Translate this page into:
Diazepam induced oxidative DNA damage in cultured human lymphocytes
⁎Corresponding author at: Department of Community Medicine and Pathology, Faculty of Medicine, The Hashemite University, P.O. Box 150459, Zarqa 13115, Jordan. azab_mohammed@hu.edu.jo (Mohammad Azab)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Diazepam is a benzodiazepine compound that is mainly used for anxiety, muscle spasms, seizures and insomnia. Several studies have shown that long-term Diazepam treatment is associated with oxidative stress. In this study, the possible genotoxic effect of Diazepam was examined in cultured human white blood cells using the sister chromatid exchanges (SCEs), chromosomal aberrations (CAs) and 8-hydroxy-deoxyguanosine (8-OHdG) assays. Treatment of cultured lymphocytes with different concentrations of Diazepam (1, 10 and 100 µg/mL) did not induce chromosomal DNA damage as measured using SCEs and CAs assays (P > 0.05). In addition, no effect was observed on mitotic and proliferative indices (P > 0.05). However, Diazepam induced oxidative DNA damage as measured by the 8-OHdG assay in a dose dependent manner (P < 0.001). In conclusion, Diazepam seems to induce oxidative DNA damage in cultured human lymphocytes. More in vivo studies are required to confirm current finding.
Keywords
Diazepam
8-OHdG
DNA damage
Chromosomal aberrations
Lymphocytes
1 Introduction
Diazepam belongs to benzodiazepine family medications with anticonvulsant, anti-anxiety, and skeletal muscle relaxing properties. Diazepam is considered as one of the most prescribed medication worldwide and is recommended by World Health Organization as a core medicine for treatment of several diseases such as anxiety, seizures, alcohol withdrawal and insomnia (Calcaterra and Barrow, 2014). The mechanism of action of Diazepam involves enhancement of the action of the neurotransmitter GABA via interacting with the benzodiazepine site on the GABA receptor, and the subsequent depression of the central nervous system (Calcaterra and Barrow, 2014; Sakai and Ishizuka, 2009). The advantages of diazepam include its rapid and potent effects compared to other similar medications. However, higher doses of Diazepam have been shown to be associated with anterograde amnesia sedation, excitement, worsen depression, physical dependence, benzodiazepine withdrawal syndrome and cognitive impairments (Duley et al., 2010).
Recently, Diazepam has been shown to cause oxidative tissue damage, especially during long periods of treatments. For example, Castro et al. (2009) showed that treatment of Diazepam increased lipid peroxidation in the cortex and cerebellum, and increased protein carbonyl formation in the striatum of mouse brain. Rats administered Diazepam showed significant decreases in glutathione levels and superoxide dismutase activity in liver (El-Sokkary, 2008). In this study, we proposed to examine the effects of Diazepam on inducing DNA damage using 8-hydroxy-deoxyguanosine (8-OHdG) assay in cultured human white blood cells. 8-OHdG is an oxidative stress biomarker that has been shown to increase in conditions and diseases that marked by elevation in oxidative stress (Kroese and Scheffer, 2014). We also examined genotoxic effect of Diazepam using sister chromatid exchanges (SCEs) and chromosomal aberrations (CAs) assays as they also can be modulated by exposure to mutagenic agents including drugs that can generate free radicals inside cells (Norppa et al., 2006).
2 Materials and methods
2.1 Subjects
Five volunteers were recruited in the study to donate blood for lymphocyte cultures. The donors were healthy non-smokers and non-alcoholic adult males (20–26 year old). About 20 mL blood was collected from each subject in heparinized collection tubes. Prior to start of the study, written informed consents was obtained from all subjects according to Institutional Review Board.
2.2 Chemicals
Diazepam, Cisplatin, Bromodeoxyuridine, DMSO and Colchicine were obtained from Sigma–Aldrich (Saint Louis, MI, USA). The 8-OHdG assay kit was purchased from Cayman Chemical (Ann Arbor, Michigan, USA). Finally, Pb-Max Culture media was obtained from Gibco-Invitrogen (United Kingdom).
2.3 Cell cultures
Blood lymphocytes cultures were initiated by adding 1 mL of freshly withdrawn blood into tissue-culture flask containing 9 mL of complete lymphocyte Pb-Max media (RPMI 1640 medium supplemented with suitable amount of fetal bovine serum, glutamine, Penicillin-Streptomycin and Phytohaemagglutinin). Diazepam was dissolved in Dimethyl sulfoxide. Experimental cultures were treated with Diazepam at a final concentration of 1, 10 and 100 µg/mL (equivalent to 3.5, 35 and 351 µM respectively) whereas control cultures were treated with vehicle (Dimethyl sulfoxide). These concentrations were selected based on previous studies that showed positive results equivalent ones (Akritopoulou et al., 2009).
