Translate this page into:
Dielectric properties and conductivity studies of some tetradentate cobalt(II), nickel(II), and copper(II) Schiff base complexes
*Corresponding author. Tel.: +91 9952704989 rselwinj@rediffmail.com (R. Selwin Joseyphus)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.

Available online 23 March 2011
Abstract
Dielectric and conductivity properties of cobalt(II), nickel(II), and copper(II)–imidazole-2-carboxaldehyde-glycylglycine (imal-glygly) complexes have been studied using impedance spectroscopy. The dielectric constant and dielectric loss factor for metal complexes have been analyzed in the frequency range from 1 Hz to 1 MHz at room temperature. The a.c. conductivity result reveals that these complexes exhibit dissimilar frequency dependent conductivity. An attempt has been made to investigate the relaxation and conductivity studies, since there are fairly few literatures available.
Keywords
Glycylglycine
Imidazole-2-carboxaldehyde
Dielectric constant
Dielectric loss factor
Impedance spectroscopy
1 Introduction
Electrically conducting organometallic polymers for complexing transition metals with conjugated bridging ligands are the current fascinating field of research (Carraher, 1981; Djebbar-Sid et al., 1997). During the past two decades, considerable attention has been paid to the chemistry of the metal complexes of Schiff bases containing nitrogen and other donors (Bhattacharyya et al., 1998; Temel et al., 2002). This may be attributed to their stability and potential applications in many fields, such as oxidation catalysis, electrochemistry, molecular materials having nonlinear optical properties and pharmaceutical applications (Lacroix et al., 2004). Schiff base complexes have been found to be important precursors for semiconducting materials (Sarkar et al., 2004). Jian-Yuan et al. (2005) have prepared the new solvent polymeric membrane electrodes characterized by using impedance measurement and demonstrate excellent sensitivity of thiocyanate ions across the membrane and diffusion process. Sun et al. (2004) studied the response mechanism of the electrode based on copper(II)–N,N′-bis-(furaldehyde)-1,2-phenylenediamine-dipicolyl complex and also studied the selectivity of thiocyanate ions across the membrane by a.c. impedance spectroscopy. Wen-Ju et al. (2006) studied a new PVC-based liquid-membrane anion-selective electrode based on the copper(II) tetradentate complex, described their response mechanism of electrode and applied to determine the iodide in medicine analysis. Qin Zhao et al. (2007) studied the selectivity for iodide, related to a direct interaction between the central manganese(III) atom and iodide and a steric effect associated with the structure of the carrier, supported by UV spectroscopy and a.c. impedance techniques. Jianyuan Dai et al. (2004) studied the bis-dimethylaminobenzaldehydecobalt(II) complex as a neutral carrier for a highly selective iodide electrode by impedance spectroscopy. Ke Qiang Ding et al. (2001) used the electrochemical impedance study of Schiff base by means of a self assembled monolayer for the complexation of copper(II) complex. The a.c. electrical studies of the complexes reveal essential structural details and valuable complementary information relevant to the electrical application of polymer materials. It is well known that the surface area of the nanomaterials is large as the grain sizes are small. Because of these excellent properties, the Schiff base complexes are keen to study the properties in various forms. However, the dielectric and bulk conductivity properties of Schiff base complexes were less reported (Ding et al., 2001; Revanasiddappa et al., 2008). Here we report the room temperature conductivity and dielectric relaxation mechanism of metal Schiff base complexes by solid state impedance analyzer.
