Translate this page into:
Development of synthetic food baits for mass trapping of Bactrocera zonata S. (Diptera: Tephritidae)
⁎Corresponding author at: Institute of Plant Protection, Muhammad Nawaz Shreef, University of Agriculture, old shujabad Road, Multan, Pakistan. shafqat.saeed@mnsuam.edu.pk (Shafqat Saeed)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The peach fruit fly, Bactrocera zonata (Saunders)(Diptera: Tephritidae) is one of the most damaging pest of fruits and is mainly managed by baits. The existing baits are less effective to manage its population and are active against only one sex. In the current study, response of male and female population of B. zonata to protein hydrolysate, jaggery, guava pulp, potassium hydroxide, papaya powder, and kachri powder was analyzed. The additive effects of ammonium compounds, ammonium acetate, trimethylamine, and putrescine as food attractants was also evaluated. A total of 32 food baits types were prepared and installed using plastic bottle traps. All the tested bait formulations attracted a higher number of male B. zonata as compared to females. The obtained results also indicated that protein hydrolysate along with jaggery, KOH, papaya, and kachri powder, and guava pulp showed minimum attractiveness to B. zonata adults. However, three local ammonium compounds mixed with base baits increased the capture of adults per trap per month and subsequent both the years. A triplet mixture of ammonium acetate, trimethylamine, and putrescine mixed with base baits showed a synergistic effect for the attraction of more B. zonata adults both males and females as compared to two or single component-based baits under field conditions. The locally available ammonium-based baits increased the attractiveness of B. zonata adults to a level comparable with GF-120 (Spinosad-based protein bait). The three-mixture compound, protein hydrolysate + jaggery + ammonium acetate + trimethylamine + putrescine for both sexes, was as effective as the key standard food bait attractant at pH level 6.82.
Keywords
Attractants
Fruit fly
GF-120
Jaggery
Protein hydrolysate
1 Introduction
The fly, Bactrocera zonata (Tephritidae: Diptera), is the most dominant and serious pest of vegetables and fruits worldwide (Bhargava and Bansal, 2018). It is distributed in the Sub-continent, Egypt, Libya, Oman, Saudi Arabia, the United Arab Emirates, Yemen, Sudan, and the Sub-Saharan region (Carey and Dowell, 1989). It causes heavy losses of an estimated $200 million on small and large farms annually. The losses due to fruit flies varied from species to species and host plant susceptibility. The guava fruit fly (Bactrocera correcta) (Bezzi) (Diptera: Tephritidae) was responsible for 60–80% of the loss. Guava and oriental fruit fly (B. dorsalis) (Hendel) (Diptera: Tephritidae) caused losses ranging from 5 to 100% (Kafi, 1986). The Ber fruit fly (Carpomya vesuviana Costa) (Diptera: Tephritidae) causes 90–100% damage. In Pakistan, the Peach fruit fly (B. zonata) is one of the most polyphagous species found in different ecological regions (Sarwar, 2006), causing 30–80% fruit losses depending on location, variety, and fruiting season (Mwatawala et al., 2006).
European Plant Protection Organization mentioned B. zonata as an A1 key quarantine pest by several countries (Liu et al., 2013). Due to their polyphagous in nature, tephritid flies attack several fruits and vegetables viz. mango, guava, citrus, peach, fig, apple, apricot, tomato, pepper, and eggplant as secondary hosts. Favorable environmental conditions (temperature, rainfall and relative humidity) lead to crop susceptibility, and losses extend from 30 to 100% (Shooker et al., 2006). Mnagement of fruit flies is much difficult in developing countries like Pakistan, due to feeding habits, behavioral changes and biological adaptability of life stages. Various types of conventional eradication techniques being used are fruit bagging, chemicals, sex pheromone traps, sterile insect technique, predators, parasitoids and entomo-pathogenic fungi (Vargas et al., 2007).
Control of fruit flies is mainly achieved by the application of broad-spectrum organophosphate insecticides. Which has resulted in the development of insecticide resistance pest resurgence, environmental pollution, maximum residual level and health hazard (Dias et al., 2018). Another drawback to the use of chemicals is that 3rd instar larvae leave rotten fruits and drop to pupate in the ground soil. Therefore, eggs and larvae in fruits and pupae in soil are well protected from insecticide surface application (Heve et al., 2017). The use of insect attractants and repellents is now one of the most important preventative methods nowadays.
So, an alternate more efficient, cost-effective and ecofriendly approach for the control of both sexes of B.zonata called synthetic proteinaceous food bait is developed for the attraction and mass trapping. Several advantages of food bait attractants are: first, both females and males are attracted; second, it is an alternative for monitoring fruit flies; and third one, synthetic lure removes the female population (Epsky et al., 2014).
Early food bait attractants were fermented sugar, yeast, molasses, and protein hydrolysate (Ahmad et al., 2007). Liquid proteinaceous mixtures with ammonium baits have been used to catch a wide range of different fruit flies (Agency., I.A.E., 2003). Many researchers also endorsed the efficacy of ammonium compounds as attractants to Anastrepha ludens and A. susupensa (Thomas et al., 2008), C. capitata and B. zonata and B. oleae (El-Metwally, 2012). For these reasons, protein hydrolysate with jaggery mixed with synthetic food attractants (ammonium acetate, trimethylamine, and putrescine) in different combinations was used to increase the volatile odours to get the maximum attraction of male and female responses (Canal et al., 2007).
The mode of action of volatile food-based attractants depends upon the odorant binding proteins (Brito et al., 2016). Synthetic food baits and plant semiochemicals, among other environmental factors, play critical roles in the life cycles of tephritidae fruit flies. Male Oriental fruit flies, Bactrocera dorsalis, are particularly drawn to ammonium, a phenylpropanoid found in food-based fragrances for attraction in “fruit fly orchids” (Tan and Nishida, 2012).
The goal of this study was to evaluate the potential of ammonium compounds viz. guava pulp, papaya powder, kachri powder, protein hydrolysate, jaggery, KOH, separate or with combinations for detecting, mass trapping, and monitoring adult B. zonata males and females in mango orchards at different population levels.
2 Material and methods
2.1 Experimental site
The study was conducted during 2019 and 2020 (January to December) in farmer orchard Durani Fruit Farm (Latitude: 30.324 and Longitude: 71.5604) near Defense Housing Authority (DHA), Multan, Punjab, Pakistan. The selected orchards having an area 30 acre and containing more and less 36 to 40 mango tree per acre. Thirty-two different attractants were tested using locally handmade plastic bottle traps (PB Traps) in a randomized complete block design with four replications per attractant. The experimental orchards were isolated from other orchards, and no insecticide application was done during study period. All the cultural practices were done simultaneously in the whole orchards.
2.2 Pheromone traps and Attractants
The locally designed pheromone traps were made by using a clean, empty two-liter plastic soda bottle (320 mm in length and 8 mm in diameter) with screwed lid. The plastic bottle trap is more cost-effective than the others commercially available trap (pheromones trap). Its design allows for easy trap service and is longer lasting since it is made of hard plastic material. Furthermore, its versatility in the use of various liquid lures based on ammonium compounds provides the trap with an additional advantage and suitable for climatic conditions for Bactrocera spp. It has been demonstrated that liquid retention systems based on water are a good option in combination with the synthetic food lures either three and two component lures (Ammonium Acetate, Trimethylamine and Putrescine) in a trap. These plastic bottle traps (PB Traps) with closed bottom had four holes of 8 cm diameter around side to allow the flies to enter inside. The trap was suspended vertically at the height of about 5–6 feet above the ground level on the tree canopy. A small cotton wick soaked with 5 mL of each attractant and placed inside center of the trap and change fresh bait having the constant pH (prepared new and check pH with pH meter) (Fig. 1). The traps were installed during the first week of January 2019 to the last week of December 2020 and baits attractants was replenished with fresh bait at fortnightly intervals during the whole years.
All traps were mounted at uniform distance of 28 m between traps and under the shady place of the tree canopy to avoid interaction between baits. Six traps were installed in each block with having each attractant. Each block having trees with traps had similar density, canopy size, and fruiting condition. Locally manufactured thirty-two synthetic food-based attractant baits of different chemicals alone or in combination as compared with GF-120. Each bait consisting of ammonia acetate, trimethylamine, putrescine, papaya powder, kachri powder, potassium hydroxide (KOH), jaggery, guava pulp, and protein hydrolysate was tested for the attraction of both sexes of B. zonata. Ammonia appears to be the principal attractant originating from these synthetic food-based attractants emits volatile chemicals that are specific for specific fruit fly’s species and also developed an ecologically standardized detection system for targeted bait killing or attractant stations.
2.3 Fruit fly populations
The captured flies (males and females) were collected separately in polythene bags for each baited trap and brought to the lab for male and female of B. zonata separation and counted on weekly basis and then added to monthly basis. Mean number of flies were monitored on the basis of catch per trap per week and per month for two years. The observations thus obtained were correlated with the meteorological parameters like temperature (minimum and maximum), humidity, and rainfall. Meteorological data used in this study were provided by Cotton Research Institute, Multan.
2.4 Sex Ratio
The captured flies were separated carefully on the basis of their sex (males and females) and also count the total number of flies in each trap. Then, separating males and females on the total number of traps captured flies for determination of sex ratio. Sex ration was calculated by the formula with little modification (Adamski-Werner et al., 2004).
Where:
R = Sex ratio of trapped flies
m = Number of male flies trapped
f = Number of female flies trapped
2.5 Proteinaceous food baits attractants
Protein hydrolysate was obtained from fish meat and then blend in one liter of distilled water by using reagent bottle. Take 250 mL sample in separate four reagent bottles, then added different types of constituents (papaya powder + kachri powder; KOH; jaggery; guava pulp) separately, to prepare four different base baits. Each of the base bait was further mixed with three types of attractants i.e., ammonium acetate, trimethylamine, and putrescine. These attractants were further mixed collectively in different combinations to check their synergistic and antagonistic effects (Eriotou-Bargiota et al., 1992). Different combination of synthetic proteinaceous food baits was used in this experiment is listed in Table 1. AA = Ammonium acetate, TMA = Trimethylamine, Pu = Putrescine, KOH = Potassium hydroxide, PH = Protein hydrolysate
S. No
Baits Name
Chemical composition of Attractants Baits
1
Bait 1
Local trap with PH + Jaggery
2
Bait 2
Local trap with PH + Jaggery + AA
3
Bait 3
Local trap with PH + Jaggery + TMA
4
Bait 4
Local trap with PH + Jaggery + Pu
5
Bait 5
Local trap with PH + Jaggery + AA + TMA
6
Bait 6
Local trap with PH + Jaggery + AA + Pu
7
Bait 7
Local trap with PH + Jaggery + TMA + Pu
8
Bait 8
Local trap with PH + Jaggery + AA + TMA + Pu
9
Bait 9
Local trap with PH + papaya powder + kachri powder
10
Bait 10
Local trap with PH + papaya powder + kachri powder + AA
11
Bait 11
Local trap with PH + papaya powder + kachri powder + TMA
12
Bait 12
Local trap with PH + papaya powder + kachri powder + Pu
13
Bait 13
Local trap with PH + papaya powder + kachri powder + AA + TMA
14
Bait 14
Local trap with PH + papaya powder + kachri powder + AA + Pu
15
Bait 15
Local trap with PH + papaya powder + kachri powder + TMA + Pu
16
Bait 16
Local trap with PH + papaya powder + kachri powder + AA + TMA + Pu
17
Bait 17
Local trap with PH + KOH
18
Bait 18
Local trap with PH + KOH + AA
19
Bait 19
Local trap with PH + KOH + TMA
20
Bait 20
Local trap with PH + KOH + Pu
21
Bait 21
Local trap with PH + KOH + AA + TMA
22
Bait 22
Local trap with PH + KOH + AA + Pu
23
Bait 23
Local trap with PH + KOH + TMA + Pu
24
Bait 24
Local trap with PH + KOH + AA + TMA + Pu
25
Bait 25
Local trap with PH + Guava pulp
26
Bait 26
Local trap with PH + Guava pulp + AA
27
Bait 27
Local trap with PH + Guava pulp + TMA
28
Bait 28
Local trap with PH + Guava pulp + Pu
29
Bait 29
Local trap with PH + Guava pulp + AA + TMA
30
Bait 30
Local trap with PH + Guava pulp + AA + Pu
31
Bait 31
Local trap with PH + Guava pulp + TMA + Pu
32
Bait 32
Local trap with PH + Guava pulp + AA + TMA + Pu
33
Bait 33 (GF-120)
Local trap with GF 120
2.6 Estimating pH Levels
Each as a fresh baits sample was taken 10 mL for the estimation of pH level of synthetic proteinaceous food baits analysis. By using High-Performance Bench Meter for Universal Applications “OHAUS Stater 5000 pH Bench Meter”.
2.7 Statistical analysis
The mean of values pest population along with standard error were calculated by using statistical software SAS 9.3 program (SAS Institute Inc, 2011a). The data means were compared according to ANOVA and subjected to applying Least Significant Difference (LSD) test at 5% level of significance. To evaluate the efficiency of synthetic food-based attractants as baits for B. zonata against weather factors, regression analysis had been done.
3 Results
3.1 Efficacy of different attractants
Different attractants showed significant differences in attracting fruit flies during both the years in mango orchard. Interaction between observation dates and attractants was found significant for number of fruit flies in mango. The obtained data indicated, that adult B. zonata showed different degrees of preference to the 32 different synthetic proteinaceous food baits attractants with GF-120 were tested in mango orchard under field conditions and result expressed as a mean number of captured flies/trap/months.