2.4 Sister-chromatid exchange assay
Details of the procedure used for the detection of SCEs in cultured lymphocytes were as previously described (Alsatari et al., 2012; Khabour et al., 2011). In brief, Bromodeoxyuridine solution was freshly prepared in distilled sterile water and was added to a final concentration of 20 μg/mL directly after culture initiation. Cultures were incubated at 37 °C in CO2 incubator for 72 h. Diazepam was added to cultures in the last 24 h of incubation time. As a positive control, Cisplatin (1 μg/mL, final concentration) was used and was added in the last 24 h of incubation time. Before harvesting of cultured lymphocytes, Colchicine (finale concentration 10 µg/mL) was added to cultures for 2 h. Cultures were then centrifuged at 1000×g for 5 min, decanted and the cellular pellet was gently re-suspended in 10 mL hypotonic solution (0.075 M KCl) at 37 °C for 20 min. The cellular suspension was centrifuged at 1000×g for 5 min and the cellular pellet was fixed with three changes of ice-cold methanol: acetic acid (3:1). The cellular suspension was then dropped on pre-chilled microscope slides to obtain metaphase spreads. The slides were stained with the fluorescent-plus-Giemsa technique as described previously (Azab et al., 2009). The slides were analyzed blindly using medical microscope at 1000 × magnification. About 250 M2 metaphase spreads (50 from each donor) were analyzed per each drug concentration for presence of SCEs (Alzoubi et al., 2014a; Khabour et al., 2016).
2.5 Chromosomal aberrations (CAs) assay
Blood lymphocytes cultures were initiated by adding1 mL of freshly withdrawn blood into tissue-culture flask containing 9 mL of complete lymphocyte Pb-Max media. Cultures were incubated at 37 °C in CO2 incubator for 72 h. Drugs were added to cultures in the last 24 h of incubation time. Colchicine (finale concentration 10 µg/mL) was added to cultures for 2 h prior to harvesting period. Cultures were then centrifuged at 1000×g for 5 min, decanted and the cellular pellet was gently re-suspended in 10 mL hypotonic solution (0.075 M KCl) at 37 °C for 20 min. The cellular suspension was centrifuged at 1000×g for 5 min and the cellular pellet was fixed with three changes of ice-cold methanol: acetic acid (3:1). The cellular suspension was then dropped on pre-chilled microscope slides to obtain metaphase spreads. The slides were stained with 2% giemsa solution (pH 6.8) for 15 min and subsequently analyzed for presence of CAs analysis (Khabour et al., 2015). The slides were analyzed blindly using medical microscope at 1000× magnification. About 500 well-spread metaphases (100 from each donor) were scored for the presence of CAs. Only breaks and exchanges were included in the analysis (Alzoubi et al., 2012).
2.6 8-OHdG assay
The 8-OHdG assay was performed as previously described (Khabour et al., 2014). In brief, blood cultures were incubated for 72 h at 37 °C. Cultures were then washed 5 times using RPMI medium. Since fetal bovine serum is very rich in 8-OHdG, Cells were then re-suspended in RPMI medium supplemented with only glutamine, Penicillin-Streptomycin and Phytohaemagglutinin. Cultures were then treated with drugs (Diazepam or positive control) and incubated at 37 °C for 6 h. Cultures were then centrifuged at 1000×g and 200 μL from the supernatant were used for 8-OHdG assay. Competitive ELISA assays for 8-OH-dG was performed according to the manufactures protocol. ELISA plate were read at 405 nm using an automated reader (ELx800 Universal microplate reader, BIO-TEK, USA). The period of treatment with Diazepam was reduced to 6 h in this assay compared with 24 h in the case of SCEs and CAs to avoid deteriorations of cultures due to the absence of fetal bovine serum. This assay has been to be potent to examine the effects of oxidative DNA damaging agent on cultured human lymphocytes (Alzoubi et al., 2014b; Azab et al., 2016; Esmadi et al., 2016; Mhaidat et al., 2016).
2.7 Cell kinetics analysis
The cytotoxic effect of drugs was examined using both the mitotic index and proliferative index. The mitotic index was determined by scoring at least 5000 cells (1000 cells from each donor) and counting the cells that were in metaphases. For the cell proliferation index, 500 metaphase cells were used. The proliferation index was calculated using the following formula = (1 × [M1] + 2 × [M2] + 3 × [≥M3])/100, where M1, M2 and M3 are the number of cells at the first, second and third metaphase, respectively (Alzoubi et al., 2014b).
2.8 Statistical analysis
All data were expressed as mean ± SEM. Graph Pad Prism software (version 5) was used for statistical analysis that includes ANOVA followed by Tukey posthoc test. A P < 0.05 was considered significant.