2 Experimental
Synthesis, spectroscopic characterization and biological studies of cobalt(II), nickel(II) and copper(II) complexes were reported (Joseyphus and Nair, 2009). The solid state complexes were pelletized using hydraulic press for impedance spectroscopy measurement. Silver paint was used to coat these complexes for uniform conductivity and to avoid air gap between the platinum electrodes and sample. The room temperature a.c. impedance analysis was carried out using SOLARTRON 1260 Impedance Gain-Phase analyzer in the frequency range of 1 Hz–1 MHz. The molar conductance measurements were carried in 0.001 M DMSO solution for the metal complexes using a coronation digital conductivity meter. The high molar conductance values of 54, 62 and 66 Ω−1 cm2 mol−1 correspond to cobalt(II), nickel(II) and copper(II) complexes. These values are very close to the expected molar conductance value which lies in the range of 40–85 Ω−1 cm2 mol−1 suggesting to 1:1 electrolytic nature of the complexes (Joseyphus and Nair, 2009). The results of cobalt(II), nickel(II) and copper(II) complexes indicate the ionic mobility of nitrate ion as evidenced by the non-involvement of the nitrate group in coordination. Proposed structure of cobalt(II), nickel(II) and copper(II)-imal-glygly complexes is shown in Fig. 1.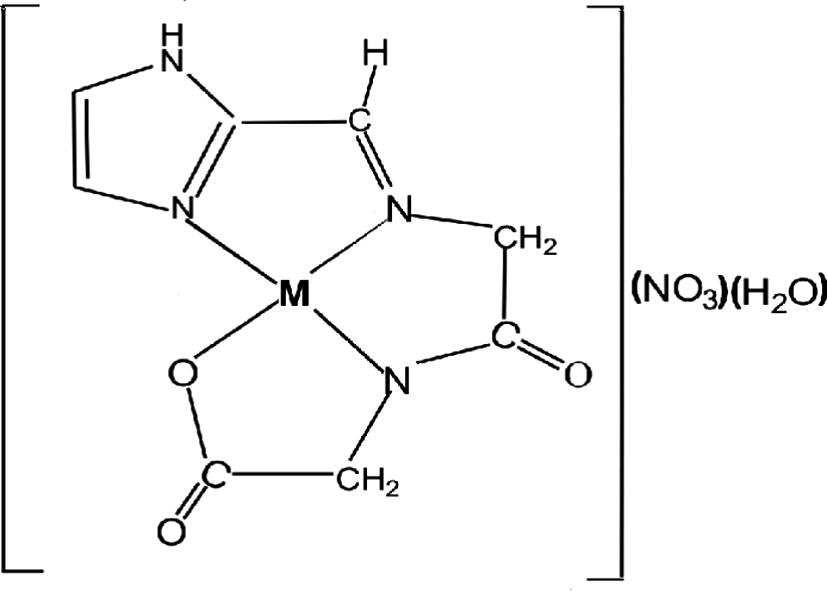
Proposed structure of cobalt(II), nickel(II) and copper(II) complexes.
3 Results and discussion
The impedance |Z∗| and phase angle θ values were used to calculate the complex impedance (Z∗) of metal complexes in the frequency range from 1 Hz to 1 MHz at room temperature. Further, the conductivity (σ), complex permittivity (ε∗) and complex electrical modulus (M∗) were extracted from the complex impedance. The detailed theoretical aspects of impedance formalism are widely found in the literature (Moynihan et al., 1973; Macedo and Bosech, 1972; West, 1975). Here, we report some interesting novel results about the impedance studies of metal complexes.