3.2 Population of Male B. zonata captured by different attractants in mango orchards during Season 2019
The attraction of B. zonata to different synthetic food baits ammonium acetate, trimethylamine, and putrescine was evaluated under field conditions and mean number of captured flies/trap/months was expressed (Table 2). In the year 2019, the recorded data indicate that adult B. zonata males exhibited degree of preference to different tested food baits without mixing pesticides. The data showed inequality in attracting of B. zonata adults between different tested ammonium food attractants. Ammonium acetate, trimethylamine and putrescine indicated highly significant attraction of B. zonata males and females as compared with two or single component mixed with different basic bait. The fly captured were also compared with GF-120 (Spinosad-based protein bait). As the data is very large from January 2019 to December 2020, therefore we consider the peak population months (June, July, August) of both the years due to two reasons first full fruiting period, second maximum abundance of fruit flies’ population attraction that attracted towards the proteinaceous food baits. Bait 1 PH + Jaggery: Bait 2 PH + Jaggery + AA: Bait 3 PH + Jaggery + TMA: Bait 4 PH + Jaggery + Pu: Bait 5 PH + Jaggery + AA + TMA: Bait 6 PH + Jaggery + AA + Pu: Bait 7 PH + Jaggery + TMA + Pu: Bait 8 PH + Jaggery + AA + TMA + Pu : Bait 9 PH + papaya powder + kachri powder: Bait 10 PH + papaya powder + kachri powder + AA: Bait 11 PH + papaya powder + kachri powder + TMA: Bait 12 PH + papaya powder + kachri powder + Pu Bait 13 PH + papaya powder + kachri powder + AA + TMA: Bait 14 PH + papaya powder + kachri powder + AA + Pu : Bait 15 PH + papaya powder + kachri powder + TMA + Pu: Bait 16 PH + papaya powder + kachri powder + AA + TMA + Pu: Bait 17 PH + KOH: Bait 18 PH + KOH + AA: Bait 19 PH + KOH + TMA: Bait 20 PH + KOH + Pu: Bait 21 PH + KOH + AA + TMA: Bait 22 PH + KOH + AA + Pu: Bait 23 PH + KOH + TMA + Pu Bait 24 PH + KOH + AA + TMA + Pu: Bait 25 PH + Guava pulp: Bait 26 PH + Guava pulp + AA: Bait 27 PH + Guava pulp + TMA: Bait 28 PH + Guava pulp + Pu: Bait 29 PH + Guava pulp + AA + TMA: Bait 30 PH + Guava pulp + AA + Pu : Bait 31 PH + Guava pulp + TMA + Pu : Bait 32 PH + guava pulp + AA + TMA + Pu: Bait 33 GF 120. Mean followed by the same letter (within each month or factor) are not significantly different (P > 0.05)
Baits Treatments
January
February
March
April
May
June
July
August
September
October
November
December
Bait 1
0.00 ± 0.00 e
2.25 ± 0.37 hi
7.19 ± 0.90 jk
15.44 ± 0.94 kl
22.19 ± 1.03 i-k
36.25 ± 3.64 g-i
17.63 ± 1.47 ij
7.25 ± 0.31 n-p
4.75 ± 0.42 h-j
1.69 ± 0.27 i-k
0.38 ± 0.15 de
0.00 ± 0.00 d
Bait 2
0.00 ± 0.00 e
4.31 ± 0.28 de
15.69 ± 0.53 g
23.75 ± 1.49 gh
29.00 ± 2.82 h
38.56 ± 1.29 g
31.44 ± 1.11 h
18.25 ± 0.62 h
9.88 ± 1.40 f-i
1.00 ± 0.34 k
0.50 ± 0.20 de
0.25 ± 0.68 cd
Bait 3
0.00 ± 0.00 e
2.31 ± 0.40 g-i
5.81 ± 0.36 kl
16.81 ± 1.03 jk
16.38 ± 1.13 l
24.00 ± 1.21 j-l
20.38 ± 0.49 i
12.00 ± 0.94 i-m
8.38 ± 0.48 h-j
0.94 ± 0.40 k
0.06 ± 0.06 e
0.00 ± 0.00 d
Bait 4
1.13 ± 0.51 bc
5.06 ± 0.57 cd
23.75 ± 0.76 b
40.81 ± 2.74c
89.25 ± 4.34 c
123.25 ± 1.31 c
123.19 ± 6.45 c
85.19 ± 2.59 c
35.44 ± 6.20 bc
6.81 ± 0.36 c
1.19 ± 0.31 bc
0.38 ± 0.26 b-d
Bait 5
0.00 ± 0.00 e
2.31 ± 0.71 g-i
5.38 ± 0.26 l
21.25 ± 0.41 ij
9.25 ± 0.56 n
13.56 ± 1.41 m-p
15.06 ± 2.64 i-l
5.63 ± 0.43 o-q
4.25 ± 0.31 h-j
1.00 ± 0.32 k
0.06 ± 0.06 e
0.00 ± 0.00 d
Bait 6
1.50 ± 0.68 ab
6.63 ± 0.88 b
25.31 ± 0.85 b
47.44 ± 2.14 b
96.25 ± 4.62 b
144.13 ± 4.73 b
144.62 ± 6.06 b
97.44 ± 4.54 b
38.19 ± 6.54 ab
10.31 ± 0.42 b
4.63 ± 0.33 a
1.88 ± 0.51 a
Bait 7
0.00 ± 0.00 e
6.06 ± 0.41 bc
8.00 ± 0.50 j
19.44 ± 1.30 ij
25.00 ± 2.09 hi
30.19 ± 1.70 g-j
18.13 ± 1.28 ij
13.69 ± 0.58 h-k
8.25 ± 0.98 h-j
1.25 ± 0.35 jk
0.06 ± 0.06 e
0.00 ± 0.00 d
Bait 8
1.81 ± 0.83 a
8.44 ± 0.50 a
29.56 ± 1.19 a
57.19 ± 1.40 a
104.06 ± 4.62 a
166.94 ± 6.89 a
168.94 ± 4.82 a
108.25 ± 5.32 a
42.63 ± 7.0 1 a
12.00 ± 0.34 a
4.81 ± 0.36 a
1.88 ± 0.51 a
Bait 9
0.00 ± 0.00 e
2.38 ± 1.06 g-i
2.50 ± 0.34 n-p
14.50 ± 0.50 k-m
18.06 ± 0.30 kl
18.56 ± 0.64 l-n
16.94 ± 0.85 i-k
11.38 ± 1.39 j-n
3.50 ± 0.58 ij
1.06 ± 0.30 jk
0.06 ± 0.06 e
0.00 ± 0.00 d
Bait 10
0.00 ± 0.00 e
2.25 ± 0.37 hi
6.25 ± 0.63 kl
14.69 ± 1.47 k-m
18.75 ± 1.17 j-l
27.81 ± 2.46 i-k
31.94 ± 2.09 h
12.88 ± 1.49 i-l
4.25 ± 0.73 h-j
1.31 ± 0.15 i-k
0.25 ± 0.17 e
0.00 ± 0.00 d
Bait 11
0.00 ± 0.00 e
1.88 ± 0.22 h-j
5.50 ± 0.29 l
12.31 ± 0.73 l-n
24.00 ± 1.21 h-j
24.50 ± 0.60 j-l
9.44 ± 0.44 k-o
6.37 ± 0.27 o-q
3.63 ± 0.51 ij
1.00 ± 0.26 k
0.06 ± 0.06 e
0.00 ± 0.00 d
Bait 12
0.00 ± 0.00 e
0.94 ± 0.21 j-l
6.25 ± 0.56 kl
5.56 ± 0.70 p-s
20.63 ± 1.04 i-l
21.38 ± 1.92 k-m
8.50 ± 0.29 l-o
6.25 ± 0.43 o-q
3.38 ± 0.42 ij
1.44 ± 0.35 i-k
0.06 ± 0.06 e
0.00 ± 0.00 d
Bait 13
0.00 ± 0.00 e
2.44 ± 0.27 g-i
2.75 ± 0.28 n-p
2.69 ± 0.46 r-t
22.00 ± 0.39 i-k
29.44 ± 2.76 h-k
13.44 ± 0.71 i-n
8.75 ± 0.62 l-o
4.31 ± 0.62 h-j
1.19 ± 0.36 jk
0.06 ± 0.06 e
0.00 ± 0.00 d
Bait 14
0.25 ± 0.17 de
3.56 ± 0.13 ef
16.13 ± 0.95 fg
21.88 ± 1.16 g-i
49.63 ± 1.02 f
62.69 ± 7.11 f
53.69 ± 3.29 g
31.25 ± 1.21 g
15.94 ± 1.81 ef
4.44 ± 0.40 de
0.44 ± 0.18 de
0.00 ± 0.00 d
Bait 15
0.00 ± 0.00 e
2.25 ± 0.25 hi
3.63 ± 0.63 mn
2.44 ± 0.47 st
19.13 ± 1.10 j-l
7.38 ± 0.75 o-q
6.69 ± 0.44 m-o
5.81 ± 0.34 o-q
4.75 ± 0.46 h-j
3.06 ± 0.39 f-h
0.06 ± 0.06 e
0.00 ± 0.00 d
Bait 16
0.38 ± 0.26 de
3.63 ± 0.30 ef
17.75 ± 0.91 ef
24.94 ± 1.18 fg
63.81 ± 1.67 e
73.38 ± 6.44 e
53.31 ± 1.85 g
29.44 ± 2.42 g
15.06 ± 1.23 e-g
3.81 ± 0.40 d-f
0.81 ± 0.21 cd
0.38 ± 0.18 b-d
Bait 17
0.00 ± 0.00 e
3.31 ± 0.15 e-g
1.69 ± 0.44 p
2.69 ± 0.50 r-t
20.63 ± 0.99 i-l
10.81 ± 1.29 n-q
6.00 ± 0.26 no
5.69 ± 0.28 o-q
4.75 ± 0.21 h-j
1.56 ± 0.34 i-k
0.06 ± 0.06 e
0.00 ± 0.00 d
Bait 18
0.00 ± 0.00 e
2.25 ± 0.11 hi
6.13 ± 0.26 kl
2.19 ± 0.52 t
23.88 ± 2.01 h-j
13.06 ± 1.98 m-p
6.88 ± 0.22 m-o
5.81 ± 0.23 o-q
5.25 ± 0.21 h-j
1.06 ± 0.23 jk
0.06 ± 0.06 e
0.00 ± 0.00 d
Bait 19
0.00 ± 0.00 e
3.50 ± 0.22 ef
2.06 ± 0.48 n-p
5.94 ± 0.69 p-r
15.88 ± 1.22 lm
14.44 ± 1.43 m-p
7.87 ± 0.26 l-o
7.00 ± 0.35 n-q
6.75 ± 0.42 h-j
1.44 ± 0.38 i-k
0.06 ± 0.06 e
0.00 ± 0.00 d
Bait 20
0.00 ± 0.00 e
2.88 ± 0.20 f-h
11.81 ± 0.57 hi
21.50 ± 0.50 hi
23.06 ± 3.22 i-k
29.63 ± 3.17 h-k
14.12 ± 1.04 i-m
9.69 ± 0.71 k-o
8.75 ± 0.82 g-j
2.38 ± 0.54 g-i
0.31 ± 0.22 de
0.00 ± 0.00 d
Bait 21
0.00 ± 0.00 e
2.13 ± 0.09 hi
1.88 ± 0.49 op
3.88 ± 0.68 q-t
10.44 ± 0.72 mn
3.12 ± 0.35 q
4.56 ± 0.95 o
2.63 ± 0.18 q
2.75 ± 0.21 j
0.81 ± 0.32 k
0.06 ± 0.06 e
0.00 ± 0.00 d
Bait 22
0.00 ± 0.00 e
2.81 ± 0.14 f-h
10.56 ± 0.40 i
8.06 ± 1.28 op
5.69 ± 0.27 n
3.25 ± 0.54 q
7.19 ± 1.12 m-o
4.00 ± 0.24 pq
3.00 ± 0.00 j
1.31 ± 0.31 i-k
0.06 ± 0.06 e
0.00 ± 0.00 d
Bait 23
0.00 ± 0.00 e
2.25 ± 0.11 hi
1.50 ± 0.32 p
6.75 ± 1.54 pq
6.31 ± 0.50 n
4.44 ± 0.68 q
7.38 ± 0.33 m-o
6.19 ± 0.28 o-q
5.25 ± 0.28 h-j
1.38 ± 0.46 i-k
0.06 ± 0.06 e
0.00 ± 0.00 d
Bait 24
0.00 ± 0.00 e
2.38 ± 0.34 g-i
6.13 ± 0.30 kl
11.69 ± 0.51 mn
19.69 ± 0.87 i-l
37.37 ± 1.24 gh
36.56 ± 2.19 h
16.00 ± 1.38 hi
10.25 ± 0.21 f-h
1.75 ± 0.56 i-k
0.31 ± 0.18 de
0.00 ± 0.00 d
Bait 25
0.00 ± 0.00 e
3.63 ± 0.26 ef
3.50 ± 0.58 m-o
10.81 ± 0.98 no
6.00 ± 0.18 n
6.13 ± 0.85 pq
8.31 ± 1.06 l-o
5.69 ± 0.48 o-q
4.75 ± 0.38 h-j
1.25 ± 0.36 jk
0.06 ± 0.06 e
0.00 ± 0.00 d
Bait 26
0.44 ± 0.27 de
4.69 ± 0.12 d
19.06 ± 0.90 de
27.38 ± 1.26 ef
69.25 ± 2.29 e
88.81 ± 5.05 d
77.69 ± 3.90 f
44.69 ± 2.54 f
19.06 ± 2.51 de
4.25 ± 0.60 de
1.25 ± 0.27 bc
0.75 ± 0.21b
Bait 27
0.00 ± 0.00 e
0.75 ± 0.34 kl
2.50 ± 0.39 n-p
6.94 ± 1.33 pq
16.13 ± 1.47 l
14.69 ± 0.94 m-o
17.69 ± 1.16 ij
12.88 ± 1.41 i-l
4.50 ± 0.72 h-j
1.19 ± 0.48 jk
0.06 ± 0.06 e
0.00 ± 0.00 d
Bait 28
0.00 ± 0.00 e
1.75 ± 0.28 i-k
12.25 ± 0.36 h
17.38 ± 1.42 jk
41.75 ± 1.70 g
37.81 ± 1.65 gh
29.69 ± 1.76 h
15.69 ± 1.37 h-j
5.81 ± 1.19 h-j
2.13 ± 0.26 h-j
1.25 ± 0.57 bc
0.38 ± 0.13 b-d
Bait 29
0.00 ± 0.00 e
0.50 ± 0.22 l
2.50 ± 0.41 n-p
5.13 ± 0.43 p-t
9.69 ± 0.57 n
8.13 ± 0.60 o-q
5.88 ± 0.76 no
5.44 ± 0.27 o-q
3.19 ± 0.52 j
0.81 ± 0.33 k
0.06 ± 0.06 e
0.00 ± 0.00 d
Bait 30
0.75 ± 0.36 cd
5.75 ± 0.21 bc
19.69 ± 0.80 d
29.75 ± 1.75 e
79.00 ± 1.35 d
92.19 ± 4.40 d
90.38 ± 5.66 e
50.63 ± 1.91 e
22.63 ± 3.15 d
4.31 ± 0.51 de
1.44 ± 0.26 b
0.63 ± 0.18 bc
Bait 31
0.00 ± 0.00 e
0.00 ± 0.00 l
4.88 ± 0.42 lm
6.56 ± 1.33 pq
17.81 ± 1.00 kl
13.25 ± 1.47 m-p
8.00 ± 0.16 l-o
7.87 ± 0.20 m-p
7.75 ± 0.21 h-j
3.44 ± 0.71 e-g
0.06 ± 0.06 e
0.00 ± 0.00 d
Bait 32
0.75 ± 0.36 cd
6.00 ± 0.18 bc
21.75 ± 0.68 c
34.81 ± 1.97 d
89.69 ± 2.81c
121.19 ± 4.91 c
110.44 ± 7.09 d
67.81 ± 1.67 d
29.31 ± 4.96 c
4.69 ± 0.71 d
1.38 ± 0.24 b
0.63 ± 0.22 bc
Bait 33GF-120
0.00 ± 0.00 e
0.00 ± 0.00 l
2.63 ± 0.26 nop
12.81 ± 0.59 l-n
7.37 ± 0.64 n
19.13 ± 2.25 l-n
11.38 ± 1.68 j-o
6.25 ± 0.75 o-q
2.38 ± 0.39 j
0.94 ± 0.19 k
0.13 ± 0.13 e
0.00 ± 0.00 d
P – Value
0.00
0.00
0.00
0.00
0.00
0.00
0.00
0.00
0.00
0.00
0.00
0.00
F- Value
3.82
26.83
179.8
125.68
223.47
205.18
253.71
289.65
21.19
43.38
38.35
9.58
Data regarding number of traps catches of male B. zonata attracted to different food baits attractants during June, in mango field was found significantly (F32,527 = 205.18, p < 0.05) different. With respect to the tested ammonium compound, data indicated that bait 8 followed by bait 6 recorded highest efficacy of attraction i.e., 166.94 ± 6.89, 144.13 ± 4.73, respectively. Whereas lowest attraction among, bait 22 and 21 showed lowest efficacy of response having attraction 3.25 ± 0.54 and 3.12 ± 0.35, respectively. Statistical analysis showed a positive response to all tested ammonium compounds during July (Table 2), remained significant (F32,527 = 253.71, p < 0.05) comes in bait 8,6 (168.94 ± 4.82; 144.62 ± 6.06, respectively). While bait 21 (4.56 ± 0.95) was exhibited the lowest attraction of catches/trap/month.