3 Results
The genotoxic effects of Diazepam on blood lymphocytes were evaluated using three assays. In the CAs and SCEs assays, cultures were treated with the different concentrations of diazepam in the last 24 h of incubation period. Fig. 1A shows changes in the levels of CAs induced by the treatment. None of the examined Diazepam concentrations (1, 10 and 100 µg/mL) induced elevation in the frequency of CAs (P = 0.93, ANOVA F = 0.1401). Similar results were obtained with the SCEs assay as shown in Fig. 1B. None of the examined Diazepam concentrations affected SCEs frequency observed in the control group (P = 0.326, ANOVA F value = 1.156). The positive control, Cisplatin, significantly increased the frequency of SCEs (10.56 ± 0.66 in Cisplatin group versus 4.87 ± 0.21 in the control group, P < 0.001) and CAs (0.092 ± 0.013 in cisplatin group versus 0.038 ± 0.014 in the control group, P < 0.01). Thus, Diazepam seems not to affect chromosomes as measured using SCEs and CAs assays.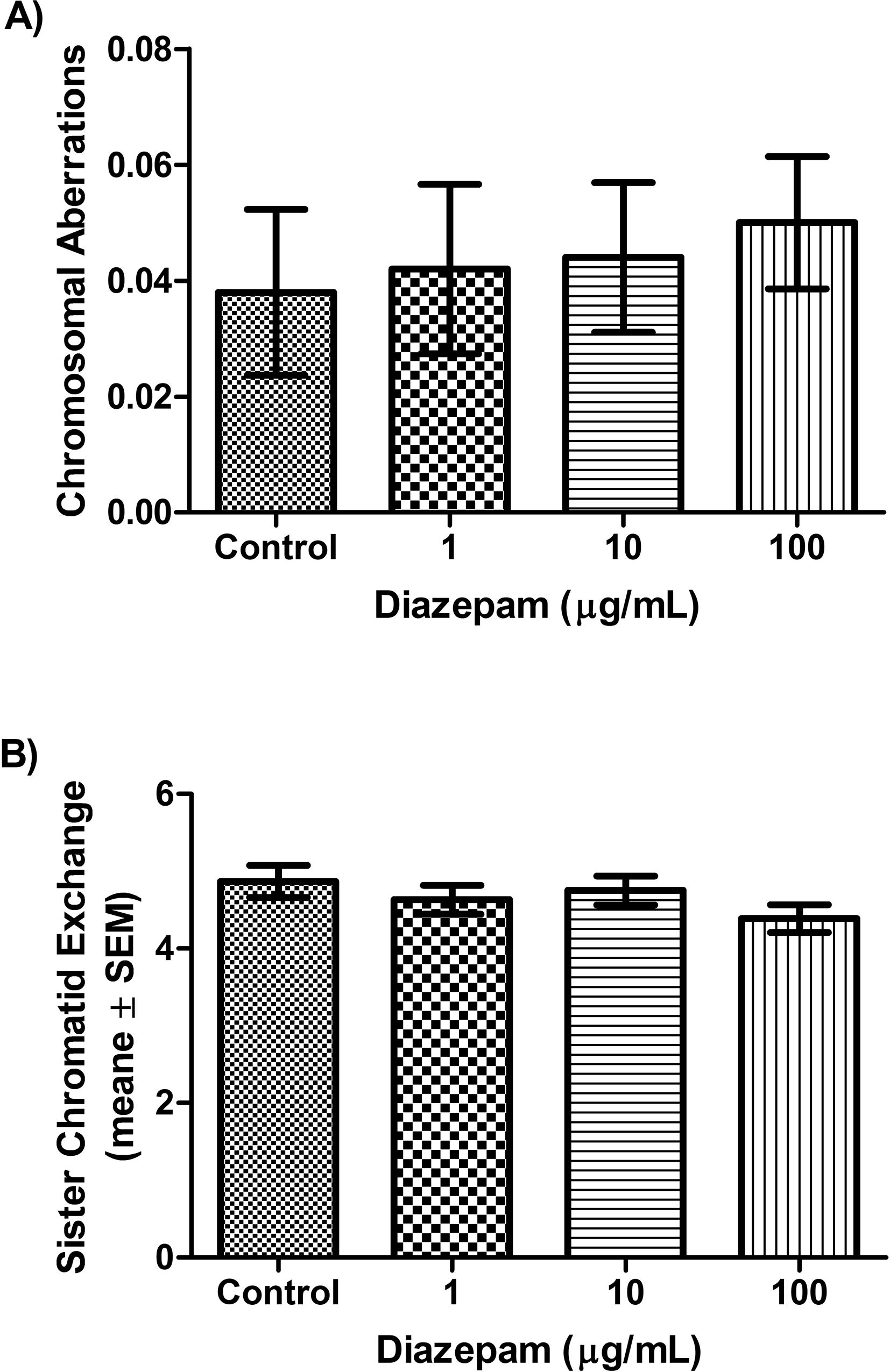
Changes in the levels of CAs and SCEs after treatment with Diazepam. A) The average of CAs/cell in control, and Diazepam groups (1, 10, 100 µg/mL) in cultured blood cells. No significant differences were detected among groups (P = 0.93). B) The average of SCEs/cell in control, and Diazepam groups (1, 10, 100 µg/mL) in cultured blood cells. No significant differences were detected among groups (P = 0.326).