Fig. 2 shows the complex impedance plot for the Schiff base complexes, from the plot it was observed that a single semicircle originates at a higher frequency side without any tail part at the low frequency side. Thus the electrode-electrolyte polarization effects are almost suppressed and the bulk effects were observed. From the infrared spectra, electronic spectra, electron spin resonance and magnetic susceptibility measurements, the four coordination donor sites are observed for Schiff base complexes. The spectral data indicate tetrahedral geometry for cobalt(II) and nickel(II) complexes where as the copper(II) complex has a square planar geometry. The molecular geometry of the complexes leads to homogeneous polarization compete single semicircle in complex impedance plane. The resistance (R) and capacitance (C) were estimated by analyzing the impedance data by using non-linear least square fitting procedure. The bulk resistance value ranging from 10 to 100 MΩ for the copper(II), nickel(II) and cobalt(II) complexes are tabulated in Table 1. The values of the resistance in this range are due to the presence of nitrate group outside the coordination sphere in the complexes. This clearly demonstrates that the complexes are ionic (Leary and Bar-Cohen, 1999; Kim and Kim, 1989) and hence the conductivity is high. The conductivity values of these complexes were calculated using the geometrical dimensions, d and A the thickness and cross-sectional area of the pellets used for the impedance measurement, given by σ = d/RA.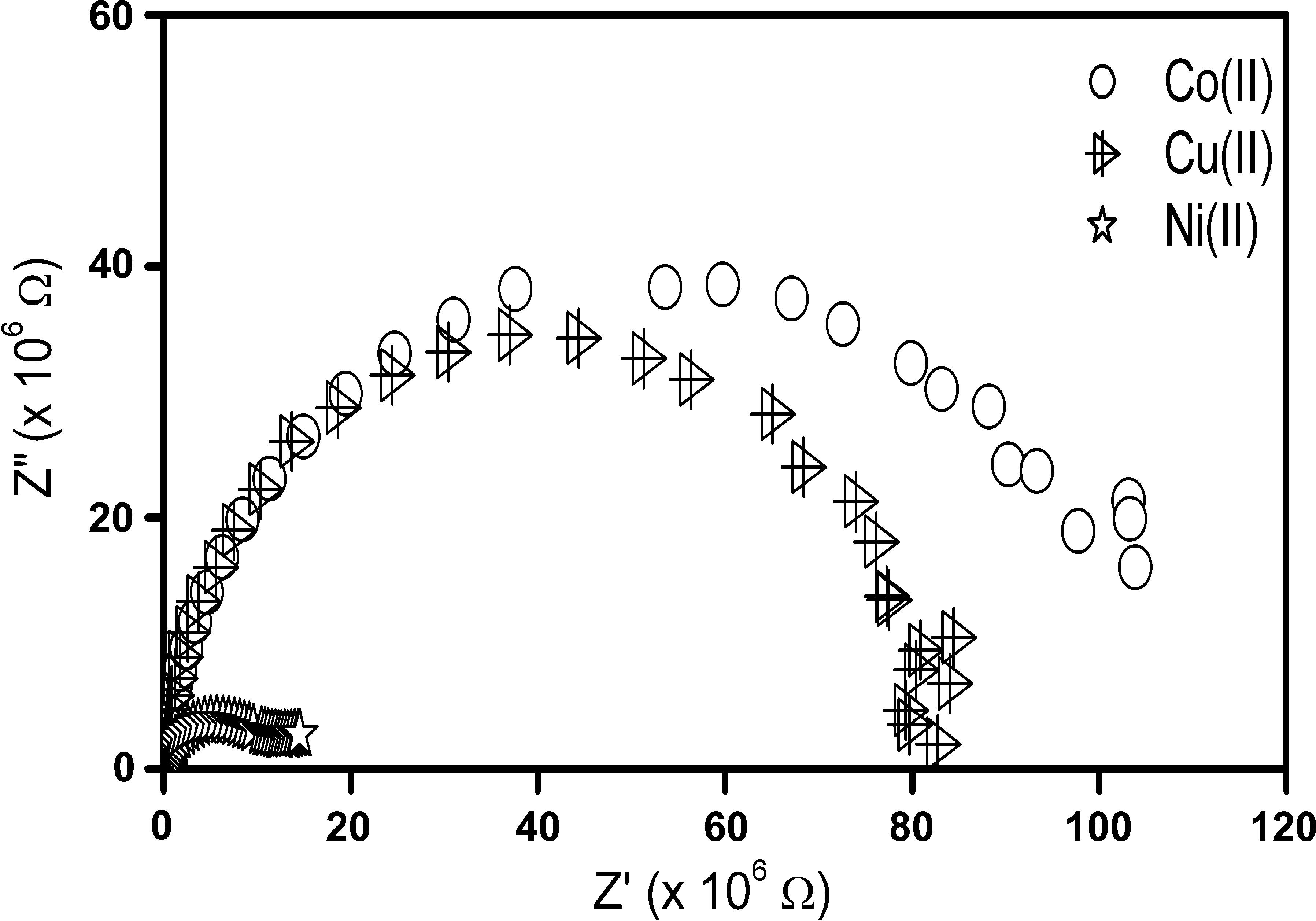
Complex impedance spectra for cobalt(II), nickel(II) and copper(II) complexes.