Number of B. zonata was significantly (F32,527 = 289.65, p < 0.05) influenced by different attractants during August (Table 2). The highest number of flies/traps/months was observed towards bait 8,6 (108.25 ± 5.32; 97.44 ± 4.54, respectively). While the lowest number of flies/traps/months towards bait 21 (2.63 ± 0.18) was observed. In the case of imported bait (GF-120 spinosad based protein bait) a very few flies in June, July and August (19.13 ± 2.25; 11.38 ± 1.68; 6.25 ± 0.75, respectively) were caught in traps.
3.3 Population response of Female B. zonata captured by different attractants in mango orchards during 2019
Among the different combinations of synthetic proteinaceous food baits data indicated that adult female B. zonata have shown different responses of attractants towards 32 baits under field conditions (Table 3). Bait 1 PH + Jaggery: Bait 2 PH + Jaggery + AA: Bait 3 PH + Jaggery + TMA: Bait 4 PH + Jaggery + Pu:Bait 5 PH + Jaggery + AA + TMA: Bait 6 PH + Jaggery + AA + Pu: Bait 7 PH + Jaggery + TMA + Pu: Bait 8 PH + Jaggery + AA + TMA + Pu : Bait 9 PH + papaya powder + kachri powder: Bait 10 PH + papaya powder + kachri powder + AA: Bait 11 PH + papaya powder + kachri powder + TMA: Bait 12 PH + papaya powder + kachri powder + Pu Bait 13 PH + papaya powder + kachri powder + AA + TMA: Bait 14 PH + papaya powder + kachri powder + AA + Pu : Bait 15 PH + papaya powder + kachri powder + TMA + Pu: Bait 16 PH + papaya powder + kachri powder + AA + TMA + Pu: Bait 17 PH + KOH: Bait 18 PH + KOH + AA: Bait 19 PH + KOH + TMA: Bait 20 PH + KOH + Pu: Bait 21 PH + KOH + AA + TMA: Bait 22 PH + KOH + AA + Pu: Bait 23 PH + KOH + TMA + Pu Bait 24 PH + KOH + AA + TMA + Pu: Bait 25 PH + Guava pulp: Bait 26 PH + Guava pulp + AA: Bait 27 PH + Guava pulp + TMA: Bait 28 PH + Guava pulp + Pu: Bait 29 PH + Guava pulp + AA + TMA: Bait 30 PH + Guava pulp + AA + Pu : Bait 31 PH + Guava pulp + TMA + Pu : Bait 32 PH + guava pulp + AA + TMA + Pu: Bait 33 GF 120. Mean followed by the same letter (within each month or factor) are not significantly different (P > 0.05)
Baits /Treatments
January
February
March
April
May
June
July
August
September
October
November
December
Bait 1
0.00 ± 0.00 c
1.31 ± 0.28 f-i
3.88 ± 0.53 d
10.06 ± 1.20 h-k
11.50 ± 0.61 f-i
16.94 ± 1.49 g-i
9.50 ± 0.37 f-i
3.63 ± 0.35 j-m
1.88 ± 0.30 fg
0.00 ± 0.00 f
0.00 ± 0.00 h
0.00 ± 0.00 e
Bait 2
0.00 ± 0.00 c
2.94 ± 0.37 bc
7.69 ± 1.17 cd
13.31 ± 1.88 gh
15.63 ± 1.57 fg
18.88 ± 0.98 g
8.94 ± 0.57 f-j
6.44 ± 0.50 g-i
3.06 ± 0.59 fg
0.00 ± 0.00 f
0.00 ± 0.00 h
0.00 ± 0.00 e
Bait 3
0.00 ± 0.00 c
2.06 ± 0.35 c-g
2.56 ± 0.29 d
12.94 ± 0.96 g-i
10.63 ± 0.55 g-k
23.31 ± 6.10 f
11.13 ± 0.48 f-h
6.69 ± 0.25 gh
2.13 ± 0.44 fg
0.00 ± 0.00 f
0.00 ± 0.00 h
0.00 ± 0.00 e
Bait 4
0.38 ± 0.22 bc
2.88 ± 0.50 bc
21.69 ± 2.22 b
41.56 ± 4.19 c
36.31 ± 2.28 c-e
42.19 ± 1.39 c
39.75 ± 2.40 c
29.31 ± 1.45 c
12.25 ± 1.85 b
1.94 ± 0.31 de
1.06 ± 0.23 e
0.00 ± 0.00 e
Bait 5
0.00 ± 0.00 c
2.38 ± 0.64 b-e
2.63 ± 0.30 d
14.56 ± 1.41 g
4.81 ± 0.47 m
6.38 ± 1.20 n-r
7.69 ± 0.91 g-k
2.25 ± 0.19 m
1.38 ± 0.26 fg
0.00 ± 0.00 f
0.00 ± 0.00 h
0.00 ± 0.00 e
Bait 6
0.69 ± 0.31 ab
2.75 ± 0.37 bc
24.25 ± 1.86 b
49.44 ± 1.91 b
40.69 ± 3.31 b-d
36.81 ± 2.34 d
44.44 ± 3.11 b
33.88 ± 2.85 b
13.88 ± 3.00 b
5.31 ± 0.84 b
2.06 ± 0.35 c
3.00 ± 0.29 b
Bait 7
0.00 ± 0.00 c
2.63 ± 0.60 bc
4.69 ± 0.59 d
13.94 ± 1.49 gh
16.75 ± 1.59 f
14.25 ± 1.06 h-j
11.81 ± 0.54 fg
5.31 ± 0.60 g-l
3.13 ± 0.46 fg
0.00 ± 0.00 f
0.00 ± 0.00 h
0.00 ± 0.00 e
Bait 8
0.88 ± 0.40 a
4.13 ± 0.26 a
50.94 ± 18.82 a
53.69 ± 1.74 a
42.31 ± 3.51 b
53.13 ± 1.41 a
49.94 ± 4.45 a
47.81 ± 2.32 a
20.88 ± 4.11 a
9.38 ± 1.00 a
3.63 ± 0.38 a
4.19 ± 0.31 a
Bait 9
0.00 ± 0.00 c
1.69 ± 0.58 d-h
1.50 ± 0.29 d
10.75 ± 0.77 g-j
9.13 ± 0.60 h-m
9.38 ± 0.81 k-o
10.31 ± 0.54 f-i
5.63 ± 0.63 g-k
1.69 ± 0.36 fg
0.00 ± 0.00 f
0.00 ± 0.00 h
0.00 ± 0.00 e
Bait 10
0.00 ± 0.00 c
1.19 ± 0.28 g-j
2.00 ± 0.40 d
12.94 ± 1.37 g-i
10.63 ± 1.69 g-k
10.06 ± 0.62 j-o
17.06 ± 1.23 e
5.50 ± 0.85 g-k
1.75 ± 1.24 fg
0.00 ± 0.00 f
0.00 ± 0.00 h
0.00 ± 0.00 e
Bait 11
0.00 ± 0.00 c
1.56 ± 0.43 e-i
2.75 ± 0.36 d
9.25 ± 0.71 i-l
8.19 ± 0.83 h-m
12.69 ± 0.47 i-k
5.81 ± 0.44 i-m
3.75 ± 0.35 i-m
0.63 ± 0.29 g
0.06 ± 0.06 f
0.19 ± 0.19 gh
0.00 ± 0.00 e
Bait 12
0.00 ± 0.00 c
0.69 ± 0.28 i-k
3.44 ± 0.27 d
4.38 ± 0.74 m-q
5.94 ± 0.72 j-m
10.44 ± 0.87 j-n
3.50 ± 0.24 k-m
3.19 ± 0.39 k-m
0.50 ± 0.24 g
0.19 ± 0.14 f
0.19 ± 0.14 gh
0.00 ± 0.00 e
Bait 13
0.00 ± 0.00 c
1.44 ± 0.34 f-i
2.56 ± 0.33 d
3.25 ± 0.79 n-q
5.19 ± 0.79 lm
11.00 ± 1.05 j-m
6.94 ± 0.51 h-l
3.25 ± 0.21 j-m
0.63 ± 0.29 g
0.00 ± 0.00 f
0.00 ± 0.00 h
0.00 ± 0.00 e
Bait 14
0.38 ± 0.26 bc
2.38 ± 0.13 b-e
15.63 ± 2.01 bc
23.31 ± 1.84 f
38.13 ± 3.88 b-e
23.38 ± 0.26 f
31.88 ± 2.77 d
16.00 ± 1.92 e
4.38 ± 0.66 d-f
2.44 ± 0.36 de
0.50 ± 0.24 fg
0.00 ± 0.00 e
Bait 15
0.00 ± 0.00 c
2.19 ± 0.38 c-f
1.63 ± 0.26 d
0.69 ± 0.20 q
8.56 ± 0.13 h-m
4.38 ± 0.73 p-r
2.56 ± 0.20 lm
2.75 ± 0.23 lm
2.00 ± 0.39 fg
0.00 ± 0.00 f
0.00 ± 0.00 h
0.00 ± 0.00 e
Bait 16
0.63 ± 0.35 ab
2.63 ± 0.24 bc
18.50 ± 1.51 b
26.44 ± 1.15 ef
41.25 ± 3.73 bc
27.94 ± 1.32 e
36.63 ± 2.26 c
12.06 ± 0.76 f
6.44 ± 0.99 cde
1.56 ± 0.34 e
1.06 ± 0.37 e
0.00 ± 0.00 e
Bait 17
0.00 ± 0.00 c
2.06 ± 0.31 c-g
1.25 ± 0.32 d
0.94 ± 0.21 po
11.25 ± 1.61 g-j
9.25 ± 0.77 k-o
3.06 ± 0.30 lm
2.69 ± 0.18 lm
2.50 ± 0.47 fg
0.00 ± 0.00 f
0.00 ± 0.00 h
0.00 ± 0.00 e
Bait 18
0.00 ± 0.00 c
1.06 ± 0.27 h-j
2.38 ± 0.36 d
1.31 ± 0.36 o-q
11.75 ± 1.42 f-h
7.06 ± 1.22 m-q
3.00 ± 0.20 lm
3.00 ± 0.26 k-m
1.63 ± 0.44 fg
0.00 ± 0.00 f
0.00 ± 0.00 h
0.00 ± 0.00 e
Bait 19
0.00 ± 0.00 c
1.69 ± 0.28 d-h
1.94 ± 0.32 d
2.69 ± 0.40 n-q
10.56 ± 1.40 g-l
5.88 ± 0.80 o-r
3.19 ± 0.25 k-m
2.94 ± 0.27 k-m
1.88 ± 0.33 fg
0.00 ± 0.00 f
0.00 ± 0.00 h
0.00 ± 0.00 e
Bait 20
0.00 ± 0.00 c
0.81 ± 0.19 h-k
4.88 ± 0.60 d
11.50 ± 1.17 g-i
11.94 ± 2.14 f-h
11.69 ± 0.68 j-l
7.75 ± 1.05 g-k
3.63 ± 0.29 j-m
1.88 ± 0.51 fg
0.00 ± 0.00 f
0.00 ± 0.00 h
0.00 ± 0.00 e
Bait 21
0.00 ± 0.00 c
0.69 ± 0.18 i-k
3.63 ± 0.72 d
1.69 ± 0.36 o-q
8.25 ± 1.12 h-m
2.19 ± 0.25 r
3.50 ± 1.39 k-m
3.94 ± 0.43 i-m
1.44 ± 0.69 fg
0.00 ± 0.00 f
0.00 ± 0.00 h
0.00 ± 0.00 e
Bait 22
0.00 ± 0.00 c
1.38 ± 0.24 f-i
3.19 ± 0.75 d
5.06 ± 1.04 m-q
4.81 ± 0.53 m
2.56 ± 0.36 r
4.88 ± 2.72 j-m
2.50 ± 0.47 m
1.44 ± 0.56 fg
0.00 ± 0.00 f
0.00 ± 0.00 h
0.00 ± 0.00 e
Bait 23
0.00 ± 0.00 c
1.56 ± 0.35 e-i
1.94 ± 0.27 d
4.81 ± 0.81 m-p
5.31 ± 0.58 k-m
2.94 ± 0.27 qr
2.19 ± 0.56 m
2.50 ± 0.27 m
2.19 ± 0.48 fg
0.00 ± 0.00 f
0.00 ± 0.00 h
0.00 ± 0.00 e
Bait 24
0.00 ± 0.00 c
1.25 ± 0.14 g-i
1.56 ± 0.24 d
6.50 ± 0.77 k-n
9.81 ± 0.78 h-m
16.13 ± 2.00 g-i
12.81 ± 1.56 ef
7.88 ± 0.75 g
3.44 ± 0.81 e-g
0.00 ± 0.00 f
0.00 ± 0.00 h
0.00 ± 0.00 e
Bait 25
0.00 ± 0.00 c
1.69 ± 0.24 d-i
2.06 ± 0.28 d
5.63 ± 0.89 l-n
4.56 ± 0.54 m
3.88 ± 0.43 p-r
3.75 ± 0.62 k-m
2.44 ± 0.34 m
1.25 ± 0.32 g
0.00 ± 0.00 f
0.00 ± 0.00 h
0.00 ± 0.00 e
Bait 26
0.63 ± 0.29 ab
2.75 ± 0.17 bc
21.38 ± 1.02 b
30.00 ± 2.07 e
34.13 ± 3.41 e
33.44 ± 2.00 d
38.81 ± 2.32 c
23.44 ± 1.50 d
8.63 ± 1.56 c
4.25 ± 1.07 c
2.75 ± 0.30 b
1.44 ± 0.34 d
Bait 27
0.00 ± 0.00 c
0.13 ± 0.13 k
2.13 ± 0.47 d
3.31 ± 0.98 n-q
8.63 ± 1.44 h-m
9.25 ± 0.73 k-o
9.25 ± 0.82 f-j
5.94 ± 1.03 g-j
0.81 ± 0.28 g
0.00 ± 0.00 f
0.00 ± 0.00 h
0.00 ± 0.00 e
Bait 28
0.00 ± 0.00 c
0.94 ± 0.14 h-k
5.69 ± 0.44 d
11.13 ± 1.23 g-j
8.56 ± 0.96 h-m
17.50 ± 1.26 gh
10.19 ± 0.60 f-i
7.00 ± 0.94 g
2.31 ± 0.70 fg
0.00 ± 0.00 f
0.00 ± 0.00 h
0.00 ± 0.00 e
Bait 29
0.00 ± 0.00 c
0.13 ± 0.09 k
2.88 ± 0.78 d
3.31 ± 0.45 n-q
6.31 ± 0.35 i-m
6.19 ± 0.71 n-r
1.25 ± 0.50 m
3.56 ± 0.47 j-m
1.38 ± 0.40 fg
0.00 ± 0.00 f
0.00 ± 0.00 h
0.00 ± 0.00 e
Bait 30
0.88 ± 0.40 a
3.13 ± 0.20 b
21.25 ± 0.95 b
30.13 ± 1.91 e
35.81 ± 2.82 de
41.75 ± 1.29 c
38.31 ± 2.20 c
26.13 ± 1.19 d
3.56 ± 0.33 e-g
2.56 ± 0.30 d
1.50 ± 0.29 d
2.06 ± 0.30 c
Bait 31
0.00 ± 0.00 c
0.06 ± 0.06 k
2.69 ± 0.20 d
4.94 ± 1.18 m-o
9.00 ± 0.85 h-m
6.94 ± 0.84 m-q
3.06 ± 0.19 lm
4.19 ± 0.43 h-m
3.31 ± 0.44 fg
0.13 ± 0.13f
0.00 ± 0.00 h