With respect to cell kinetic analysis, treatments of blood lymphocytes with different concentrations of Diazepam in the last 24 h of culture period neither affected the mitotic index (P = 0.96, ANOVA F = 0.07981, Fig. 2A) nor the proliferative index (P = 0.152, ANOVA F = 2.11, Fig. 2B). Thus Diazepam seems to have no effects on cell kinetic parameters as measured by mitotic index and proliferative index.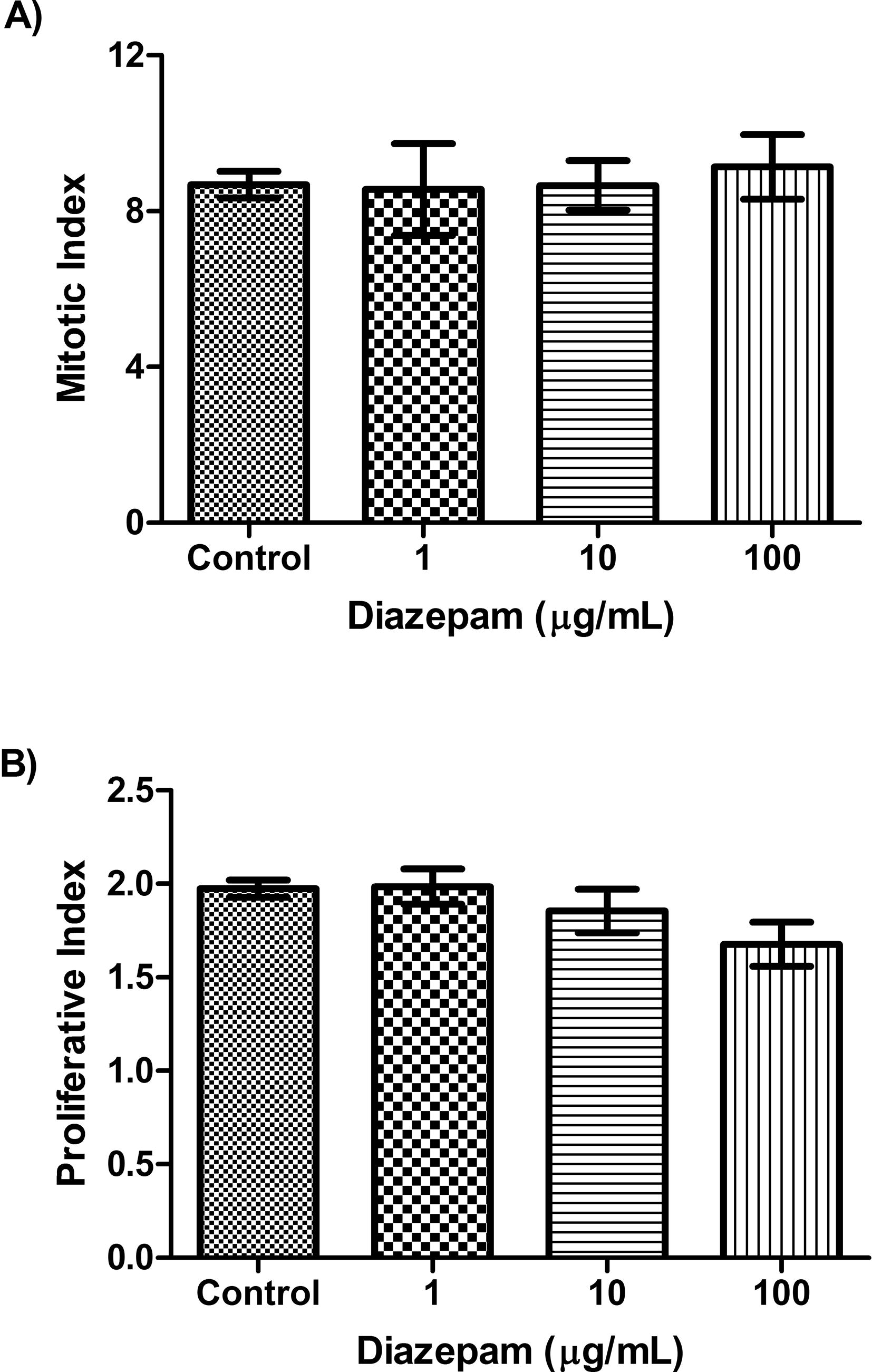
Changes in the levels of mitotic index and proliferative index after treatment with Diazepam. The average value of A) Mitotic index, and B) Proliferative index in control, and Diazepam groups (1, 10, 100 µg/mL) in cultured blood cells. No significant differences were detected among groups (P > 0.05).