Metal complexes
Resistance (×107 Ω)
Capacitance (×10−11 F)
Conductivity at RT (×10−6 Scm−1)
FWHM of M′′ (β)
Relaxation time (ms)
Cobalt(II) complex
10.241
3.3741
0.25452
0.6
7.2
Nickel(II) complex
1.1536
3.8506
1.3762
0.56
0.54
Copper(II) complex
7.8955
1.7169
1.2828
0.75
5.1
Fig. 3 shows the plot for frequency dependent dielectric constant of Schiff base complexes. From the results, high dielectric constant value of 158 at 1 kHz was observed, for nanocrystalline copper(II) complex. This high value of dielectric constant for copper(II) complex is mainly due to the difference in geometry with respect to nickel(II) and cobalt(II) complexes. A very small variation in dielectric constant values at the low frequency region (Fig. 3) was also evident. The dielectric loss factors as a function of frequency for Schiff base complexes are shown in Fig. 4. The peak loss was observed for these set of Schiff base complexes and shows that a high value of 0.813 at 1 kHz for nickel(II) complex was observed. This indicates the energy loss is more in this complex.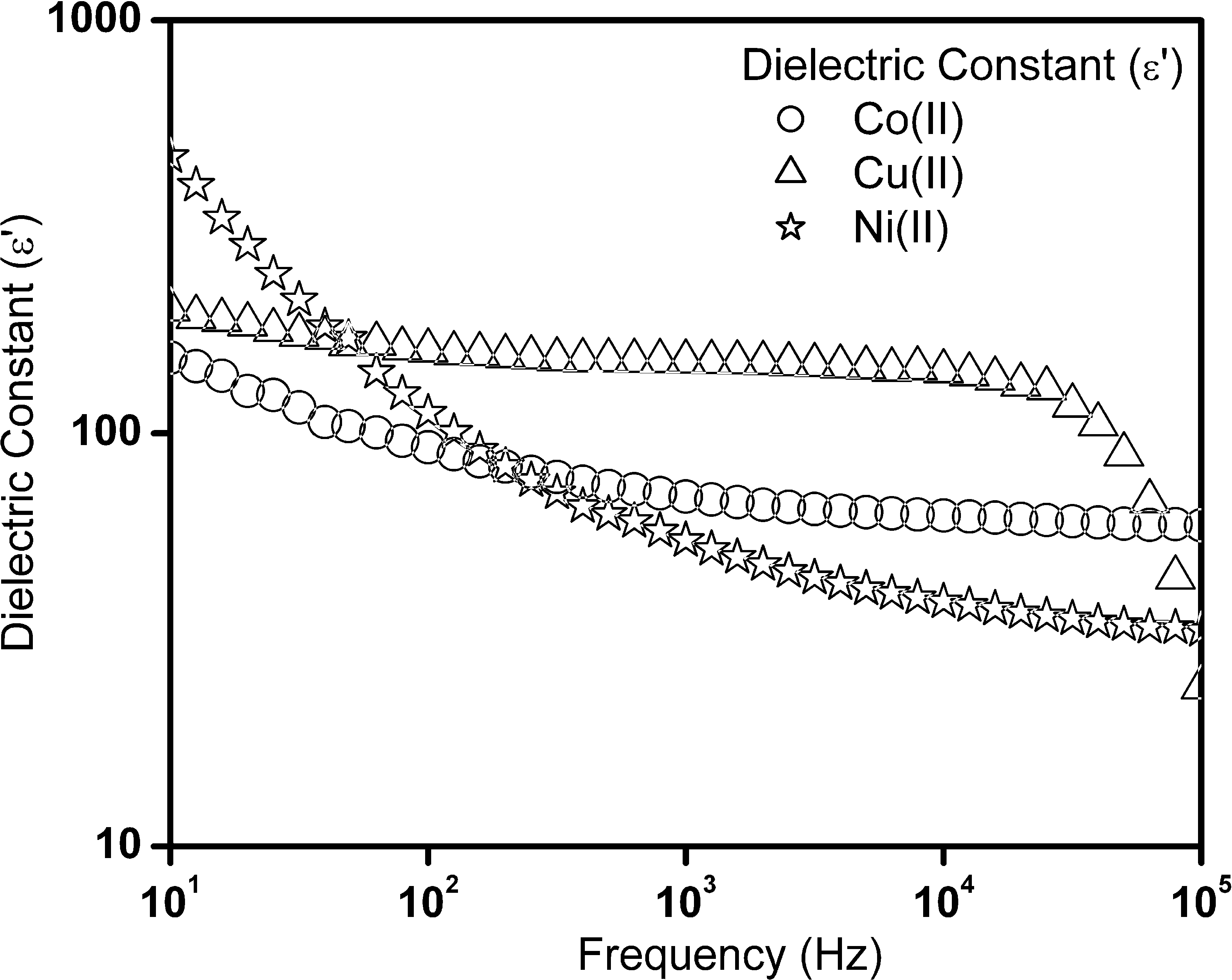
Dielectric constant (ε′) as a function of frequency for cobalt(II), nickel(II) and copper(II) complexes.