0.00 ± 0.00 e
Bait 32
0.88 ± 0.40 a
2.56 ± 0.34 b-d
24.19 ± 1.56 b
35.25 ± 1.98 d
51.13 ± 4.29 a
46.56 ± 1.73 b
36.19 ± 3.51 cd
31.44 ± 1.20 bc
7.13 ± 1.16 cd
2.50 ± 0.51 d
0.75 ± 0.30 ef
0.00 ± 0.00 e
GF-120 (Control)
0.00 ± 0.00 c
0.31 ± 0.22 jk
1.31 ± 0.22 d
7.25 ± 0.73 j-m
4.75 ± 0.47 m
7.56 ± 1.07 l-p
4.13 ± 0.78 k-m
3.63 ± 0.49 j-m
1.31 ± 0.41 fg
0.13 ± 0.13 f
0.00 ± 0.00 h
0.00 ± 0.00 e
P – Value
0.00
0.00
0.00
0.00
0.00
0.00
0.00
0.00
0.00
0.00
0.00
0.00
F- Value
3.43
9.29
10.25
103.57
52.10
81.55
81.03
138.72
15.42
37.56
31.38
80.27
Data regarding the number of traps catches of female B. zonata attracted to different food baits attractants (Table 3) during June, in mango field was found significantly (F32,527 = 81.55, p < 0.05) different. With respect to the tested ammonium compound, data indicated that the highest relative catches/trap/months belonged to bait 8, 32 (53.13 ± 1.41; 46.56 ± 1.73, respectively). Whereas baits 22, 21 (2.56 ± 0.36; 2.19 ± 0.25, respectively) showed lowest catches and both are statistically similar.
Statistical analysis showed during July, the interaction between attractants and month (Table 3) remained significantly (F32,527 = 81.03, p < 0.05) comes in bait 8, 6 (49.94 ± 4.45; 44.44 ± 3.11, respectively). While bait 29 exhibited lowest attraction having value i.e., 1.25 ± 0.50. Number of female B. zonata was significantly (F32,527 = 138.72, p < 0.05) influenced by different attractants during August (Table 3). The highest number of catches/traps/months was observed towards bait 8 and 6 (47.81 ± 2.32; 33.88 ± 2.85, respectively). All the others baits showed a reasonable response towards the catches of fruit flies, while baits 5 showed the lowest odor attraction i.e., 2.25 ± 0.19. In the case of imported bait GF-120 (Spinosad protein bait) a very few flies in June, July and August (7.56 ± 1.07; 4.13 ± 0.78; 3.63 ± 0.49, respectively) were caught in traps observed.
3.4 Population of Male B. zonata captured by different attractants in mango orchards during Season 2020
According to data collected in the year 2020, adult B. zonata males demonstrated various degrees of affinity to several tested food baits attractants under field settings without mixing pesticides.
Data regarding number of traps catches of male B. zonata attracted to different food baits attractants (Table 4) during June, in mango field was found significantly (F32,527 = 383.76, p < 0.05) different. The highest relative catches/trap/months belonged to bait 8,6 (57.00 ± 0.18; 51.75 ± 0.21, respectively). Whereas, baits 22 (3.00 ± 0.48) showed the lowest catches/trap/month. Statistical analysis showed during July, the interaction between attractants and month (Table 4) remained significantly (F32,527 = 367.38, p < 0.05) comes in bait 8, 6 (73.60 ± 0.56; 69.40 ± 0.28, respectively). While, bait 29 (3.00 ± 0.18) was exhibited lowest attraction of catches/trap/months. Number of male B. zonata was significantly (F32,527 = 553.55, p < 0.05) influenced by different attractants during August (Table 4). The highest number of catches/traps/months was observed towards bait 8,6 (73.75 ± 0.56; 69.25 ± 0.28, respectively). While lowest number of catches/traps/months towards bait 29 (3.25 ± 0.18) was observed. Bait 1 PH + Jaggery: Bait 2 PH + Jaggery + AA: Bait 3 PH + Jaggery + TMA: Bait 4 PH + Jaggery + Pu: Bait 5 PH + Jaggery + AA + TMA: Bait 6 PH + Jaggery + AA + Pu: Bait 7 PH + Jaggery + TMA + Pu: Bait 8 PH + Jaggery + AA + TMA + Pu : Bait 9 PH + papaya powder + kachri powder: Bait 10 PH + papaya powder + kachri powder + AA: Bait 11 PH + papaya powder + kachri powder + TMA: Bait 12 PH + papaya powder + kachri powder + Pu Bait 13 PH + papaya powder + kachri powder + AA + TMA: Bait 14 PH + papaya powder + kachri powder + AA + Pu : Bait 15 PH + papaya powder + kachri powder + TMA + Pu: Bait 16 PH + papaya powder + kachri powder + AA + TMA + Pu: Bait 17 PH + KOH: Bait 18 PH + KOH + AA: Bait 19 PH + KOH + TMA: Bait 20 PH + KOH + Pu: Bait 21 PH + KOH + AA + TMA: Bait 22 PH + KOH + AA + Pu: Bait 23 PH + KOH + TMA + Pu Bait 24 PH + KOH + AA + TMA + Pu: Bait 25 PH + Guava pulp: Bait 26 PH + Guava pulp + AA: Bait 27 PH + Guava pulp + TMA: Bait 28 PH + Guava pulp + Pu: Bait 29 PH + Guava pulp + AA + TMA: Bait 30 PH + Guava pulp + AA + Pu : Bait 31 PH + Guava pulp + TMA + Pu : Bait 32 PH + guava pulp + AA + TMA + Pu: Bait 33 GF 120. Mean followed by the same letter (within each month or factor) are not significantly different (P > 0.05)
Baits / Treatments
January
February
March
April
May
June
July
August
September
October
November
December
Bait 1
0.00 ± 0.00 c
1.25 ± 0.56 f-h
3.25 ± 0.11 j-l
3.25 ± 0.10 mn
11.25 ± 0.21 ij
12.50 ± 0.29 kl
20.25 ± 0.34 jk
21.50 ± 0.29 jk
7.00 ± 0.41 lm
5.00 ± 0.26 o
5.75 ± 0.28 l-n
0.00 ± 0.00 f
Bait 2
0.00 ± 0.00 c
1.50 ± 0.53 e-g
7.50 ± 0.29 g
5.00 ± 0.16 j-l
11.25 ± 0.21 ij
21.75 ± 0.21 f
22.00 ± 0.18 ij
31.75 ± 0.21 f
11.75 ± 0.21 hi
16.00 ± 0.32 i
2.25 ± 0.28 q
1.00 ± 0.45 de
Bait 3
0.00 ± 0.00 c
1.75 ± 0.78 ef
3.50 ± 0.13 j-l
8.25 ± 0.52 fg
7.75 ± 0.80 l
15.00 ± 0.32 g-j
23.00 ± 0.32 ij
23.00 ± 0.32 h-j
13.00 ± 0.18 gh
15.75 ± 0.28 i
8.00 ± 0.18 jk
0.00 ± 0.00 f
Bait 4
0.00 ± 0.00 c
3.75 ± 0.34 b
13.00 ± 0.32 bc
21.00 ± 0.32 b
33.00 ± 0.29 b
51.25 ± 0.28 b
61.00 ± 0.18 c
68.25 ± 0.21 b
43.50 ± 0.29 c
40.00 ± 0.32 cd
17.00 ± 0.32 b
1.25 ± 0.56 de
Bait 5
0.00 ± 0.00 c
1.00 ± 0.45 f-h
3.75 ± 0.21 jk
7.75 ± 0.19 fg
7.50 ± 0.18 lm
14.25 ± 1.65 h-k
10.00 ± 0.53 l-n
10.50 ± 0.53 no
10.50 ± 0.53 h-k
10.50 ± 0.53 lm
10.50 ± 0.53 ef
1.50 ± 0.67 c-e
Bait 6
1.75 ± 0.78 a
6.00 ± 0.26 a
8.50 ± 0.13 fg
21.75 ± 0.19 b
34.00 ± 0.59 ab
51.75 ± 0.21 b
69.40 ± 0.28 b
69.25 ± 0.28 b
49.50 ± 0.29 b
48.25 ± 0.21 a
16.75 ± 0.11 b
1.75 ± 0.46 b-d
Bait 7
0.00 ± 0.00 c
2.25 ± 0.11 de
15.50 ± 0.76 a
6.75 ± 0.34 g-i
14.50 ± 0.39 fg
15.50 ± 0.39 g-i
13.00 ± 0.48 l
13.00 ± 0.48 m
13.00 ± 0.48 gh
13.00 ± 0.48 jk
13.00 ± 0.48 c
0.00 ± 0.00 f
Bait 8
0.75 ± 0.34 b
6.50 ± 0.13 a
13.25 ± 0.11 b
23.50 ± 0.11 a
35.50 ± 0.86 a
57.00 ± 0.18 a
73.60 ± 0.56 a
73.75 ± 0.56 a
53.50 ± 0.29 a
49.75 ± 0.38 a
20.50 ± 0.29 a
3.75 ± 0.59 a
Bait 9
0.00 ± 0.00 c
0.50 ± 0.22 hi
3.25 ± 0.11 j-l
12.00 ± 0.36 e
20.00 ± 0.42 d
20.00 ± 0.86 f
23.75 ± 1.99 hi
23.75 ± 1.99 h-j
23.75 ± 1.99 f
23.75 ± 1.99 h
5.00 ± 0.77 no
1.50 ± 0.67 c-e
Bait 10
0.00 ± 0.00 c
0.75 ± 0.34 g-i
8.75 ± 0.34 fg
12.00 ± 0.16 e
14.25 ± 0.29 gh
16.25 ± 0.38 gh
30.60 ± 0.55 g
30.00 ± 0.55 f
15.00 ± 0.80 g
15.00 ± 0.80 ij
8.75 ± 0.11 h-j
0.00 ± 0.00 f
Bait 11
0.00 ± 0.00 c
1.50 ± 0.67 e-g
0.00 ± 0.00 m
8.25 ± 0.52 fg
16.50 ± 0.13 e
10.00 ± 0.80 mn
20.25 ± 1.65 jk
20.25 ± 1.65 kl
11.00 ± 0.66 h-j
11.00 ± 0.66 k-m
11.00 ± 0.66 de
0.00 ± 0.00 f
Bait 12
0.00 ± 0.00 c
0.50 ± 0.22 hi
0.00 ± 0.00 m
3.00 ± 0.84 m-o
12.50 ± 0.38 hi
12.50 ± 0.29 kl
18.50 ± 2.22 k
18.50 ± 2.22 l
11.75 ± 0.38 hi
11.75 ± 0.38 kl
6.50 ± 0.29 lm
1.25 ± 0.56 de
Bait 13
0.00 ± 0.00 c
1.00 ± 0.45 f-h
3.50 ± 0.29 j-l
3.50 ± 0.57 lm
13.75 ± 0.22 gh
11.75 ± 0.21 lm
22.50 ± 1.94 ij
22.50 ± 1.94 i-k
12.00 ± 0.58 hi
12.00 ± 0.58 kl
12.00 ± 0.58 cd
2.25 ± 0.59 bc
Bait 14
0.00 ± 0.00 c
2.25 ± 1.01 de
0.00 ± 0.00 m
8.75 ± 0.10 f
13.50 ± 0.79 gh
41.50 ± 0.39 e
43.50 ± 0.29 f
43.50 ± 0.29 e
29.25 ± 0.46 e
29.25 ± 0.46 f
8.50 ± 0.47 ij
1.25 ± 0.56 de
Bait 15
0.00 ± 0.00 c
1.50 ± 0.67 e-g
5.50 ± 0.29 hi
4.50 ± 0.26 j-m
7.50 ± 0.50 lm
8.25 ± 0.99 no
4.00 ± 0.00 qr
4.00 ± 0.00 q
4.00 ± 0.00 n
0.00 ± 0.00 o
0.00 ± 0.00 op
0.00 ± 0.00 f
Bait 16
0.00 ± 0.00 c
2.75 ± 0.72 cd
0.00 ± 0.00 m
5.25 ± 0.38 i-k
9.75 ± 1.66 jk
41.00 ± 0.26 e
24.40 ± 0.48 hi
24.00 ± 0.48 g-i
36.75 ± 0.38 d
36.75 ± 0.38 e
8.50 ± 0.47 ij
2.50 ± 0.39 b
Bait 17
0.00 ± 0.00 c
0.75 ± 0.34 g-i
3.00 ± 0.18 kl
3.75 ± 1.03 k-m
12.75 ± 0.64 g-i
12.75 ± 1.66 j-l
5.00 ± 0.00 qr
5.00 ± 0.00 q
5.00 ± 0.00 mn
0.00 ± 0.00 o
0.00 ± 0.00 no
0.75 ± 0.34 ef
Bait 18
0.00 ± 0.00 c
1.00 ± 0.45 f-h
0.00 ± 0.00 m
1.50 ± 0.44 op
7.25 ± 1.45 lm
14.75 ± 2.47 g-k
8.00 ± 0.00 n-p
8.00 ± 0.00 p
8.00 ± 0.00 kl
0.00 ± 0.00 n