Finally, the potential of Diazepam to induce oxidative DNA damage was assessed using 8-OHdG assay. Cultures were treated with Diazepam in this experiment for 6 h. The results showed that Diazepam induced oxidative DNA damage in a dose dependent manner (P < 0.001, ANOVA F = 11.30, Fig. 3). The percentage of increases in 8-OHdG were 39%, 48% and 52% for 1, 10 and 100 µg/mL Diazepam respectively. Cisplatin (1 μg/mL), the positive control induced 106% increase in OHdG (275.3 ± 11.28 ng/mL in Cisplatin group versus 133.6 ± 8.51 ng/mL in the control group, P < 0.001). Thus, Diazepam seems to induce oxidative DNA damage in cultured human lymphocytes.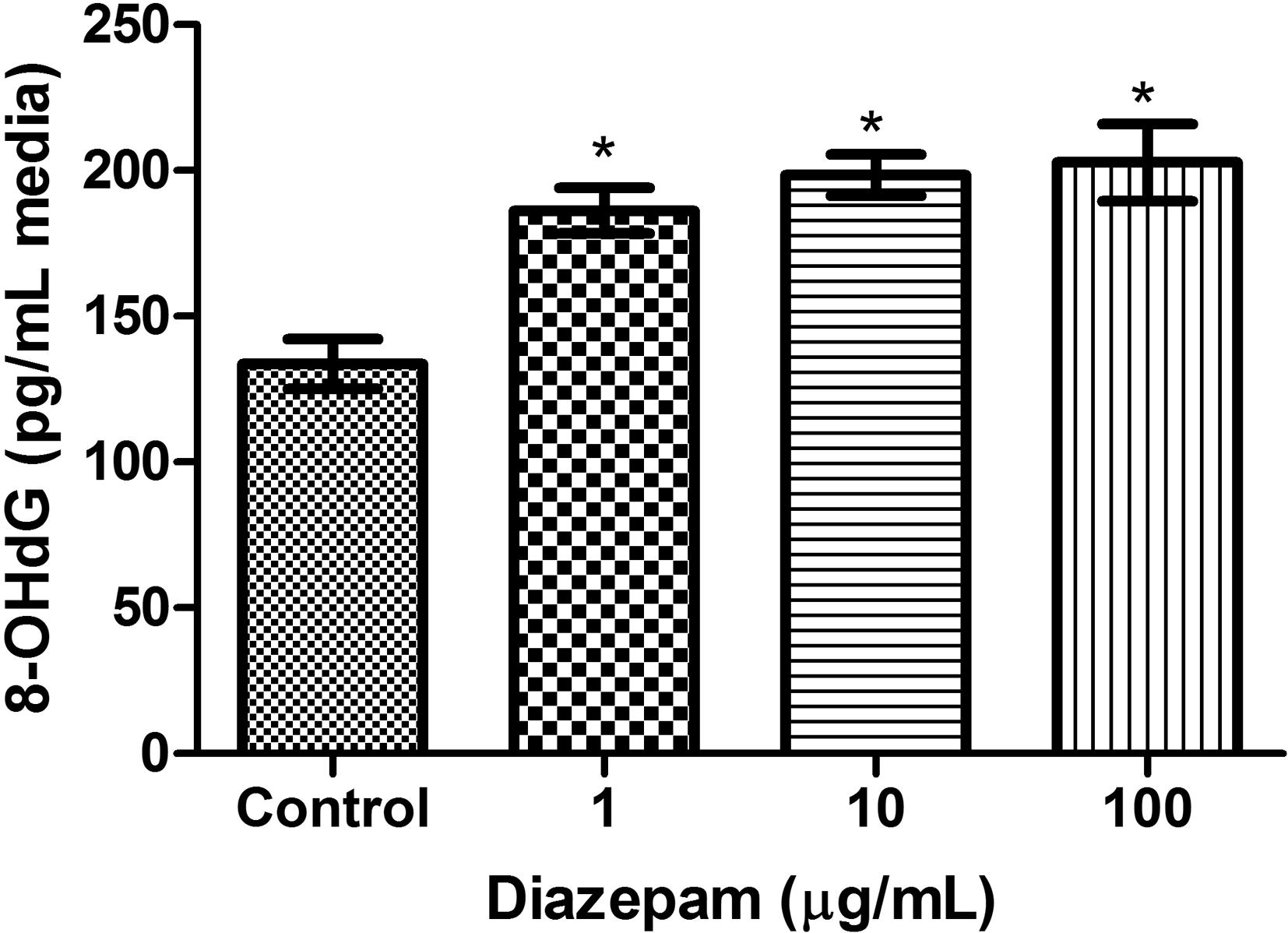
Changes in the levels of 8-OHdG after treatment with Diazepam. Levels of 8-OHdG values in in control, and Diazepam groups (1, 10, 100 µg/mL) in cultured blood cells. The level of 8-OHdG in Diazepam treated groups were significantly higher than control group. *Indicate significant difference (P < 0.001).