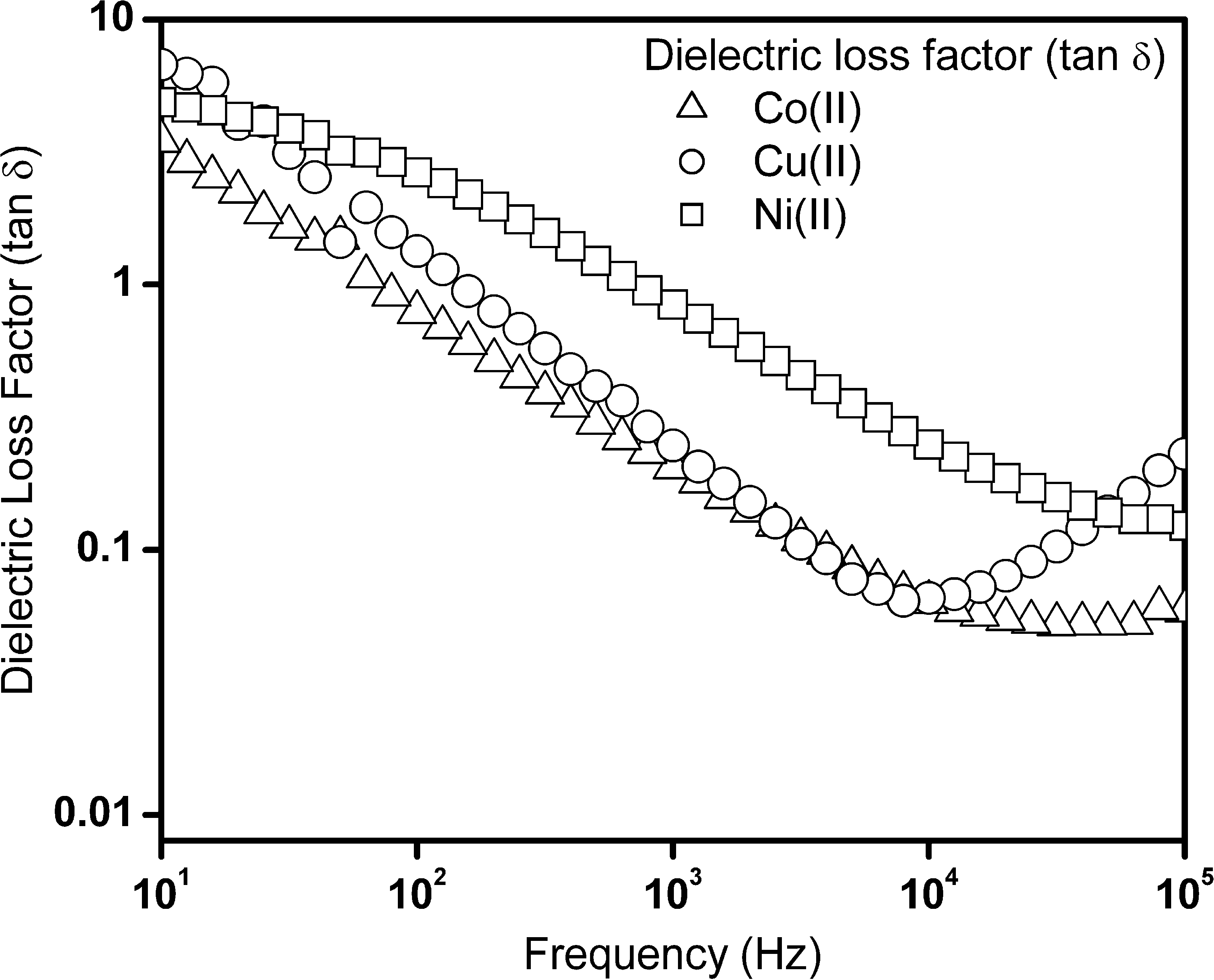
Dielectric loss factor (tan δ) as a function of frequency for cobalt(II), nickel(II) and copper(II) complexes.