0.00 ± 0.00 jk
0.00 ± 0.00 f
Bait 19
0.00 ± 0.00 c
0.50 ± 0.22 hi
3.00 ± 0.18 kl
6.75 ± 1.97 g-i
14.00 ± 0.29 gh
16.75 ± 1.59 g
9.00 ± 0.00 m-o
9.00 ± 0.00 op
9.00 ± 0.00 j-l
0.00 ± 0.00 mn
0.00 ± 0.00 g-j
1.25 ± 0.34 de
Bait 20
0.00 ± 0.00 c
0.75 ± 0.34 g-i
4.50 ± 2.01 ij
6.00 ± 0.00 h-j
14.50 ± 1.45 fg
15.50 ± 0.59 g-i
24.40 ± 0.48 hi
24.00 ± 0.48 g-i
13.00 ± 0.18 gh
13.00 ± 0.18 jk
6.75 ± 0.42 kl
0.00 ± 0.00 f
Bait 21
0.00 ± 0.00 c
0.50 ± 0.22 hi
2.25 ± 0.34 l
1.25 ± 0.50 p
2.75 ± 0.38 op
12.50 ± 0.29 kl
11.50 ± 0.29 lm
10.50 ± 1.12 no
10.50 ± 1.12 h-k
10.50 ± 1.12 lm
9.50 ± 1.06 f-i
0.00 ± 0.00 f
Bait 22
0.00 ± 0.00 c
0.75 ± 0.34 g-i
3.50 ± 0.65 j-l
6.75 ± 0.71 g-i
4.75 ± 0.21 no
3.00 ± 0.48 q
6.50 ± 0.13 o-q
11.00 ± 1.29 m-o
11.00 ± 1.29 h-j
11.00 ± 1.29 k-m
9.25 ± 1.05 f-j
0.00 ± 0.00 f
Bait 23
0.00 ± 0.00 c
1.00 ± 0.45 f-h
0.00 ± 0.00 m
1.75 ± 0.70 n-p
1.75 ± 0.18 p
5.25 ± 0.38 pq
6.50 ± 0.47 o-q
9.50 ± 0.43 n-p
9.50 ± 0.43 i-l
9.50 ± 0.43 mn
9.00 ± 0.37 g-j
0.00 ± 0.00 f
Bait 24
0.00 ± 0.00 c
0.75 ± 0.34 g-i
6.00 ± 0.91 hi
7.50 ± 0.57 f-h
7.00 ± 0.74 lm
6.25 ± 0.21 op
5.25 ± 0.21 p-r
11.00 ± 1.29 m-o
11.00 ± 1.29 h-j
11.00 ± 1.29 k-m
9.25 ± 1.05 f-j
0.00 ± 0.00 f
Bait 25
0.00 ± 0.00 c
1.25 ± 0.56 f-h
11.75 ± 0.21 cd
7.75 ± 0.50 fg
4.50 ± 0.21 n-p
6.00 ± 1.02 op
10.00 ± 0.41 mn
10.00 ± 0.41 n-p
10.00 ± 0.41 i-k
10.00 ± 0.41 l-n
10.00 ± 0.41 e-h
0.00 ± 0.39 f
Bait 26
0.00 ± 0.00 c
2.75 ± 0.42 cd
2.75 ± 1.23 kl
14.25 ± 0.59 d
8.25 ± 0.38 kl
44.50 ± 0.29 d
49.25 ± 0.69 e
49.25 ± 0.69 d
38.25 ± 0.21 d
38.25 ± 0.21 de
9.75 ± 0.46 e-i
1.50 ± 0.00 c-e
Bait 27
0.00 ± 0.00 c
0.75 ± 0.34 g-i
10.75 ± 0.28 de
11.25 ± 0.77 e
8.75 ± 0.34 kl
16.00 ± 1.02 gh
11.75 ± 2.11 lm
11.75 ± 0.50 mn
11.75 ± 2.11 hi
11.75 ± 2.11 kl
8.75 ± 0.59 h-j
0.00 ± 0.00 f
Bait 28
0.00 ± 0.00 c
2.75 ± 1.23 cd
0.00 ± 0.00 m
15.00 ± 0.36 d
5.75 ± 0.98 mn
12.75 ± 0.28 jkl
26.25 ± 0.21 h
26.25 ± 0.21 g
26.25 ± 0.21 f
26.25 ± 0.21 g
6.25 ± 0.21 l-n
0.00 ± 0.00 f
Bait 29
0.00 ± 0.00 c
0.00 ± 0.00 i
9.50 ± 0.22 ef
3.75 ± 0.54 k-m
14.00 ± 0.28 gh
9.00 ± 0.55 n
3.00 ± 0.18 r
3.25 ± 0.18 q
2.75 ± 0.28 n
3.25 ± 0.21 o
2.75 ± 0.28 pq
0.00 ± 0.53 f
Bait 30
0.00 ± 0.00 c
2.75 ± 0.11 cd
9.50 ± 0.22 ef
14.50 ± 0.41 d
19.25 ± 0.92 d
48.75 ± 0.21 c
45.00 ± 3.50 f
25.00 ± 0.48 gh
41.75 ± 3.23 c
40.50 ± 0.13 bc
10.25 ± 0.50 e-g
3.50 ± 0.00 a
Bait 31
0.00 ± 0.00 c
0.50 ± 0.22 hi
11.75 ± 0.72 cd
3.25 ± 0.57 mn
16.25 ± 0.28 ef
13.50 ± 2.07 i-l
5.25 ± 0.84 p-r
5.25 ± 0.84 q
5.25 ± 0.84 mn
5.25 ± 0.84 o
5.25 ± 0.84 m-o
0.00 ± 0.63 f
Bait 32
0.00 ± 0.00 c
3.50 ± 0.13 bc
8.50 ± 0.13 fg
17.25 ± 0.19 c
25.75 ± 0.2 2c
51.25 ± 0.28 b
56.00 ± 0.32 d
56.00 ± 0.32 c
42.50 ± 0.29 c
42.50 ± 0.29 b
8.50 ± 0.65 ij
4.00 ± 0.00 a
GF-120 (Control)
0.00 ± 0.00 c
0.00 ± 0.00 i
0.75 ± 0.34 m
1.25 ± 0.30 p
3.50 ± 0.28 op
6.25 ± 0.11 op
4.00 ± 0.26 qr
4.00 ± 0.26 q
4.00 ± 0.26 n
4.00 ± 0.26 o
4.00 ± 0.26 op
0.00 ± 0.00 f
P – Value
0.00
0.00
0.00
0.00
0.00
0.00
0.00
0.00
0.00
0.00
0.00
0.00
F- Value
4.97
21.28
87.39
112.24
177.62
383.76
376.38
553.55
265.04
379.24
61.57
14.91
3.5 Population repones of Female B. zonata captured by different attractants in mango orchards during 2020
Data regarding number of traps catches of female B. zonata attracted to different food baits attractants (Table 5) during June, in mango field was found significantly (F32,527 = 341.23, p < 0.05) different. The highest relative catches/trap/months belonged to bait 8,4 (23.00 ± 0.18; 22.75 ± 0.64, respectively). Whereas baits 31 (0.50 ± 0.22) showed lowest catches/trap/month. Statistical analysis showed during July female fruit flies, the interaction between attractants and month (Table 5) remained significantly (F32,527 = 125.10, p < 0.05) comes in bait 8 ,32 (22.50 ± 0.29; 21.80 ± 0.21, respectively). While, bait 19 and 23 (1.00 ± 0.26; 1.00 ± 0.26) was exhibited lowest catches/trap/months and similar value of attraction. Number of female B. zonata was significantly (F32,527 = 218.93, p < 0.05) influenced by different attractants during August (Table 5). Highest number of catches/traps/months was observed towards bait 8,32,4 (22.50 ± 0.29; 21.75 ± 0.21; 20.75 ± 0.11, respectively). While the lowest number of catches/traps/months towards bait 23 (1.00 ± 0.26) was observed both having similar responses statistically. Bait 1 PH + Jaggery: Bait 2 PH + Jaggery + AA: Bait 3 PH + Jaggery + TMA: Bait 4 PH + Jaggery + Pu: Bait 5 PH + Jaggery + AA + TMA: Bait 6 PH + Jaggery + AA + Pu: Bait 7 PH + Jaggery + TMA + Pu: Bait 8 PH + Jaggery + AA + TMA + Pu : Bait 9 PH + papaya powder + kachri powder: Bait 10 PH + papaya powder + kachri powder + AA: Bait 11 PH + papaya powder + kachri powder + TMA: Bait 12 PH + papaya powder + kachri powder + Pu Bait 13 PH + papaya powder + kachri powder + AA + TMA: Bait 14 PH + papaya powder + kachri powder + AA + Pu : Bait 15 PH + papaya powder + kachri powder + TMA + Pu: Bait 16 PH + papaya powder + kachri powder + AA + TMA + Pu: Bait 17 PH + KOH: Bait 18 PH + KOH + AA: Bait 19 PH + KOH + TMA: Bait 20 PH + KOH + Pu: Bait 21 PH + KOH + AA + TMA: Bait 22 PH + KOH + AA + Pu: Bait 23 PH + KOH + TMA + Pu Bait 24 PH + KOH + AA + TMA + Pu: Bait 25 PH + Guava pulp: Bait 26 PH + Guava pulp + AA: Bait 27 PH + Guava pulp + TMA: Bait 28 PH + Guava pulp + Pu: Bait 29 PH + Guava pulp + AA + TMA: Bait 30 PH + Guava pulp + AA + Pu : Bait 31 PH + Guava pulp + TMA + Pu : Bait 32 PH + guava pulp + AA + TMA + Pu: Bait 33 GF 120. Mean followed by the same letter (within each month or factor) are not significantly different (P > 0.05)
Baits / Treatments
January
February
March
April
May
June
July
August
September
October
November
December
Bait 1
0.00 ± 0.00 c
1.00 ± 0.18 d-g
2.75 ± 0.21 ij
3.75 ± 0.10 j-l
13.50 ± 0.29 c
3.00 ± 0.18 k-m
10.00 ± 0.41 fg
10.00 ± 0.41 h
7.00 ± 0.18 i
2.75 ± 0.11 lm
1.25 ± 0.34 jk
0.00 ± 0.00 e
Bait 2
0.00 ± 0.00 c
1.50 ± 0.67 d-f
11.50 ± 0.13 c
5.00 ± 0.16 h-j
12.75 ± 0.28 cd
2.50 ± 0.13 mn
18.00 ± 0.32 c
18.00 ± 0.32 c
8.00 ± 0.18 i
8.00 ± 0.18 gh
1.25 ± 0.34 jk
0.50 ± 0.22 d
Bait 3
0.00 ± 0.00 c
0.75 ± 0.34 e-g
3.00 ± 0.18 hi
0.00 ± 0.00 m
6.50 ± 0.22 h
3.75 ± 0.38 j-l
8.80 ± 0.38 g
8.75 ± 0.38 i
7.25 ± 0.38 i
7.25 ± 0.38 gh
6.25 ± 0.38 de
0.00 ± 0.00 e
Bait 4
0.00 ± 0.00 c
0.50 ± 0.22 fg
12.00 ± 0.18 bc
11.50 ± 0.11 ef
21.25 ± 0.11 b
22.75 ± 0.64 a
14.75 ± 0.59 d
14.75 ± 0.59 de
11.25 ± 0.28 fg
5.50 ± 0.29 ij
7.25 ± 0.38 cd
0.75 ± 0.34 cd
Bait 5
0.00 ± 0.00 c
0.75 ± 0.34 e-g
3.00 ± 0.18 hi
6.00 ± 0.40 h
4.75 ± 0.21 ij
6.25 ± 0.38 h
4.50 ± 0.70 h
4.50 ± 0.70 k
4.50 ± 0.70 jk
4.50 ± 0.70 jk
5.25 ± 0.38 e-g
0.75 ± 0.34 cd
Bait 6
0.75 ± 0.34 b
1.75 ± 0.28 de
10.25 ± 0.34 d
13.75 ± 0.19 d
33.75 ± 0.56 a
22.50 ± 0.43 a
15.75 ± 0.28 d
15.75 ± 0.28 d
15.75 ± 0.28 ab
8.00 ± 0.18 gh
8.00 ± 0.18 bc
0.75 ± 0.21 cd
Bait 7
0.00 ± 0.00 c
0.00 ± 0.00 g
2.00 ± 0.63 jk
3.75 ± 0.52 j-l
8.25 ± 0.21 fg
8.00 ± 0.18 g
4.75 ± 0.50 h
4.75 ± 0.50 k
4.75 ± 0.50 j
4.75 ± 0.50 j
4.75 ± 0.50 fg
0.00 ± 0.00 e
Bait 8
1.25 ± 0.56 a
3.25 ± 0.21 c
14.75 ± 0.38 a
19.75 ± 0.19 a
32.75 ± 0.34 a
23.00 ± 0.18 a
22.50 ± 0.29 a
22.50 ± 0.29 a
16.00 ± 0.18 a
10.75 ± 0.28 c-e
8.50 ± 0.13 b
1.75 ± 0.28 a
Bait 9
0.00 ± 0.00 c
1.25 ± 0.56 d-f
0.00 ± 0.00 m
0.25 ± 0.10 m
3.00 ± 0.80 lm
3.00 ± 0.80 k-m
9.00 ± 0.67 g
9.50 ± 0.67 hi
9.50 ± 0.67 h
9.50 ± 0.67 ef
2.75 ± 0.42 hi
1.00 ± 0.45 bc
Bait 10
0.00 ± 0.00 c
2.00 ± 0.37 d
3.25 ± 0.28 hi
12.00 ± 0.16 ef
4.50 ± 0.39 i-k
10.25 ± 0.50 e
14.40 ± 0.47 d
14.50 ± 0.47 e
6.75 ± 0.28 i
6.75 ± 0.28 hi
2.50 ± 0.13 hi
0.00 ± 0.00 e
Bait 11
0.00 ± 0.00 c
1.75 ± 0.46 de
7.00 ± 0.63 f
0.00 ± 0.00 m
4.50 ± 0.13 i-k
6.50 ± 0.83 h
8.75 ± 0.64 g
8.75 ± 0.18 j
7.75 ± 0.21 i
7.75 ± 0.21 gh
7.75 ± 0.21 bc
0.00 ± 0.00 e
Bait 12
0.00 ± 0.00 c
0.75 ± 0.34 e-g
0.00 ± 0.00 m
3.25 ± 0.44 kl
3.50 ± 0.13 k-m
3.50 ± 0.13 j-m
5.60 ± 0.50 h
5.25 ± 0.50 k
5.25 ± 0.50 j
5.25 ± 0.50 j
2.50 ± 0.13 hi
0.75 ± 0.34 cd
Bait 13
0.00 ± 0.00 c
2.00 ± 0.55 d
3.75 ± 0.21 hi
0.00 ± 0.00 m
5.00 ± 0.41 i
5.00 ± 0.41 i
1.60 ± 0.22 i
2.25 ± 0.11 lm