4 Discussion
In the current investigation, we examined genotoxicity of Diazepam in cultured human lymphocytes. The results showed for the first time that Diazepam induced oxidative DNA damage in a dose dependent manner.
Diazepam is a widely used medication for the treatment of a spectrum of acute and chronic brain and muscle related conditions (Okoromah and Lesi, 2004; Pellock, 2004). Diazepam has three rings and belongs to benzodiazepine family that acts via modulating GABA affinity for the GABA receptor (Calcaterra and Barrow, 2014; Sakai and Ishizuka, 2009).
The results of the present investigation showed for the first time that Diazepam can induce oxidative DNA damage in vitro in cultured human lymphocytes as measure by 8-OHdG assay. Significant elevation in the 8-OHdG was observed directly after 6 h of treatment. The effect of Diazepam is dose dependent reaching a plateau at high concentrations (as no statistical difference was noticed between 10 μg/mL and 100 μg/mL concentrations). This is in agreement with previous literature that pointed to the ability of Diazepam to cause oxidative stress after treatments of rats for 30 days. For example, rats treated with Diazepam (0.5 or 1.0 mg/kg) for 30 days enhanced TBARS and protein carbonyl levels and altered enzymatic activity of oxidative stress enzymes including catalase, glutathione peroxidase (GPx), and superoxide dismutase (SOD) in the brain as well as decreased glutathione levels and superoxide dismutase activity in the liver (Eger et al., 2016). Similar findings in rats were reported using a 3 mg/kg dose of Diazepam and 30 days of treatments (El-Sokkary, 2008). In mouse model, treatment of Diazepam (1 mg/kg day for 6 days) intraperitoneally increased lipid peroxidation in the cortex and cerebellum, and increased protein carbonyl formation in the striatum, while at a higher concentration (30 mg/kg day for 6 days), it significantly lowered SOD activity (Prabhakar et al., 2011). Finally, exposure of euryhaline fish Gambusia holbrooki to Diazepam for 3 days significantly altered glutathione homeostasis and enzymatic activity of glutathione peroxidase, glutathione reductase, super-oxide dismutase and glutathione-S-transferases in the gills and liver tissues (Nunes et al., 2008). Thus, taking together the previous literature and current findings, Diazepam seems to induces tissue damage including DNA via induction of oxidative stress in body cells. However, our data using cell cultures showed that a short period of exposure to Diazepam for 6 h is enough to induced oxidative damage. It is worth mentioning that we used high concentrations of Diazepam than the therapeutic ones. Therefore, replication of the finding with lower doses is strongly recommended. Alternatively, 8-OHdG marker can be examined in body fluids of patients treated with Diazepam to confirm the in vitro findings.
The results showed that Diazepam did not induce significant changes in the spontaneous level of SCEs and structural CAs. This is in agreement with the majority of previous studies that showed no changes in the frequency of SCEs and CAs assays after treatment with Diazepam (reviewed by Brambilla et al., 2007). For example, treatment of patients with Diazepam during open-heart surgery did not exert genotoxic effects as measured by chromosomal aberrations in blood lymphocytes (Karahalil et al., 2005). Similarly, no increase in CAs frequency was detected in the peripheral blood of 20 healthy young adults after receiving a single 12- to 20-mg intravenous dose of Diazepam (White et al., 1974). Diazepam also did not induce increase in the micronucleus assay using in vitro cultured human lymphocytes (Carlo et al., 1989). It also did not induce CAs in rats treated with high oral doses (1 mmol/kg) of Diazepam (Carlo et al., 1989). In addition, using comet assay, no DNA damage effect for Diazepam in equine lymphocytes was detected during or after inhalational anesthesia (Strasser et al., 2012). A longitudinal population-based study of 42,500 cases that investigated the association between medication use and cancer risk of people aged over 20 years did not found an association between Diazepam with carcinogenicity and concluded that Diazepam is safer compared to other Benzodiazepines (Iqbal et al., 2015). In contrast to our findings, however, some studies reported an increase in the frequency of SCEs in cultured human lymphocytes after 72 h of incubation with Diazepam (Akritopoulou et al., 2009; Ekonomopoulou et al., 2011). Another study showed that Diazepam drug abuse is associated with shorter leukocyte telomere length in Chinese Han population (Yang et al., 2013). In addition, high sensitivity for aneuploidy induction by Diazepam during male meiosis was reported (Baumgartner et al., 2001). Our results also showed lack of effect of Diazepam on both mitotic and proliferative indices. However, a significant decrease of proliferation rate index of lymphocytes after 72 h of incubation was reported (Ekonomopoulou et al., 2011). The discrepancy of our results and some of previous reports (Akritopoulou et al., 2009; Ekonomopoulou et al., 2011) could be due to the duration of treatment, which was for 24 h in our case versus 72 h in the other cases. In support of this, treatments of cultured lymphocytes with similar drugs (Alprazolam and Lorazepam) for 72 h caused a dose-dependent increase of SCE frequency a decrease of proliferative index (Iakovidou-Kritsi et al., 2009).