The log σ versus frequency plot at room temperature for cobalt(II), nickel(II) and copper(II) complexes are shown in Fig. 5. The conductivity values above the critical frequency shows the frequency dependent behavior. This result signifies the presence of conductivity dispersion as dominant in these complexes. This also indicates the possibility of charge carrier migration in metal complexes. The cobalt(II) complex indicates the variation of conductivity at the low frequency region, this may be due to amorphicity of this material and the effects were not being observed in the nickel(II) and copper(II) complexes.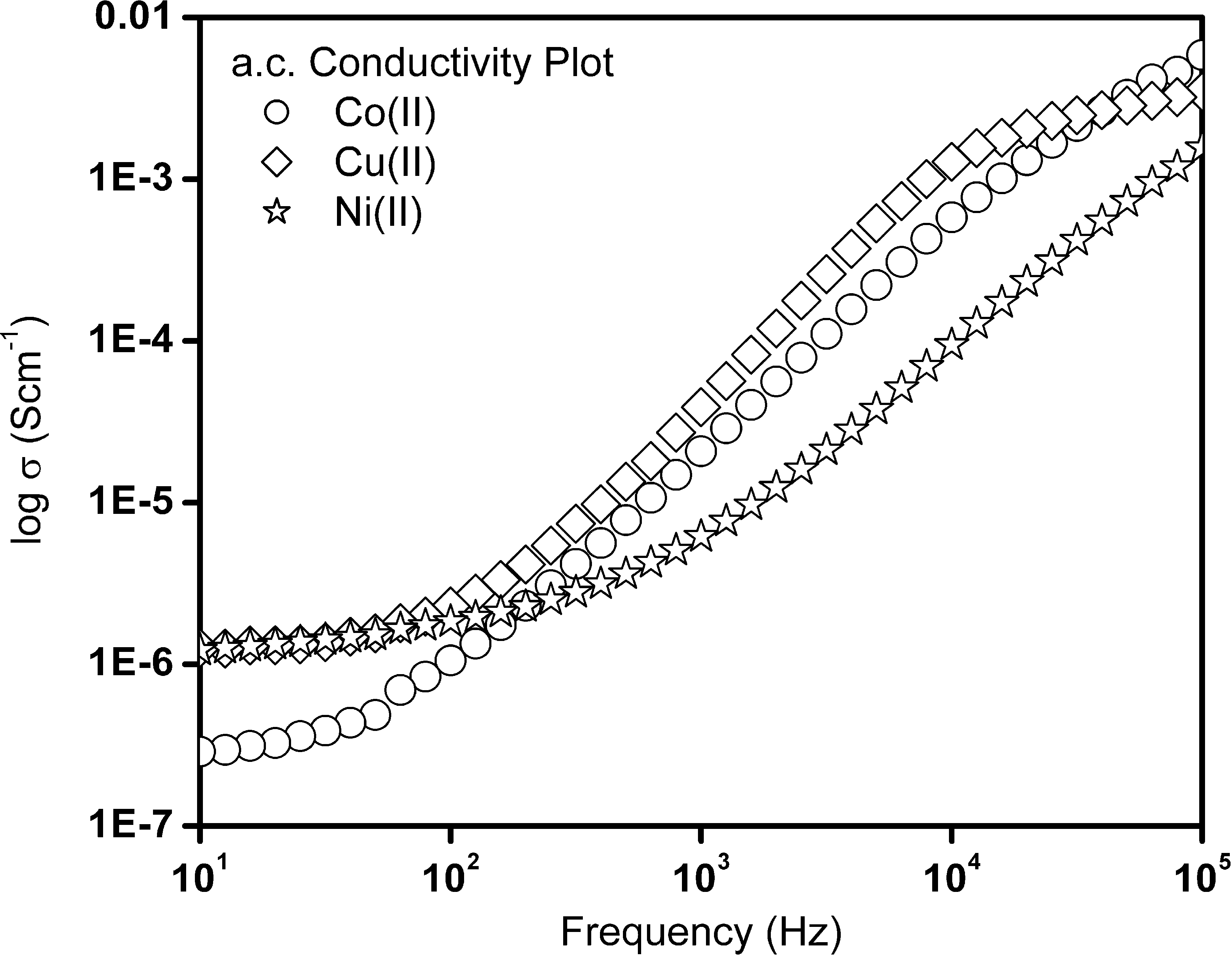
The conductivity as a function of frequency for the cobalt(II), nickel(II) and copper(II) complexes.