4.75 ± 0.21 j
4.75 ± 0.21 j
4.75 ± 0.21 fg
1.00 ± 0.26 bc
Bait 14
0.00 ± 0.00 c
8.00 ± 1.33 a
0.00 ± 0.00 m
7.75 ± 0.25 g
7.00 ± 0.48 h
12.25 ± 0.21 d
21.00 ± 0.11 b
20.75 ± 0.11b
14.50 ± 0.47 bc
14.50 ± 0.47 a
2.75 ± 0.21 hi
0.75 ± 0.34 cd
Bait 15
0.00 ± 0.00 c
1.75 ± 0.46 de
2.75 ± 0.11 ij
3.75 ± 0.19 j-l
3.25 ± 0.28 lm
5.75 ± 0.28 k-m
1.25 ± 0.34 i
1.25 ± 0.34 mn
1.25 ± 0.34 mn
1.25 ± 0.34 no
4.50 ± 0.53 g
0.00 ± 0.00 e
Bait 16
0.00 ± 0.00 c
3.25 ± 0.11 c
0.00 ± 0.00 m
8.50 ± 0.11 g
12.75 ± 0.46 cd
22.25 ± 0.34 ab
10.25 ± 1.44 fg
5.50 ± 0.22 k
13.00 ± 0.26 de
13.00 ± 0.26 b
5.75 ± 0.46 ef
1.25 ± 0.21 b
Bait 17
0.00 ± 0.00 c
0.75 ± 0.34 e-g
0.00 ± 0.00 m
3.50 ± 0.53 kl
2.75 ± 0.56 lm
6.25 ± 0.38 h
1.50 ± 0.43 i
1.50 ± 0.43 l-n
1.50 ± 0.43 mn
1.50 ± 0.43 m-o
2.25 ± 0.28 h-j
0.50 ± 0.22 d
Bait 18
0.00 ± 0.00 c
2.00 ± 0.55 d
0.00 ± 0.00 m
4.25 ± 0.34 i-k
3.50 ± 0.29 k-m
3.50 ± 0.29 j-m
1.25 ± 0.34 i
1.25 ± 0.34 mn
1.25 ± 0.34 mn
1.25 ± 0.34 no
2.25 ± 0.21 h-j
0.00 ± 0.00 e
Bait 19
0.00 ± 0.00 c
1.50 ± 0.43 def
2.75 ± 0.21 ij
3.00 ± 0.71 kl
2.50 ± 0.13 m
1.75 ± 0.78 n
1.00 ± 0.26 i
1.00 ± 0.26 n
1.75 ± 0.28 mn
1.75 ± 0.28 m-o
2.50 ± 0.13 hi
0.00 ± 0.00 e
Bait 20
0.00 ± 0.00 c
4.00 ± 0.88 c
0.00 ± 0.00 m
12.00 ± 0.95 ef
5.00 ± 0.52 i
9.25 ± 0.28 ef
10.25 ± 1.44 fg
5.25 ± 0.50 k
8.00 ± 0.88 i
8.00 ± 0.88 gh
4.25 ± 0.21 g
0.00 ± 0.00 e
Bait 21
0.00 ± 0.00 c
1.25 ± 0.28 d-f
1.75 ± 0.78 kl
0.00 ± 0.00 m
3.75 ± 0.34 j-l
5.00 ± 0.18 i
1.75 ± 0.64 i
1.50 ± 0.29 l-n
2.00 ± 0.41 l-n
2.00 ± 0.26 l-o
1.75 ± 0.64 i-k
0.00 ± 0.00 e
Bait 22
0.00 ± 0.00 c
1.25 ± 0.56 d-f
0.00 ± 0.00 m
5.25 ± 0.25 hi
10.00 ± 0.26 e
2.75 ± 0.28 l-n
2.50 ± 0.74 i
2.50 ± 0.39 l
2.50 ± 0.39 lm
2.50 ± 0.74 l-n
2.50 ± 0.74 hi
0.00 ± 0.00 e
Bait 23
0.00 ± 0.00 c
1.25 ± 0.34 d-f
0.00 ± 0.00 m
5.00 ± 0.43 h-j
4.50 ± 0.70 i-k
4.00 ± 0.18 i-k
1.00 ± 0.26 i
1.00 ± 0.26n
1.00 ± 0.26 n
1.00 ± 0.26 o
1.00 ± 0.26 k
0.00 ± 0.00 e
Bait 24
0.00 ± 0.00 c
1.00 ± 0.45 d-g
0.00 ± 0.00 m
10.75 ± 0.52 f
6.75 ± 0.21 h
3.75 ± 0.21 j-l
12.80 ± 0.28 e
12.75 ± 0.28 f
10.50 ± 0.59 gh
10.50 ± 0.59 de
8.75 ± 0.92 b
0.00 ± 0.00 e
Bait 25
0.00 ± 0.00 c
1.00 ± 0.26 d-g
12.50 ± 0.29 b
8.00 ± 0.49 g
7.25 ± 0.46 gh
2.75 ± 0.62 l-n
4.80 ± 0.50 h
4.75 ± 0.50 k
4.75 ± 0.50 j
4.75 ± 0.50 j
4.75 ± 0.50 fg
0.00 ± 0.21 e
Bait 26
0.00 ± 0.00 c
5.75 ± 1.32 b
0.00 ± 0.00 m
12.25 ± 1.24 e
6.25 ± 0.59 h
21.25 ± 0.11 b
11.25 ± 0.69 ef
11.25 ± 0.69 g
11.25 ± 0.69 fg
11.25 ± 0.69 cd
3.00 ± 0.18 h
0.75 ± 0.00 cd
Bait 27
0.00 ± 0.00 c
0.00 ± 0.00 g
8.50 ± 0.39 e
3.75 ± 1.03 j-l
7.25 ± 0.28 gh
4.50 ± 0.13 ij
4.75 ± 0.84 h
4.75 ± 0.84 k
4.75 ± 0.84 j
4.75 ± 0.84 j
5.75 ± 0.53 ef
0.00 ± 0.00 e
Bait 28
0.00 ± 0.00 c
1.75 ± 0.46 de
0.00 ± 0.00 m
11.00 ± 0.86 ef
6.25 ± 0.67 h
8.25 0.21 fg
12.00 ± 0.88 e
12.00 ± 0.88 fg
12.00 ± 0.88 ef
12.00 ± 0.88 bc
4.50 ± 0.59 g
0.00 ± 0.00 e
Bait 29
0.00 ± 0.00 c
0.00 ± 0.00 g
9.25 ± 0.42 e
2.75 ± 0.30 l
3.00 ± 0.48 lm
3.00 ± 0.48 k-m
1.60 ± 0.53 i
1.75 ± 0.53 l-n
3.25 ± 0.28 kl
3.25 ± 0.28 kl
1.75 ± 0.53 ijk
0.00 ± 0.21 e
Bait 30
0.00 ± 0.00 c
3.50 ± 0.13 c
0.00 ± 0.00 m
15.25 ± 0.41 c
8.50 ± 0.56 f
18.50 ± 0.13 c
11.25 ± 0.69 ef
11.25 ± 0.69 g
11.25 ± 0.69 fg
11.25 ± 0.69 cd
5.00 ± 0.37 fg
0.75 ± 0.00 cd
Bait 31
0.00 ± 0.00 c
0.00 ± 0.00 g
6.00 ± 0.41 g
5.00 ± 0.58 h-j
0.50 ± 0.22 n
0.50 ± 0.22 o
1.75 ± 0.28 i
1.25 ± 0.28 l-n
2.50 ± 0.47 lm
1.75 ± 0.53 m-o
1.25 ± 0.56 jk
0.00 ± 0.21 e
Bait 32
0.00 ± 0.00 c
4.25 ± 0.11 c
6.00 ± 0.37 g
17.25 ± 0.57 b
12.25 ± 0.34 d
22.75 0.64 a
21.80 ± 0.21 ab
21.75 ± 0.21 ab
13.75 ± 0.42 cd
8.50 ± 0.13 fg
10.50 ± 0.70 a
0.75 ± 0.00 cd
GF-120 (Control)
0.00 ± 0.00 c
0.00 ± 0.00 g
1.00 ± 0.45 l
3.00 ± 0.16 kl
5.00 ± 0.18 i
3.25 ± 0.56 k-m
5.00 ± 0.26 h
5.00 ± 0.26 k
5.00 ± 0.26 j
5.00 ± 0.26 j
5.00 ± 0.26 fg
0.00 ± 0.00 e
P – Value
0.00
0.00
0.00
0.00
0.00
0.00
0.00
0.00
0.00
0.00
0.00
0.00
F- Value
5.13
17.55
240.73
127.82
374.51
341.23
125.1
218.93
101.53
68.71
34.88
8.4
3.6 Efficiency of different food baits attractants against B. zonata during the session 2019 and 2020 at mango orchard
The analysis of variance revealed substantial variations in the overall effectiveness of the attractants to catch B. zonata in mango orchards throughout 2019–20. The majority of the treatments attracted more males than females. Female B. zonata catches/trap/month were lower than males for the majority of treatments, and the same pattern was seen in both years. The means of both the years of all ammonium compounds against males, females and total relative attractancy for attraction B. zonata adults during the experiments is shown (Table 6). Bait 1 PH + Jaggery: Bait 2 PH + Jaggery + AA: Bait 3 PH + Jaggery + TMA: Bait 4 PH + Jaggery + Pu:Bait 5 PH + Jaggery + AA + TMA: Bait 6 PH + Jaggery + AA + Pu: Bait 7 PH + Jaggery + TMA + Pu: Bait 8 PH + Jaggery + AA + TMA + Pu : Bait 9 PH + papaya powder + kachri powder: Bait 10 PH + papaya powder + kachri powder + AA: Bait 11 PH + papaya powder + kachri powder + TMA: Bait 12 PH + papaya powder + kachri powder + Pu Bait 13 PH + papaya powder + kachri powder + AA + TMA: Bait 14 PH + papaya powder + kachri powder + AA + Pu : Bait 15 PH + papaya powder + kachri powder + TMA + Pu: Bait 16 PH + papaya powder + kachri powder + AA + TMA + Pu: Bait 17 PH + KOH: Bait 18 PH + KOH + AA: Bait 19 PH + KOH + TMA: Bait 20 PH + KOH + Pu: Bait 21 PH + KOH + AA + TMA: Bait 22 PH + KOH + AA + Pu: Bait 23 PH + KOH + TMA + Pu Bait 24 PH + KOH + AA + TMA + Pu: Bait 25 PH + Guava pulp: Bait 26 PH + Guava pulp + AA: Bait 27 PH + Guava pulp + TMA: Bait 28 PH + Guava pulp + Pu: Bait 29 PH + Guava pulp + AA + TMA: Bait 30 PH + Guava pulp + AA + Pu : Bait 31 PH + Guava pulp + TMA + Pu : Bait 32 PH + guava pulp + AA + TMA + Pu: Bait 33 GF 120. Mean followed by the same letter (within each month or factor) are not significantly different (P > 0.05)
Baits Name
2019
2020
Male
Female
Total
Male
Female
Total
Bait 1
9.58 ± 0.85 g-l
4.89 ± 0.43 g-j
14.47 ± 1.25 g-j
7.58 ± 0.52 k-p
4.58 ± 0.32 h-j
12.17 ± 0.78 jk
Bait 2
14.39 ± 1.01 f
6.41 ± 0.52 g
20.79 ± 1.48 f
10.98 ± 0.71 g-i
7.25 ± 0.46 ef
18.23 ± 1.09 fg
Bait 3
8.92 ± 0.64 h-m
5.95 ± 0.71 gh
14.88 ± 1.24 f-j
9.92 ± 0.57 h-k
4.35 ± 0.26 ij
14.27 ± 0.79 h-j
Bait 4
44.62 ± 3.43 c
19.11 ± 1.34 cd
63.73 ± 4.57 c
29.42 ± 1.64 b
10.19 ± 0.54 c
39.60 ± 2.05 c
Bait 5
6.48 ± 0.55 i-o
3.51 ± 0.35 i-m
9.98 ± 0.87 i-l
7.35 ± 0.37 l-p
3.73 ± 0.20 j-l
11.08 ± 0.54 kl
Bait 6
51.53 ± 3.93 b
21.43 ± 1.43 b
72.96 ± 5.08 b
31.54 ± 1.77 b
12.23 ± 0.67 b
43.77 ± 2.22 b
Bait 7
10.84 ± 0.78 f-i
6.04 ± 0.49 gh
16.88 ± 1.24 f-h
9.96 ± 0.43 h-k
3.81 ± 0.23 j-l
13.77 ± 0.62 i-k
Bait 8
58.88 ± 4.49 a
28.41 ± 2.29 a
87.28 ± 6.07 a
34.29 ± 1.85 a
4.73 ± 0.69 a
49.02 ± 2.37 a
Bait 9
7.41 ± 0.57 h-n
4.17 ± 0.34 h-l
11.58 ± 0.88 h-k
13.10 ± 0.80 g
4.10 ± 0.33 ij
17.21 ± 1.08 f-h
Bait 10
10.03 ± 0.85 f-k
5.09 ± 0.48 g-i
15.13 ± 1.27 f-i
12.56 ± 0.71 g
6.42 ± 0.38 fg
18.98 ± 1.05 f
Bait 11
7.39 ± 0.63 h-n
3.74 ± 0.32 i-m
11.13 ± 0.91 h-k
9.15 ± 0.57 i-m
4.90 ± 0.27 hi
14.04 ± 0.75 i-k
Bait 12
6.20 ± 0.56 j-o
2.70 ± 0.25 k-m
8.90 ± 0.76 j-l
8.06 ± 0.56 j-o
2.94 ± 0.18 l-n
11.00 ± 0.70 kl
Bait 13
7.26 ± 0.71 i-n
2.85 ± 0.27 j-m
10.11 ± 0.93 i-l
9.73 ± 0.60 ik
2.90 ± 0.16 l-n
12.63 ± 0.66 i-k
Bait 14
21.66 ± 1.71 e
13.20 ± 1.05 f
34.85 ± 2.61 e
18.44 ± 1.24 e
9.08 ± 0.54 d
27.52 ± 1.74 d
Bait 15
4.60 ± 0.39 m-o
2.06 ± 0.19 lm
6.66 ± 0.56 kl
3.94 ± 0.21 st
2.02 ± 0.13 n-p
5.96 ± 0.29 mn
Bait 16
23.89 ± 1.91 e
14.59 ± 1.13 f
38.48 ± 2.90 e
15.94 ± 1.09 f
7.96 ± 0.48 e
23.90 ± 1.50 e
Bait 17
4.77 ± 0.44 m-o
2.75 ± 0.30 k-m
7.52 ± 0.71 kl
4.90 ± 0.36 q-t
1.83 ± 0.16 op
6.73 ± 0.45 mn
Bait 18
5.55 ± 0.54 k-o
2.60 ± 0.29 k-m
8.15 ± 0.81 kl
5.35 ± 0.39 p-s
1.71 ± 0.13 op
7.06 ± 0.44 mn
Bait 19
5.41 ± 0.41 l-o
2.56 ± 0.26 k-m
7.97 ± 0.63 kl
7.27 ± 0.44 m-q
1.63 ± 0.13 p
8.90 ± 0.51 lm
Bait 20
10.34 ± 0.80 f-j
4.51 ± 0.41 g-k
14.85 ± 1.16 f-j
10.17 ± 0.62 h-j
5.50 ± 0.35 gh
15.67 ± 0.84 g-i
Bait 21
2.69 ± 0.24 o
2.11 ± 0.24 lm
4.80 ± 0.44 l
6.00 ± 0.42 o-s