The results showed that Diazepam did not show any genotoxic effects in the SCE and CA assay while positive effect was detected using 8-OHdG test. The discrepancy in these finding could be explained by which different markers are induced. CAs reflect DNA strand breaks that can be formed by agents that directly interact with the DNA backbones (Hagmar et al., 2004). Induction of CAs is considered as strong biomarker for cancer risk (Hagmar et al., 2004). SCEs on the other hand arise during mitosis by agents that interfere with DNA recombination (Norppa et al., 2006). However, agents that induce oxidative stress, which in most of the cases does not require direct interactions with DNA, increases 8-OHdG (Valavanidis et al., 2009).
It is worth to mention that previous literature have shown oxidative DNA damage induced by drugs can be overcome by co-treatment with antioxidants (Lewinska et al., 2008; Muzandu et al., 2005). Interestingly, melatonin and vitamin C administration ameliorate Diazepam-induced decreases in GSH levels and SOD activity and elevation in lipid peroxidation in livers of Diazepam-administered rats (El-Sokkary, 2008). Thus, administration of antioxidants to patients who take Diazepam might protect against some of the side effects of this medication. This speculation requires extensive in vitro and preclinical studies before it applied to patients, as some times combinations of drugs can cause adverse effects instead of expected beneficial effect (Cabarkapa et al., 2016).
Among the limitations of the study are that higher concentrations of Diazepam than that used in patients were examined and for only short time windows (≤24 h). Thus, future studies that examined lower concentrations of diazepam and for extended time windows are strongly recommended. In addition, expansion of the genotoxicity assays to include comet and micronucleus ones is strongly recommended.
5 Conclusion
Results showed for the first time that high doses of Diazepam for 6 h can induce oxidative DNA damage in cultured human lymphocytes.
Declaration of interest
Nothing to declare.
Acknowledgments
This study was funded by a grant from the Deanship of Research at the Hashemite University/Jordan to MA. The authors thank Jordan University of Science and Technology for providing the laboratory facility to conduct the research experiments.
References
- Cytogenetic activity of diazepam in normal human lymphocyte cultures. Genet. Test Mol. Biomarkers. 2009;13:227-231.
- [Google Scholar]
- Assessment of DNA damage using chromosomal aberrations assay in lymphocytes of waterpipe smokers. Int. J. Occup. Med. Environ. Health. 2012;25:218-224.
- [Google Scholar]
- Evaluation of vitamin B12 effects on DNA damage induced by pioglitazone. Mutat. Res.. 2012;748:48-51.
- [Google Scholar]
- Evaluation of vitamin B12 effects on DNA damage induced by paclitaxel. Drug Chem. Toxicol.. 2014;37:276-280.
- [Google Scholar]
- Tempol prevents genotoxicity induced by vorinostat: role of oxidative DNA damage. Cytotechnology. 2014;66:449-455.
- [Google Scholar]
- Effect of every-other-day fasting on spontaneous chromosomal damage in rat’s bone-marrow cells. J. Toxicol. Environ. Health A. 2009;72(5):295-300.
- [Google Scholar]
- Assessment of genotoxicity of pyrethrin in cultured human lymphocytes. Drug Chem. Toxicol. 2016:1-5.
- [Google Scholar]
- Detection of aneuploidy in rodent and human sperm by multicolor FISH after chronic exposure to diazepam. Mutat. Res.. 2001;490:11-19.
- [Google Scholar]
- Genotoxicity and carcinogenicity studies of benzodiazepines. Pharmacol. Res.. 2007;56:443-458.
- [Google Scholar]
- Unexpected effect of dry olive leaf extract on the level of DNA damage in lymphocytes of lead intoxicated workers, before and after CaNa2EDTA chelation therapy. Food Chem. Toxicol. 2016
- [Google Scholar]
- Classics in chemical neuroscience: diazepam (valium) ACS Chem. Neurosci.. 2014;5:253-260.
- [Google Scholar]
- Absence of liver DNA fragmentation in rats treated with high oral doses of 32 benzodiazepine drugs. Fundam. Appl. Toxicol.. 1989;12:34-41.
- [Google Scholar]
- Neuropeptide S produces hyperlocomotion and prevents oxidative stress damage in the mouse brain: a comparative study with amphetamine and diazepam. Pharmacol. Biochem. Behav.. 2009;91:636-642.