The modulus function against frequency for metal complexes is shown in Fig. 6. Modulus formalism (Moynihan et al., 1973; Macedo and Bosech, 1972; Hodge, 1976) is particularly suitable to extract bulk properties of electrical conductivity. The modulus data were fitted by using modulus fitting procedure adopted by Moynihan et al. and least-squares iterative routine software package (Baskaran, 1997). The extracted values from modulus fitting, β is less than unity (0.6, 0.56 and 0.75) values for cobalt(II), nickel(II) and copper(II) complexes. The frequency dependent M′′ spectra show distorted shape and the conductivity relaxation was found to be non Debye type since the values of β are less than unity. This distortion and peak maximum indicate the switching from short-range mobility into long-range mobility with decrease in the frequency. The observed average relaxation times are 7.2, 0.54 and 5.1 ms for cobalt(II), nickel(II) and copper(II) complexes, respectively. The interesting results of small average relaxation time and long range of mobility was observed for nickel(II) complex. The presence of very low relaxation frequency result indicates that the small mobility range of conductivity was observed for cobalt(II) complex.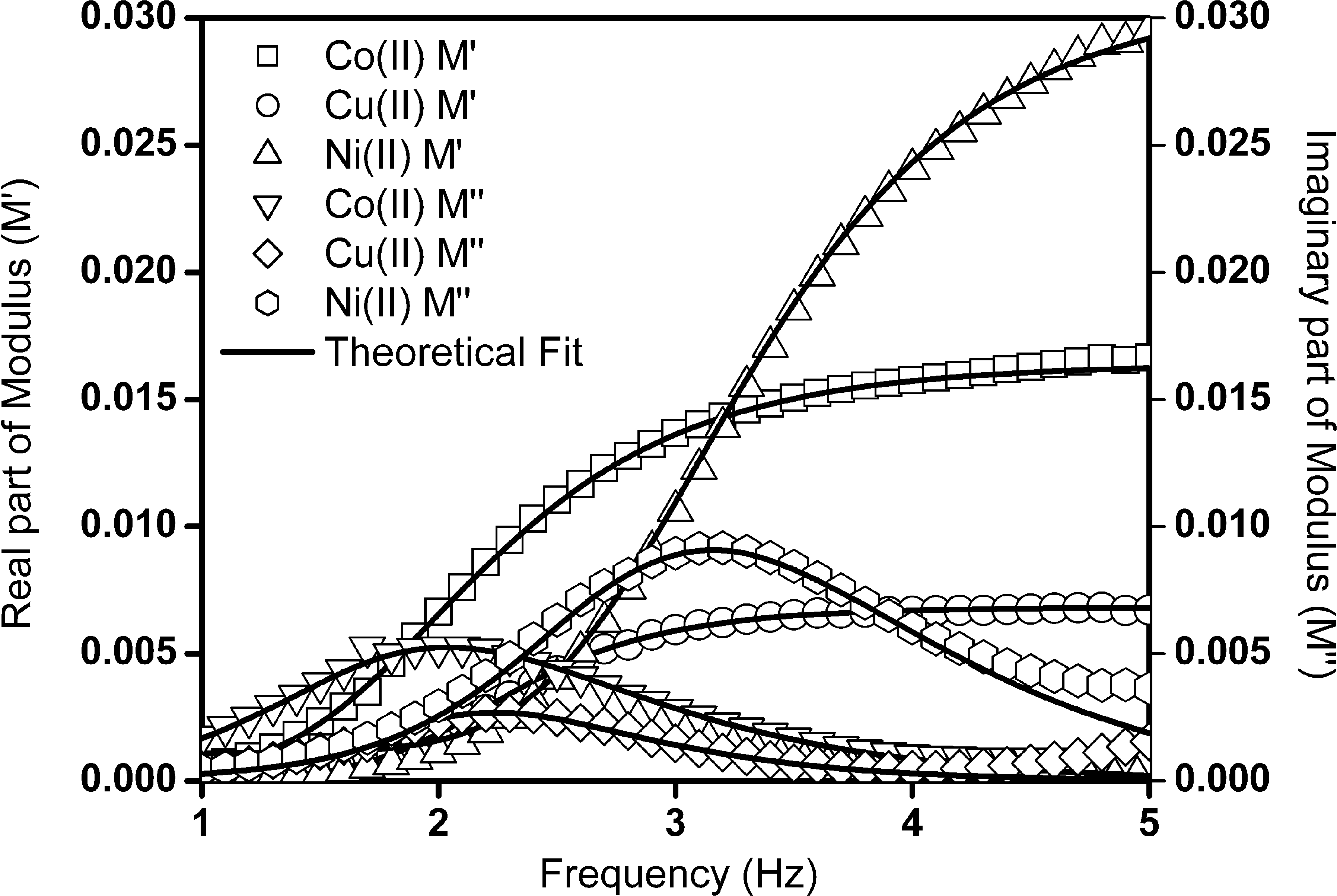
Electrical modulus plot as a function of frequency for cobalt(II), nickel(II) and copper(II) complexes. Solid lines show the best theoretical fit of equation (4).
4 Conclusion
High dielectric constant values obtained for nanocrystallite copper(II) complex at 1 kHz and low dielectric loss values at 1 kHz were observed for cobalt(II) and nickel(II) complexes. The presence of long range of ion mobility in nickel(II) complex was observed from electrical modulus formalisms. The presence of non-Debye nature of these complexes was deduced from (β values) modulus formalism. Peculiar nature in conductivity at the low frequency region for cobalt(II) complex may be due to the amorphicity of this complex and this effect is not seen in nickel(II) and copper(II) complexes.
Acknowledgment
We thank Dr. N. Ponpandian, Department of Nanoscience and Technology, Bharathiar University, Coimbatore 641 046, India for his help in this work.
References
- A.c. conductivity and relaxation processes in silver selenochromate glass. Solid State Ionics. 1997;98(3-4):217.
- [Google Scholar]
- J. Chem. Soc. Dalton Trans. 1998:3149.
- J. Chem. Ed.. 1981;58:921.
- Anal. Sci.. 2004;20:1661.
- Chinese Chem. Lett.. 2001;12:1101.
- Synthesis, characterization and electrochemical behaviour of some copper(II) complexes with linear and tripodal tetradentate ligands derived from Schiff bases. Polyhedron. 1997;16:2175.
- [Google Scholar]
- Impedance and modulus spectroscopy of polycrystalline solid electrolytes. J. Electroanal. Chem.. 1976;74(2):125.
- [Google Scholar]
- Anal. Lett.. 2005;38:389.
- J. Coord. Chem.. 2009;62:319.
- Bull. Korean Chem. Soc.. 1989;10:495.
- Synthesis, crystal structures, and molecular hyperpolarizabilities of a new Schiff base ligand, and its copper(II), nickel(II), and cobalt(II) metal complexes. Inorg. Chim. Acta. 2004;357(13):3825.
- [Google Scholar]
- Leary, S., Bar-Cohen, Y., 1999. Proceedings of SPIE’s, 6th Annual International Symposium on Smart Structures and Materials, San Diego, CA 1.
- Phys. Chem. Glass.. 1972;13:171.
- Phys. Chem. Glass.. 1973;14:122.
- Eur. J. Chem.. 2008;5:797.
- Mater. Chem. Phys.. 2004;88:357.
- Annal. Bioannal. Chem.. 2004;378:490.
- Spect. Lett. An Inter. J. Rapid Comm.. 2002;35:219.
- Anal. Sci.. 2006;22:1345.
- J. Electroceramics. 1975;1:65.
- Anal. Sci.. 2007;23:1331.