1.73 ± 0.15 op
7.72 ± 0.49 mn
Bait 22
3.83 ± 0.28 no
2.15 ± 0.29 lm
5.98 ± 0.51 kl
5.63 ± 0.37 p-s
2.65 ± 0.23 m-o
8.27 ± 0.49 l-n
Bait 23
3.46 ± 0.25 no
1.95 ± 0.17 m
5.41 ± 0.39 kl
4.56 ± 0.30 r-t
1.65 ± 0.15 p
6.21 ± 0.34 mn
Bait 24
11.84 ± 0.96 f-h
4.95 ± 0.46 g-j
16.79 ± 1.37 f-h
6.25 ± 0.36 n-s
6.46 ± 0.38 fg
12.71 ± 0.66 i-k
Bait 25
4.18 ± 0.29 no
2.10 ± 0.18 lm
6.28 ± 0.44 kl
6.77 ± 0.33 m-r
4.60 ± 0.28 h-j
11.38 ± 0.55 j-l
Bait 26
29.78 ± 2.34 d
16.80 ± 1.13 e
46.58 ± 3.31 d
21.56 ± 1.42 d
7.85 ± 0.49 e
29.42 ± 1.81 d
Bait 27
6.44 ± 0.54 i-o
3.29 ± 0.33 i-m
9.73 ± 0.85 i-l
8.60 ± 0.49 i-n
4.06 ± 0.26 i-k
12.67 ± 0.67 i-k
Bait 28
13.82 ± 1.09 fg
5.28 ± 0.44 g-i
19.10 ± 1.47 fg
12.29 ± 0.79 gh
6.65 ± 0.40 f
18.94 ± 1.15 f
Bait 29
3.44 ± 0.26 no
2.08 ± 0.20 lm
5.53 ± 0.43 kl
4.25 ± 0.32 st
2.48 ± 0.20 m-p
6.73 ± 0.46 mn
Bait 30
33.09 ± 2.58 d
17.26 ± 1.20 de
50.35 ± 3.68 d
21.73 ± 1.30 d
8.04 ± 0.45 e
29.77 ± 1.66 d
Bait 31
5.80 ± 0.44 k-o
2.86 ± 0.26 j-m
8.66 ± 0.67 j-l
5.96 ± 0.44 o-s
1.75 ± 0.17 op
7.71 ± 0.51 mn
Bait 32
40.70 ± 3.23 c
19.88 ± 1.46 bc
60.58 ± 4.51 c
26.31 ± 1.53 c
11.63 ± 0.57 b
37.94 ± 2.02 c
GF-120
5.25 ± 0.50 l-o
2.53 ± 0.24 k-m
7.78 ± 0.73 kl
2.65 ± 0.16 t
3.10 ± 0.17 klm
5.75 ± 0.32 n
P Value
0.00
0.00
0.00
0.00
0.00
0.00
F Value
86.30
85.33
96.97
96.95
91.74
106.93
Among different combinations of synthetic food-based attractants baits 8,6 (58.88 ± 4.49; 51.53 ± 3.93, respectively) was showed maximum attraction (F32,527 = 86.30, p < 0.05) for capturing the B. zonata male (Table 6) during the whole year. While the minimum attraction towards bait 21 (2.69 ± 0.24) was observed in the year 2019. In the year 2020, response among different combinations of synthetic food-based attractants baits 8,6 (34.29 ± 1.85; 31.54 ± 1.77, respectively) was showed maximum attraction (F32,527 = 96.95, p < 0.05) for capturing the B. zonata male (Table 6) during the whole year. While the minimum attraction towards bait 33 (2.65 ± 0.16) was observed in the year 2020.
During the year the female highest response (F32,527 = 85.33, p < 0.05) towards bait 8,6 (28.41 ± 2.29; 21.43 ± 1.43, respectively) was noted. While lowest response towards bait 23 (1.95 ± 0.17) was noted (Table 6) in the year 2019. During the year 2020, the female highest response (F32,527 = 91.74, p < 0.05) towards bait 8,6 (4.73 ± 0.69; 12.23 ± 0.67, respectively) was noted. While lowest response towards bait 19 (1.63 ± 0.13) was noted (Table 6). Total most significant (F32,527 = 96.97, p < 0.05) peak population response towards bait 8,6 (87.28 ± 6.07; 72.96 ± 5.08, respectively) was observed. Likewise, lowest peak population (Table 6) towards bait 21 (4.80 ± 0.44) was observed in the year 2019. Total most significant (F32,527 = 106.93, p < 0.05) peak population response towards bait 8,6 (49.02 ± 2.37; 43.77 ± 2.22, respectively) was observed. Likewise, lowest peak population (Table 6) towards bait 33 (5.75 ± 0.32) was observed in the year 2020.
3.7 Number of B. zonata in mango field 2019–20
During both years, the number of B. zonata in mango orchards varied significantly depending on attractants and observational dates. Number of populations of B. zonata increased gradually from the month of March to the end of August where the number of populations were in its peak maximum value i.e., May (49.45), June (57.9), and July (50.49) in the year 2019, and also same observation in May (21.37), June (29.58), and July (31.62) catches/traps/months in the year 2020. From September to November, its population decreased but from December to February its population decreased drastically in both years. So, the interaction between attractants (catches/traps) and months of the years for the trapped more B. zonata are closely associated (Fig. 2). Population during the year 2019 was 263.15 and during the year 2020 i.e., 204.63 the reduction %age was 12.63% (Fig. 3). So, installation of these food baits attractants for more than two years gets maximum suppression of B. zonata in mango fields.
Collection of fruit fly (Bactrocera zonata) field data through plastic bottle trap baited with synthetic food-based attractants/lures
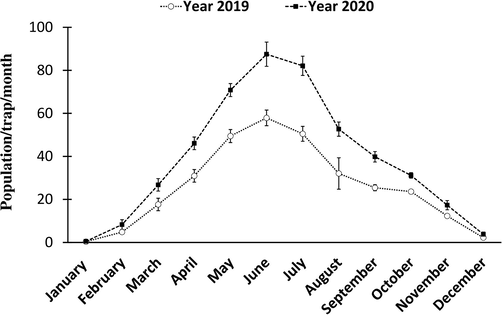
Means number of B. zonata population fluctuation level by all tested ammonium compounds in mango orchards during the session 2019 and 2020 of all treatment at P = 5% significant level.
3.8 Regression analysis with weather factors
The regression analysis between fruit fly catching and temperature (maxi and mini), relative humidity, and rainfall worked out. Relationship between fruit fly traps catches population maximum temperature (Fig. 4a) and minimum temperature (Fig. 4b) in mango orchards (y = 0.4617x + 22.788, R2 = 0.6684 and y = 0.471x + 13.243, R2 = 0.6666, respectively). The fruit fly infestation showed significant positive correlation with maximum and minimum temperature and negative relation with rainfall (Fig. 4c) and (Fig. 4d) relative humidity (y = -0.4899x + 91.701 R2 = 0.9156 and y = -0.0021x + 1.3745 R2 = 0.0002, respectively).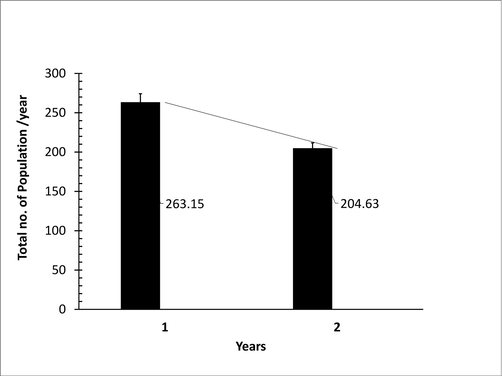
Population reduction trend due to the combined effect of different attractants during the 2019 and 2020.
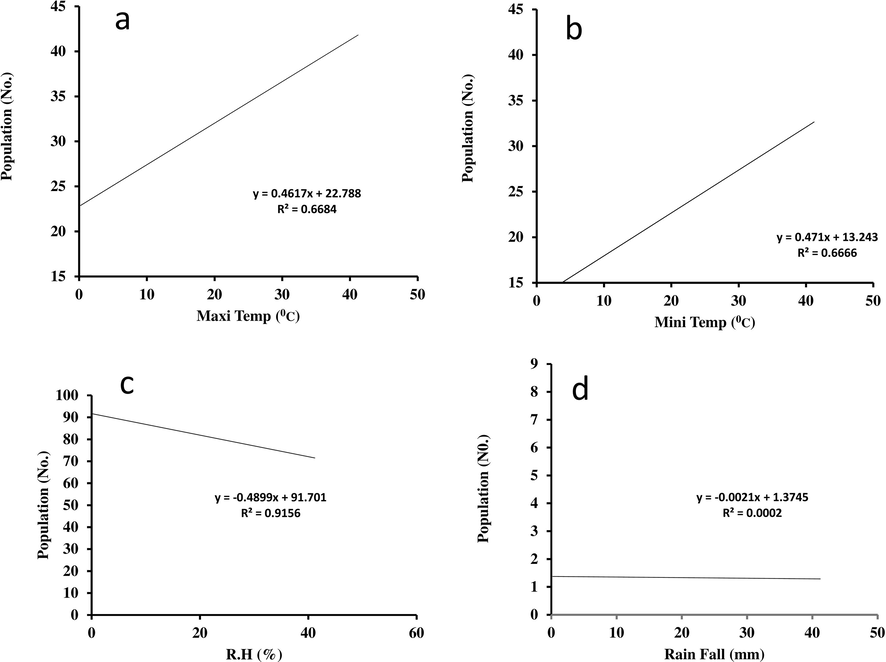
The relationship between Plastic Bottle traps (PB Traps) baited with different proteinaceous food attractants and adults captured/traps/year in mango field evaluated.
4 Discussion
Ammonia-based formulations play a key role in the attraction of fruit flies by adding ammonium acetate, trimethylamine, and putrescine. In previous studies, it has been shown that protein hydrolysate plus molasses or jaggery (sugar baits) increases the effectiveness and is important for the attraction of flies (Irsad and Haq, 2019). The attractiveness of sugar baits to insect pests of fruits is assumed to be due to fermentation processes for the production of attractants (Landolt, 1995). In the present study, a significant increase in the response of B. zonata to 32 synthetic baits was tested when ammonium acetate, trimethylamine, and putrescine were added to the remaining substances. The addition of these food-based attractants resulted in an increased number of attractive or a decrease in male/female or both responsiveness (Pinero et al., 2015).
The quantity of males was caught significantly with respect to females in all the attractants, and very few flies were caught/trapped/month in the control treatment. Efficiently baiting for B. zonata is an essential requirement for the eco-friendly management of mango orchards. Our experiments were designed to find the most efficient attractants against this pest. When protein hydrolysate was combined with different host fruits (juices/pulp of guava, mango, grapes, and pineapple), jaggery, KOH, papaya, and kachri powder, its effectiveness varied. In mango orchards, protein hydrolysate mixed with guava pulp and jaggery, mixed with ammonia-based food attractants, increased the rate of decomposition and produced volatile compounds which affect the attraction of fruit flies. Substances that release ammonia play an important role in the attraction of fruit flies towards host plants (Hull and Cribb, 2001). The synthetic proteinaceous food-based attractants composed of three chemicals (ammonium acetate, trimethylamine, and putrescine) baits performed better efficacy than that of GF-120 (Spinosad protein-based bait) and Bio Lure through the control of Ceratitis capitata through largely mass trapping (Piñero et al., 2017). Odorant Binding Proteins carry external odorants that enter through the pores of the sensilla into Odorant Receptors on odorant receptor neurons, therefore beginning olfactory signal transduction. Tephritidae fruit flies have been widely researched in numerous areas, including ecology, behaviour, and physiology. Phytophagous insects, in general, respond to environmental signals, including smell and visual cues linked to their host plants (Bernays and Chapman, 1994).
Local plastic bottle traps (PB Traps) are essential for accurate quantification of the B. zonata population and smooth running of the experiment during both seasons. Our results indicated (Table 6), that ammonium acetate, trimethylamine, and putrescine, alone or in combination with jaggery and protein hydrolysate, were the most preferable attractants (bait 8,6,4) for B. zonata, both males and females. These results are comparable with the results reported that ammonium acetate releasing ammonia and acetic acid when decomposition of organic matter occurs was better for attracting Tephritidae fruit flies (Thomas et al., 2008).
It is a general hypothesis that traps baited with protein hydrolysate mixed with ammonium acetate, trimethylamine, and putrescine attract more female Bactrocera spp. than those baited with male fruit flies of different species. But our results disagreed, this difference may be due to fruiting period, ecological attributes, climatic factors, and different responses between species. It was determined that B. zonata females and males fruitflies respond better to ammonium acetate, trimethylamine and putrescine than to the conventional protein baits (Torula Yeast and hydrolysate protein) (Howland et al., 1965; Anonae Kenya and Mauritius, 2007). Different concentrations of ammonium acetate, trimethylamine and putrescine proved to be better attractants compared with specific species, corresponding attractant and location (Quilici et al., 2007). B. zonata’s male and female have more attraction lures containing more than two components like ammonium acetate + trimethylamine + putrescine (Seewooruthun et al., 2007). In capturing female B. zonata, the three component lures, AA + PT + TMA, outperformed the single AA attractant (Sookar et al., 2006). So, due to its pestiferous nature, B. zonata is the main key pest of mango orchards in Pakistan, and none of the other pests trapped.
Our finding is similar in both years (Table 6), that during summer and spring seasons, ammonium compounds capture 2–3 times more male flies than females of B. oleae (Katsoyannos et al., 2007), while in contrast, summer catches more females than males (Mazomenos et al., 2002). Such responses could be interpreted by considering the effects of fruiting periods, ecological distribution, olfactory odour preference, climatic factors (variation of environmental conditions), and attraction responses different among different species towards liquid baits in the dry season versus the wet season. For example, Anastrepha spp. was attracted to liquid lures more in the dry season than in the wet season. Similarly, Rhagoletis pomonella Walsh (apple maggot fly) responded better to ammonium carbonate lure in the dry climate than to fruit volatiles (Yee et al., 2014).
Traps baited with ammonium acetate and putrescine increased the attractiveness of more adult flies as baited with ammonium acetate, trimethylamine, and putrescine alone. Our result was consistent with that of previous studies, which showed that traps baited with three components of synthetic food-based attractants showed remarkable performance for capturing B. zonata (Heath et al., 1997).
As compared to baits containing ammonium acetate, trimethylamine, and putrescine, our results were comparable. McPhail traps baited with ammonium acetate, trimethylamine, and putrescine were the most efficient at capturing the highest numbers of Anstrepha oblique, C. capitata, and B. zonata as compared to baits containing ammonium acetate + putrescine under field conditions evaluated (Moust Among the fruits, different pulps of guava, grapes, bananas, papaya, and pineapple were used with different food-based attractants for the attraction of fruit flies (Bharathi et al., 2004). Protein hydrolysate with guava pulp increased the attractiveness of the baits due to microbial fermentation and emits phagostimulant semiochemicals that are attractive to tephritids (Jang and Light, 1996). Guava pulp contains a wide range of volatile compounds such as aldehydes, alcohols, sesquiterpenes that increase the severity of fruit fly damage to fruits (Liu et al., 2013).
Our findings suggest that odorant-binding proteins (OBPs) interact with odorants and maintain the integrity of the insect olfactory system by stimulating and activating the Odorant Receptor (Larter et al., 2016). Similarly, behavioral and antennal responses were observed in the tobacco cutworm Spodoptera litura and in B. dorsalis to attractants (Liu et al., 2017) and in Bactrocera species such as Tephritidae, Ceratitidini, and Carpomyini fruit flies showed strong binding capacity to OBPs.
Furthermore, regression analysis between fly trapped data and weather factors was done to analyze the impact of weather on the population. The positive regression between temperature and fruit flies’ population was reported by (AnithaKumari et al., 2010). The maximum and minimum temperatures showed significant effects on the fruit fly population (Raghuvanshi et al., 2012). Rainfall and humidity showed a negative correlation with rainfall and relative humidity (Vayssières et al., 2009).
Therefore, according to our finding, our result agreed that bait 32 and 30 (guava pulp + protein hydrolysate + ammonium acetate + trimethylamine + putrescine) and (protein hydrolysate + guava pulp + ammonium acetate + putrescine) attracted more fruitfly (XIE and ZHANG, 2005).
5 Conclusion
The experiment was carried out to produce important, new, and increasing information regarding the response of the peach fruit fly, B. zonata, to different types of synthetic food-based attractants. Our results have shown that B. zonata’s response towards ammonia-based lures remains the best for both male and female B. zonata attractants, and can be used for monitoring as well as mass trapping approach techniques. However, these attractants capture a very large number of males and also a substantial number of females. So, that local trap (PB Trap) baited with bait 8 (protein hydrolysate + jaggery + ammonium acetate + trimethylamine + putrescine) captured more B. zonata flies as compared with other treatment combinations followed by bait 6,32 and 30 (protein hydrolysate + jaggery + ammonium acetate + putrescine), (protein hydrolysate + guava pulp + ammonium acetate + trimethylamine + putrescine), and (protein hydrolysate + guava pulp + ammonium acetate + putrescine) also attracted more flies respectively. Based on these, it is apparent that synthetic food-based attractants with three component lures, ammonium acetate, trimethylamine, and putrescine, in a trap, captured the most flies in the field test and could be used in mass trapping.
Acknowledgments
This research was funded by Higher Education Commission (HEC) Indigenous scholarship (Phase II) batch V (PIN No.518-112695-2AV5-073). The authors wish to thank Ejaz Muhammad Umar (General Manager, Hexone Chemical) to provide chemical lab for the preparation and manufacturing of different synthetic food baits attractants and Durani fruit farm, DHA (Muhammad Ilyas Khan Durani) for conducting experiment.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Diflunisal analogues stabilize the native state of transthyretin. Potent inhibition of amyloidogenesis. J. Med. Chem.. 2004;47(2):355-374.
- [Google Scholar]
- Agency., I.A.E., 2003. Trapping guidelines for area-wide fruit fly programmes, p. 47.
- Development of female attractants with local food-based chemicals for Bactrocera zonata (Saunders) In: Development of Improved Attractants and Their Integration into Fruit Fly SIT Management Programmes. 2007. p. :189.
- [Google Scholar]
- Influence of abiotic factors on the incidence of hopper and chemical control strategies in mango. Karnataka Journal of. Agricultural Sciences. 2010;22
- [Google Scholar]
- Behavior: the process of host-plant selection. Host-plant selection by phytophagous insects 1994:95-165.
- [Google Scholar]
- Attractiveness of some food baits to the melon fruit fly, Bactrocera cucurbitae (Coquillett)(Diptera: Tephritidae) Int. J. Trop. Insect Sci.. 2004;24:125-134.
- [Google Scholar]
- Anonae Kenya, C., and R. Mauritius. 2007. attractants compared with the conventional protein baits (Torula Yeast and hydrolysate protein) for some fruit flies of economic importance. The species, corresponding attractant and location are the following. Development of Improved Attractants and Their Integration into Fruit Fly SIT Management Programmes.1.
- Bhargava, A., Bansal, A., 2018. Fruits and vegetables quality evaluation using computer vision: A review. Journal of King Saud University-Computer and Information Sciences.
- A look inside odorant-binding proteins in insect chemoreception. J. Insect Physiol.. 2016;95:51-65.
- [Google Scholar]
- Response of Anastrepha species (Diptera: Tephritidae) to synthetic attractants in Colombia. IAEA-TECDOC Development of improved attractants and their integration into fruit fly. SIT management programmes (Austria). 2007;1574:129-141.
- [Google Scholar]
- Fruit fly management research: A systematic review of monitoring and control tactics in the world. Crop Prot.. 2018;112:187-200.
- [Google Scholar]
- Response of the olive fruit fly, Bactrocera oleae Rossi to some ammonium compounds and certain food attractants under field conditions in olive orchards. Journal of Plant Protection and Pathology. 2012;3(5):491-502.
- [Google Scholar]
- Trapping and the Detection, Control, and Regulation of Tephritid Fruit Flies. Dordrecht: Springer Netherlands; 2014. p. :75-118.
- [CrossRef]
- Antagonistic and synergistic peptide analogs of the tridecapeptide mating pheromone of Saccharomyces cerevisiae. Biochemistry. 1992;31(2):551-557.
- [Google Scholar]
- Heath, R.R., Epsky, N.D., Dueben, B.D., Rizzo, J., Jeronimo, F., 1997. Adding methyl-substituted ammonia derivatives to a food-based synthetic attractant on capture of the Mediterranean and Mexican fruit flies (Diptera: Tephritidae). Journal of Economic Entomology 90, 1584-1589.
- Biological control potential of entomopathogenic nematodes for management of Caribbean fruit fly, Anastrepha suspensa Loew (Tephritidae) Pest Manag. Sci.. 2017;73(6):1220-1228.
- [Google Scholar]
- Olfaction in the Queensland fruit fly, Bactrocera tryoni. II: Response spectra and temporal encoding characteristics of the carbon dioxide receptors. J. Chem. Ecol.. 2001;27:889-906.
- [Google Scholar]
- Irsad, P.Q.R., Haq, E., 2019. Fruit Fly (Bactrocera spp.): A Major Threat to Guava Production and Its Integrated Management. Recent Trends in, 57.
- Jang, E.B., Light, D.M., 1996. Olfactory semiochemicals of tephritids. Fruit fly pests: A world assessment of their biology and management, 73-90.
- Howland, A., P. Vail and T. Henneberry. 1965. Effect of chemosterilants on fertility of cabbage loopers. Journal of Economic Entomology. 58:635-637.
- Progress and problems in controlling fruit flies infestation. RAPA, Bangkock: FAO; 1986. p. :16-19.
- Field evaluation of trap types and lures for Bactrocera oleae (diptera: tephritidae). experiments conducted in Chios, Greece. In: Development of Improved Attractants and Their Integration into Fruit Fly SIT Management Programmes. 2007. p. :33.
- [Google Scholar]
- Attraction of Mocis latipes (Lepidoptera: Noctuidae) to sweet baits in traps. Florida Entomologist. 1995;78(3):523.
- [CrossRef] [Google Scholar]
- Larter, N.K., Sun, J.S., Carlson, J.R., 2016. Organization and function of Drosophila odorant binding proteins. Elife 5, e20242.
- BdorOBP2 plays an indispensable role in the perception of methyl eugenol by mature males of Bactrocera dorsalis (Hendel) Sci. Rep.. 2017;7:1-14.
- [Google Scholar]
- Recent spread and climatic ecological niche of the invasive guava fruit fly, Bactrocera correcta, in mainland China. J. Pest. Sci.. 2013;86(3):449-458.
- [Google Scholar]
- Attract and kill of the olive fruit fly Bactrocera oleae in Greece as a part of an integrated control system. International Organization for Biological Control. 2002;25:137-146.
- [Google Scholar]
- Seasonality and host utilization of the invasive fruit fly, Bactrocera invadens (Dipt., Tephritidae) in central Tanzania. J. Appl. Entomol.. 2006;130(9-10):530-537.
- [Google Scholar]
- Ammonium acetate enhances the attractiveness of a variety of protein-based baits to female Ceratitis capitata (Diptera: Tephritidae) J. Econ. Entomol.. 2015;108(2):694-700.
- [Google Scholar]
- Attraction of Bactrocera cucurbitae and Bactrocera dorsalis (Diptera: Tephritidae) to beer waste and other protein sources laced with ammonium acetate. Florida Entomologist. 2017;100(1):70-76.
- [Google Scholar]
- DEVELOPMENT OF IMPROVED ATTRACTANTS AND THEIR INTEGRATION INTO FRUIT FLY SIT MANAGEMENT PROGRAMMES: FINAL REPORT FOR THE PERIOD. Development of Improved Attractants and Their Integration into Fruit Fly SIT Management Programmes.. 2007;73
- Raghuvanshi, A.K., Satpathy, S., Mishra, D.S., 2012. Role of abiotic factors on seasonal abundance and infestation of fruit fly, Bactrocera cucurbitae (Coq.) on bitter gourd. Journal of Plant Protection Research 52.
- Occurrence of insect pests on guava (Psidium guajava) tree. Pakistan Journal of Zoology. 2006;38:197.
- [Google Scholar]
- SAS/STAT Product Documentation. Cary, NC: SAS Institute Inc.; 2011. Available at
- Evaluation of food-based attractants for fruit fly trapping in Mauritius. In: Development of Improved Attractants and Their Integration into Fruit Fly SIT Management Programmes. 2007. p. :195.
- [Google Scholar]
- Use of maize as a trap crops for the control of melon fly, B. cucurbitae (Diptera: Tephritidae) with GF-120. Bio-control and other control methods. (http\www.. 2006;fcla. edu/FlaEnt/fe87p354 Pdf)(accessed 12 October 2018)
- [Google Scholar]
- Sookar, P., S. Permalloo, M. Alleck and S. Seewooruthun. 2006. Development of improved attractants and their integration into fruit fly management programmes.
- Methyl eugenol: its occurrence, distribution, and role in nature, especially in relation to insect behavior and pollination. J. Insect Sci.. 2012;12(56):1-60.
- [Google Scholar]
- Ammonia formulations and capture of Anastrepha fruit flies (Diptera: Tephritidae) Journal of entomological science. 2008;43:76-85.
- [Google Scholar]
- Vargas, R.I., Leblanc, L., Putoa, R., Eitam, A., 2007. Impact of introduction of Bactrocera dorsalis (Diptera: Tephritidae) and classical biological control releases of Fopius arisanus (Hymenoptera: Braconidae) on economically important fruit flies in French Polynesia. Journal of economic entomology 100, 670-679.
- Correlation of fruit fly (Diptera Tephritidae) infestation of major mango cultivars in Borgou (Benin) with abiotic and biotic factors and assessment of damage. Crop Prot.. 2009;28(6):477-488.
- [Google Scholar]
- Study advance on biology and ecology of Bactrocera: Bactrocera dorsalis (Hendel) In: and its control [J]. Ecologic science 1. 2005.
- [Google Scholar]
- Ammonium carbonate is more attractive than apple and hawthorn fruit volatile lures to Rhagoletis pomonella (Diptera: Tephritidae) in Washington State. Environ. Entomol.. 2014;43(4):957-968.
- [Google Scholar]