- [Google Scholar]
- Magnesium sulphate versus diazepam for eclampsia. Cochrane Database Syst. Rev. 2010:CD000127.
- [Google Scholar]
- Acute administration of diazepam provokes redox homeostasis imbalance in the rat brain: prevention by simvastatin. J. Biochem. Mol. Toxicol.. 2016;30:506-512.
- [Google Scholar]
- A comparative study on the cytogenetic activity of three benzodiazepines in vitro. Genet. Test Mol. Biomarkers. 2011;15:373-378.
- [Google Scholar]
- Melatonin and vitamin C administration ameliorate diazepam-induced oxidative stress and cell proliferation in the liver of rats. Cell Prolif.. 2008;41:168-176.
- [Google Scholar]
- Synthesis, characterization and biological activity of some unsymmetrical Schiff base transition metal complexes. Drug Chem. Toxicol.. 2016;39:41-47.
- [Google Scholar]
- Epidemiological evaluation of cytogenetic biomarkers as potential surrogate end-points for cancer. IARC Sci. Publ. 2004:207-215.
- [Google Scholar]
- In vitro genotoxicity of two widely used benzodiazepines: alprazolam and lorazepam. Aristotle Univ. Med. J.. 2009;36:39-44.
- [Google Scholar]
- Is long-term use of benzodiazepine a risk for cancer? Medicine (Baltimore). 2015;94:e483.
- [Google Scholar]
- Diazepam and propofol used as anesthetics during open-heart surgery do not cause chromosomal aberrations in peripheral blood lymphocytes. Mutat. Res.. 2005;581:181-186.
- [Google Scholar]
- Assessment of genotoxicity of waterpipe and cigarette smoking in lymphocytes using the sister-chromatid exchange assay: a comparative study. Environ. Mol. Mutagen.. 2011;52:224-228.
- [Google Scholar]
- Tempol protects human lymphocytes from genotoxicity induced by cisplatin. Int. J. Clin. Exp. Med.. 2014;7:982-988.
- [Google Scholar]
- Assessment of genotoxicity associated with Behcet's disease using sister-chromatid exchange assay: vitamin E versus mitomycin C. Cytotechnology. 2015;67:1051-1057.
- [Google Scholar]
- Evaluation of DNA damage induced by norcantharidin in human cultured lymphocytes. Drug Chem. Toxicol.. 2016;39:303-306.
- [Google Scholar]
- 8-hydroxy-2′-deoxyguanosine and cardiovascular disease: a systematic review. Curr. Atheroscler Rep.. 2014;16:452.
- [Google Scholar]
- The nitroxide antioxidant Tempol affects metal-induced cyto- and genotoxicity in human lymphocytes in vitro. Mutat. Res.. 2008;649:7-14.
- [Google Scholar]
- Assessment of genotoxicity of vincristine, vinblastine and vinorelbine in human cultured lymphocytes: a comparative study. Balkan J. Med. Genet.. 2016;19:13-20.
- [Google Scholar]
- Lycopene and beta-carotene ameliorate catechol estrogen-mediated DNA damage. Jpn. J. Vet. Res.. 2005;52:173-184.
- [Google Scholar]
- Chromosomal aberrations and SCEs as biomarkers of cancer risk. Mutat. Res.. 2006;600:37-45.
- [Google Scholar]
- Behaviour and biomarkers of oxidative stress in Gambusia holbrooki after acute exposure to widely used pharmaceuticals and a detergent. Ecotoxicol. Environ. Saf.. 2008;71:341-354.
- [Google Scholar]
- Safety of Diastat, a rectal gel formulation of diazepam for acute seizure treatment. Drug Saf.. 2004;27:383-392.
- [Google Scholar]
- Bacopa monniera selectively attenuates suppressed Superoxide dismutase activity in Diazepam induced amnesic mice. Ann. Neurosci.. 2011;18:8-13.
- [Google Scholar]
- Impact of rat P450 genetic polymorphism on diazepam metabolism. Expert Opin. Drug Metab. Toxicol.. 2009;5:1421-1433.
- [Google Scholar]
- Immunomodulation during and after castration under inhalation anaesthetic without genotoxic effects on equine lymphocytes. Res. Vet. Sci.. 2012;92:306-310.
- [Google Scholar]
- 8-hydroxy-2′ -deoxyguanosine (8-OHdG): a critical biomarker of oxidative stress and carcinogenesis. J. Environ. Sci. Health C. 2009;27:120-139.
- [Google Scholar]
- Chromosomal aberration rates and intravenously given diazepam. A negative study. JAMA. 1974;230:414-417.
- [Google Scholar]
- Drug addiction is associated with leukocyte telomere length. Sci. Rep.. 2013;3:1542.
- [Google Scholar]







