional properties of dietary fiber from mango peel var. Hilacha (Mangifera indica L) Effect of Convection Drying. Biotechnologia En Al Sector Agropecuario y agroindustrial. 2014;12(1):153-160.
- [Google Scholar]
- Antifungal activity and chemical composition of ginger essential oil against ginseng pathogenic fungi. Curr. Res. Environ. Appl. Mycol. 2018;8:194-203.
- [Google Scholar]
- Kart, D., Çağındı, Ö., 2017. Determination of antioxidant properties of dry rose tea. Int. J. Secondary Metabolite, 4(3, Special Issue 2), 384-390.
- Retention of caffeic acid derivatives in dried Echinacea purpurea. J. Agric. Food Chem.. 2000;48(9):4182-4186.
- [Google Scholar]
- Evaluation of quality of fruits and vegetables. In: Irving G.W. Jr., Hoover S.R., eds. Food Quality. Washington, DC: American Association for the Advancement of Science; 1965. p. :9-18.
- [Google Scholar]
- Nutritional content, functional properties and conservation of edible flowers. Arch. Latinoam. Nutr.. 2013;63(3):197-208.
- [Google Scholar]
- Phytochemicals and enzyme inhibitory capacities of the methanolic extracts from the Italian apple cultivar Mela Rosa dei Monti Sibillini. Pharmaceuticals. 2020;13(6):127.
- [Google Scholar]
- Drying methods affect bioactive compound contents and antioxidant capacity of Bletilla striata (Thunb.) Reichb. F. Flower. Ind. Crops Products. 2021;164:113388
- [Google Scholar]
- Influence of drying method on some bioactive compounds and the composition of volatile components in dried pink rock rose (Cistus creticus L.) Molecules. 2020;25(11):2596.
- [Google Scholar]
- Effect of drying methods on physico-chemical properties and antioxidant activity of Dendrobium officinale. J. Food Meas. Charact.. 2018;12:1-10.
- [Google Scholar]
- Effect of drying method on the phenolic content and antioxidant activity of spearmint. Czech J. Food Sci.. 2013;31:509-513.
- [Google Scholar]
- Antioxidant and antibacterial activities of Rosa damascena flower extracts. Food Sci Tech Int.. 2004;10(4):277-281.
- [Google Scholar]
- Composition analysis of carotenoids and phenolic compounds and antioxidant activity from hibiscus calyces (Hibiscus sabdariffa L.) by HPLC/DAD/MS/MS. Phytochem. Anal. 2019;30(2):208-217.
- [Google Scholar]
- A review on nutritional, bioactive, toxicological properties and preservation of edible flowers. Future Foods. 2021;4:100078
- [Google Scholar]
- Bioactive and antimicrobial properties of oven-dried beetroot (pulp and peel) using different solvents. Processes. 2021;9(4):588.
- [Google Scholar]
- Effect of temperature and storage duration of flowers on essential oil content and composition of damask rose (Rosa× damascena Mill.) under western Himalayas. J. Appl. Res. Med. Aromat. Plants. 2016;3(1):10-17.
- [Google Scholar]
- The effect of drying methods on the energy consumption, bioactive potential and colour of dried leaves of Pink Rock Rose (Cistus creticus) J. Food Sci. Technol.. 2019;56:2386-2394.
- [Google Scholar]
- Analysis and stability of carotenoids in the flowers of daylily (Hemerocallis d isticha) as affected by various treatments. J. Agric. Food Chem.. 2000;48(12):5962-5968.
- [Google Scholar]
- Edible flowers: bioactive profile and its potential to be used in food development. Food Res. Int.. 2020;129:108868
- [Google Scholar]
- Tocopherol, carotene, phenolic contents and antibacterial properties of rose essential oil, hydrosol and absolute. Curr. Microbiol.. 2009;59:554-558.
- [Google Scholar]
- Evaluation of antimicrobial potential and phytochemicals in Acmella (A. oleracea) flower pod extracts subjected to different drying techniques. J. Food Process. Preserv.. 2021;45(6):e15570.
- [Google Scholar]
- Comparison of the total antioxidant content of 30 widely used medicinal plants of New Mexico. Life Sci.. 2002;70(9):1035-1040.
- [Google Scholar]
- Rose petal tea as an antioxidant-rich beverage: cultivar effects. J. Food Sci.. 2006;71(1):S42-S47.
- [Google Scholar]
- Processing and analytical characterisation of pulp-enriched cloudy apple juices. LWT Food Sci. Technol.. 2008;41(10):2057-2063.
- [Google Scholar]
- Effect of drying methods on the antioxidant capacity, colour and phytochemicals of Portulaca oleracea L Leaves. J. Nutr. Food Sci.. 2014;4:322.
- [Google Scholar]
- Total phenolics and antioxidants profiles of commonly consumed edible flowers in China. Int. J. Food Prop.. 2018;21(1):1524-1540.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2023.103025.
Appendix A
Supplementary material
The following are the Supplementary data to this article:







