Translate this page into:
Development of screen- printed carbon electrode-based immunosensors for the electrochemical detection of dengue virus antigen
⁎Corresponding author. wmohammad@jazanu.edu.sa (Waquar Ahsan)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objective
This study describes the development of a cost-effective and sensitive immunosensor for rapid detection of the dengue virus (DENV) antigen in human plasma.
Methods
Screen-printed carbon electrodes (SPCEs) were used to fabricate the immunosensor by immobilizing the DENV antibodies to its surface using 1-ethyl-3-(3-dimethylamino propyl)carbodiimide hydrochloride/N-hydroxysulfosuccinimide (EDC/s-NHS) as linker. The detection of the antigen–antibody interaction was achieved by linear sweep voltammetry (LSV) using potentiostat instrument and a sensor connector. The developed method was validated as per the ICH guidelines for linearity, sensitivity and accuracy.
Results
The developed immunosensors showed excellent sensitivities in detecting the DENV antigens in both phosphate buffer saline (PBS) maintained at pH 7.4 as well as diluted human plasma. The limit of detection (LOD) values obtained in PBS was 0.11 nM; whereas in human plasma, it was calculated to be 0.16 nM. Good linearity was obtained for both PBS and human plasma over a wide range of concentrations showing promising applications of the immunosensor in both qualitative and quantitative detection of dengue virus antigen. Also, the developed immunosensor was able to detect the dengue antigen in 5 min showing rapid detection.
Conclusion
A sensitive, reliable and cost-effective biosensor was developed which was able to detect the dengue virus antigen selectively and rapidly.
Keywords
Dengue
DENV antigen
Immunosensor
SPCE
Electrochemical
Diagnosis
1 Introduction
Dengue, a viral infection has grown rapidly in recent past and an estimated 100–400 million infections are reported every year (Bhatt et al., 2013; Messina et al., 2019). Although most of the infections are mild and asymptomatic; however, severe dengue has been a primary cause of critical illness and death in various parts of Asia and Latin America (World Health Organization, 2009; Basurko et al., 2018). There still is no specific treatment available for dengue and the fatalities can only be reduced by early detection of the disease progression. Several methods are available for the dengue virus (DENV) detection including virus isolation methods and serological methods broadly. Viral isolation methods include virus culture and the amplification of viral nucleic acid using reverse transcriptase-polymerase chain reaction (RT-PCR) technique. On the other hand, serological methods include tests such as immunoglobulin M (IgM) and immunoglobulin G (IgG) capture enzyme-linked immunosorbent assay (ELISA) (Linares et al., 2013; Sanjaya et al., 2020; Ma et al., 2022). Although these techniques are accurate; 5–9 days post-infection are generally required to achieve a detectable concentration of IgM and IgG antibodies using ELISA (Watthanaworawit et al., 2011; Morales et al., 2021; Yow et al., 2021). Additionally, ELISA technique is not cost-effective specially if is used for smaller number of samples.
Another method of detecting DENV antigens is by using the Rapid Diagnostic Test (RDT) devices which is less sensitive than ELISA and used for the point-of-care testing. These devices however are prone to the storage conditions which may result in wrong findings (Stephen et al., 2014; Chamnanchanunt et al., 2021; Mahajan et al., 2021). To overcome the problems with ELISA and RDTs, several immunosensors were developed previously which could offer a number of advantages including easier management, effective miniaturization and possible on-site detection and monitoring of the disease (Dias et al., 2013; Bachour Junior, 2021; Tran and Park, 2021; Goharshadi et al., 2022). Depending upon the transducers used in the development of these immunosensors, they can be optical, electrochemical and piezoelectric; out of which electrochemical-based immunosensors have gained much attraction owing to their high sensitivity, accuracy and simplicity (Kassim et al., 2011; Habib et al., 2021; Mahajan et al., 2021).
The screen-printed carbon electrodes (SPCEs) are being utilized for the development of electrochemical-biosensors owing to various advantages associated with SPCEs including cost-effectiveness, simplicity, easy scale-up, versatility, portability, and small size (Taleat et al., 2014; Sher et al., 2021; Ameku et al., 2022). Among various electrode materials, carbon remains an attractive choice as it is relatively inexpensive and is associated with lesser background currents (Baniukevic et al., 2013; Wu et al., 2022; Yunus et al., 2022). The immobilization of antibodies is another crucial factor in the design of immunosensors as it affects the specificity and sensitivity of immunosensors (Cavalcanti et al., 2012; Fortunati et al., 2022; Polli et al., 2022). In this study, we selected the SPCEs to design our immunosensor and the anti-DENV proteins were immobilized using EDC/-sNHS linker in (2-(N-morpholino) ethanesulfonic acid (MES), as this is one of the most widely accepted techniques yielding highly sensitive biosensors. The EDC/-sNHS linker requires carboxylic acid (–COOH) acid group to react which was added to the carbon surface using 1-pyrene carboxylic acid (1-PCA). The functionalization and immobilization steps used in this study were simple and did not require costly chemicals or reagents.
2 Materials and methods
2.1 Materials
All the chemicals, reagents and solvents including 1-pyrene-carboxylic acid (1-PCA, 95 %), 2-(N-morpholino)ethanesulfonic acid (MES, ≥99.5 %), 1-ethyl-3-(3-dimethylamino propyl)carbodiimide hydrochloride (EDC, ≥98 %), N-hydroxysulfosuccinimide (sNHS, ≥98 %), amino-polyethylene glycol5-alcohol (amino-PEG, >95 %), Tris (>99 %), methanol (99.8 %), 1X phosphate buffer saline (PBS) were purchased form Sigma Aldrich (Steinheim, Germany). Dengue antigen DENV type 2 envelope protein [His] was purchased from Creative Diagnostics (New York, USA) and monoclonal anti-dengue virus envelope protein antibody produced in mouse was purchased from Sigma Aldrich (Steinheim, Germany). Non-serogenic human plasma was obtained from King Fahad Central Hospital, Jazan, Saudi Arabia.
2.2 Instruments
The SPCEs, PalmSens 4 potentiostat and sensor connector were purchased from PlamSens BV (Houten, Netherlands). The SPCEs consisted of three electrodes comprising of working electrode and counter electrode made of carbon and a pseudo-reference electrode made of silver. The PalmSens 4 potentiostat was connected to the SPCEs using sensor connector and was interfaced with the PSTrace 5.8 software for linear sweep voltammetric (LSV) studies.
2.3 Preparation of solutions
The 3 mM 1-PCA solution was prepared by dissolving 7.38 mg of 1-PCA in 10 mL of methanol. MES solution (50 mM) was prepared by dissolving 976 mg of MES in 100 mL water and the pH was adjusted to 6 using 0.1 M sodium hydroxide (NaOH) solution. Amino-PEG solution (3 mM) was prepared by dissolving 7.11 mg of amino-PEG in 10 mL of PBS maintained at pH 7.4. Tris solution (1 M) was prepared by dissolving 1.21 g of tris in 10 mL PBS buffer (pH 7.4).
2.4 Functionalization of blank SPCEs
The blank chips were treated with 20 µL of 3 mM 1-PCA solution in methanol for 2 h in order to functionalize the chips with carboxylic acid (–COOH) groups. The chips were then rinsed with 100 % methanol for 5 min and dried under nitrogen.
2.5 Immobilization of anti-DENV envelope monoclonal antibody
The functionalized chips were calibrated, and the baseline correction were made by 50 mM MES solution (pH 6) for a period of 5 min. Immediately, a mixture containing the above 50 mM MES solution (5 mL) with 2 mg of EDC and 6 mg of sNHS were applied to each chip and incubated at room temperature for 20 min. The chips were then rinsed twice in the prepared 50 mM MES solution before immobilizing the antibodies. The commercially obtained antibody solution was diluted using PBS (pH 7.4) to achieve 50 nM concentration. An aliquot (60 µL) of the resulting solution was placed on the chip followed by incubation at room temperature for 15 min. The residual sNHS ester functional groups were quenched using 3 mM amino-PEG solution in 10 mL PBS buffer (pH 7.4) for 15 min. Quenching of remaining –COOH groups were achieved using 1 M Tris solution for 15 min to reduce the non-specific interactions. Chips were finally rinsed 5 times using PBS (pH 7.4).
2.6 Instrumental conditions
Detection of antigens using the developed immunosensors was performed by LSV using PalmSens 4 potentiostat instrument. The software PSTrace 5.8 was used to control the detection settings and after optimization and several trials, the following parameters were found to be most appropriate for the detection of antigens: tequilibration = 10 s; Ebegin = 0.2 V; Eend = 2.5 V; Estep = 0.016 V and Scan rate = 0.016 V/s.
2.7 Preparation of quality control (QC) and calibration standard solutions
DENV type 2 Envelope protein [His] was obtained from the supplier as 2.94 mg/mL solution which was further diluted using 1X PBS buffer (pH 7.4) to obtain working standard solutions of antigen (AgWS-1). Concentrations of 1, 5, 10, 20 and 50 nM antigen were prepared to construct the calibration curve in PBS buffer. Measured volumes of AgWS-1 solution was spiked to diluted (1:100 using PBS) blank human plasma to prepare the working calibration standard solutions in human plasma (AgWS-2) of concentrations 0.5, 1, 2, 4, 8 and 16 nM. Three concentrations of 1, 6, and 12 nM were prepared in diluted blank human plasma as low quality control (LQC), medium quality control (MQC), and high quality control (HQC) samples, respectively, to validate the developed method. These samples were used to assess the intra- and inter-day precision and accuracy.
2.8 Detection of DENV type-2 envelope antigen in buffer background
An aliquot (60 µL) of AgWS-1 were placed on the treated chips, incubated for 5 min and measured using LSV method by PalmSens 4 potentiostat instrument keeping PBS buffer as blank. The measurements were made using PSTrace 5.8 software and the change in potential due to the antigen–antibody interaction was measured. The experimental setup used for the detection of DENV antigen is given in Fig. 1.
Experimental setup used for the detection of DENV antigen using the immobilized carbon chips connected to potentiostat and measuring the change in potential using LSV by the software PSTrace 5.8.
2.9 Detection of DENV type-2 envelope antigen in human plasma background
The DENV type-2 envelope protein was detected using the same method as described above using AgWS-2 solutions and the calibration curve was plotted using the peak area of voltammograms obtained for each concentration.
2.10 Method validation
The developed method for the detection of DENV antigen was evaluated for linearity, sensitivity, reproducibility, precision and accuracy using the three quality control samples. The sensitivity of the method was analyzed by determining the limit of quantification (LOQ) and limit of detection (LOD) values from the calibration plots obtained for both PBS buffer as well as human plasma. To check the linearity, various non-zero concentrations were prepared in a series and detected in triplicate. The calibration curve was plotted using the peak area on Y-axis versus antigen concentrations at X-axis. The equation for regression was computed using the least square regression analysis and the slope and intercept were determined. The correlation coefficient (R2) was measured to prove the linear fit of the curve.
The reproducibility, accuracy and precision of the method was determined using the LQC (1 nM), MQC (6 nM) and HQC (12 nM) samples analyzed in triplicates at three different times within the same day for intra-day, whereas at three consecutive days for inter-day precision and accuracy. Precision values were presented as the percentages of relative standard deviation (%RSD) values from the analysis of QC samples. On the other hand, accuracy of the method was computed as % mean relative error (%RE) and was determined by comparing the analyzed concentration with the nominal concentration. %RSD values less than 15 % and %RE values ± 15 % was set as acceptance criteria.
3 Results and discussion
3.1 Development of immunosensor
Blank SPCEs were functionalized with –COOH groups using 1-PCA by means of passive adsorption. The solution of 1-PCA prepared in methanol was placed on the chip for 2 h which led to the adsorption of 1-PCA on to the surface of carbon exposing the –COOH groups (Fig. 2). The exposed –COOH groups were utilized for the immobilization of anti-DENV antibodies using EDC/s-NHS chemistry. The solution of EDC and s-NHS prepared in MES buffer were used to react with –COOH groups of 1-PCA resulting in the deployment of free –sNHS groups on the surface.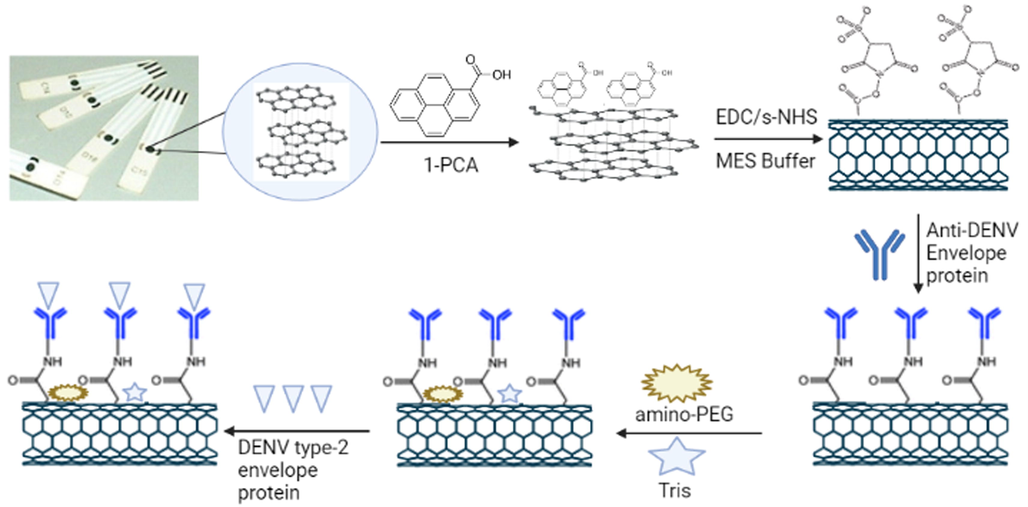
Flow diagram of DENV antigen detection using developed immunosensors. Blank SPCE chips were functionalized using 1-PCA by passive adsorption method and the DENV antibodies were immobilized using EDC/sNHS. Amino-PEG and Tris were used to block the unbound –COOH and –sNHS groups to reduce the non-specific interactions and finally the DENV antigens were detected using LSV.
The –sNHS groups were utilized to immobilize the anti-DENV envelope protein antibodies covalently to the carbon surface. The developed immunosensors were then treated with amino-PEG to block the unreacted –COOH groups followed by treatment with tris solution to block the excess –sNHS groups. Blocking the free –COOH and –sNHS groups resulted in reducing the non-specific interactions as the antigens could bind to these groups giving non-specific results. The developed immunosensor chips were then washed repeatedly using PBS buffer (pH 7.4) in order to remove all unbound reagents and antibodies and finally dried under nitrogen and stored in a sealed container in refrigerator till further use.
3.2 Detection of DENV envelope antigens in PBS buffer (pH 7.4)
The immobilized immunosensor chip was placed in the sensor connector which was attached to the Potentiostat connected to the computer. Blank reading was taken with PBS buffer (pH 7.4) followed by addition of increasing concentrations of antigens and voltammograms were obtained in each case. Clear deviation was seen in the voltammogram in comparison to blank upon treatment with antigen solution which kept on increasing with the increasing antigen concentrations (Fig. 3A). To measure the deviation, each peak was subtracted from the blank peak and the resulting overlay voltammograms are shown in Fig. 3B.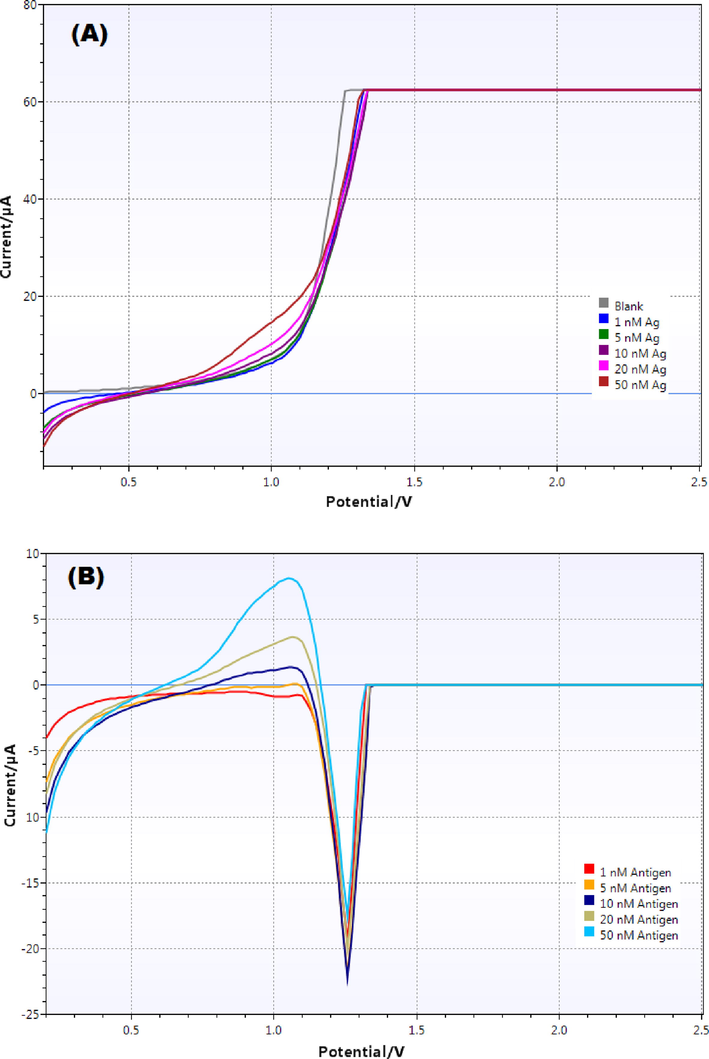
(A) Overlay linear sweep voltammograms obtained with different concentrations of antigen (blank, 1, 5, 10, 20 and 50 nM) in PBS buffer (pH 7.4) and (B) Difference voltammograms (Blank - antigen) obtained for various concentrations.
As evident from the figure, both peak height as well as peak area increased with increasing antigen concentration. Each peak was integrated using the PSTrace software and the corresponding peak area values were measured which was used to construct the calibration curve. The equation for linear regression was obtained to be “y = 0.0597 × – 0.1369” with a correlation coefficient (R2) value equal to 0.9915 (Fig. 4). The LOD and LOQ values were obtained from the calibration curve and were calculated to be 0.11 nM and 0.35 nM respectively. This showed high sensitivity of the developed immunosensors.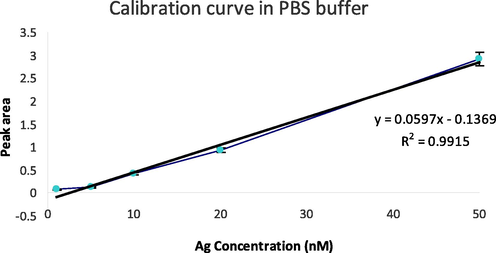
Calibration curve obtained using various antigen concentrations (1, 5, 10, 20 and 50 nM) in PBS buffer (pH 7.4).
3.3 Detection of DENV envelope antigens in human plasma
The developed method in PBS buffer was applied to the detection of antigens spiked to the human plasma. Human plasma was first diluted 100 times using PBS buffer (pH 7.4) and different concentrations of antigen were spiked to achieve final concentrations of 0.5, 1, 2, 4, 8 and 16 nM. Antigens were detected using the developed immunosensor using the same method used for PBS buffer. It was observed that the antigen in human plasma could be detected after 5 min of treatment with the antibodies immobilized on the immunosensor. Therefore, after addition of each sample, antigens and antibodies were allowed to interact for at least 5 min and then the voltammograms were obtained. Before analyzing the samples, a blank reading was taken using plain diluted plasma.
The linear sweep voltammograms for each antigen concentration was obtained and the overlay voltammogram is shown in Fig. 5A. It is evident from the figure that the deviation in peaks was directly proportional to the antigen concentrations. To give a better picture of the extent of deviation, each sample peak was subtracted from the blank and the overlay subtracted voltammograms are shown in Fig. 5B. The peak height as well as the peak area increased upon increasing the antigen concentration. Each peak was integrated, and individual peak areas were calculated which were used to construct the calibration curve in human plasma.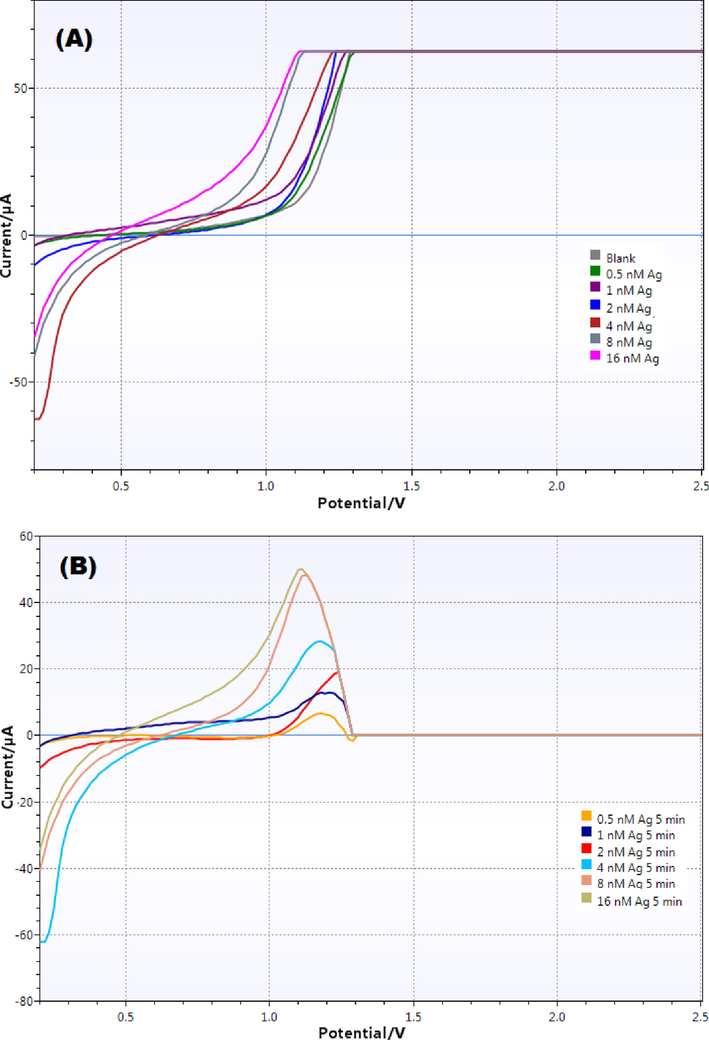
(A) Overlay linear sweep voltammograms obtained with different concentrations of antigen (blank, 0.5, 1, 2, 4, 8 and 16 nM) in diluted human plasma (1:100 using PBS pH 7.4); and (B) Difference voltammograms (Blank - antigen) obtained for various antigen concentrations.
The calibration curve showed almost linear relationship between the peak area and antigen concentrations and the equation for linear regression was obtained to be “y = 65.37x + 43.741” with coefficient of determination (R2) value = 0.9867 showing good linearity over wide range of antigen concentrations (Fig. 6). The LOD and LOQ values were also calculated from the linearity plot and were found to be 0.16 nM and 0.47 nM respectively showing excellent sensitivity of the developed immunosensor for detection of dengue antigens in human plasma.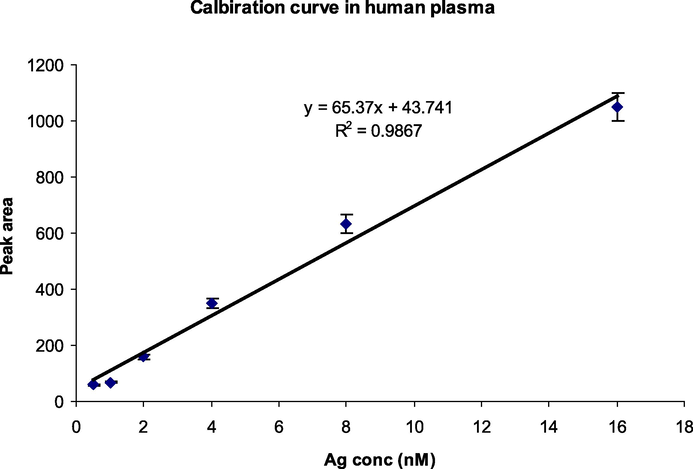
Calibration curve obtained using various antigen concentrations (0.5, 1, 2, 4, 8 and 16 nM) in human plasma 100X diluted with PBS buffer (pH 7.4).
3.4 Method validation
The method showed good linearity in both PBS and human plasma as the R2 values were calculated to be 0.9915 for PBS and 0.9867 for human plasma over a broad range of antigen concentrations. The sensitivity of the method was also observed to be good as the LOD values in PBS buffer was 0.11 nM whereas, in human plasma it was calculated to be 0.16 nM. The precision and accuracy data obtained from measuring the QC samples are provided in Table 1. The %RSD values calculated during the intra-day analysis were obtained to be 1.32 – 4.13 %; whereas, it was found to be in the range of 0.68 – 2.54 % in the inter-day analysis showing good precision of the developed method in the detection of DENV antigen in human plasma. The accuracy of the method was also observed to be high as the recovery values calculated after the intra-day analysis was 99.7 – 107.9 % and 99.8 – 104.1 % after the inter-day analysis. The %RE values were measured to be in the range of 0.59 – 4.21 % for all the DENV-Ag concentrations in human plasma. The results of precision and accuracy measurements were well within the prescribed range which indicated that the developed biosensing method was reliable and reproducible for the detection of DENV antigen.
QC sample
Intra-day analysis
Inter-day analysis
Measured conc (nM ± SD)
%RSD
% Recovery
%RE
Measured conc (nM ± SD)
%RSD
% Recovery
%RE
LQC (1.0 nM)
1.04 ± 0.061
4.13
103.8
2.21
1.01 ± 0.023
2.54
101.5
4.21
MQC (6.0 nM)
6.08 ± 0.032
3.52
107.9
1.76
6.04 ± 0.044
2.10
104.1
0.59
HQC (12.0 nM)
11.73 ± 0.032
1.32
99.7
2.43
11.88 ± 0.011
0.68
99.8
1.68
The developed immunosensor was successfully applied for the detection of DENV envelope antigen in both PBS buffer as well as human plasma with a very low detection limit of 0.11 and 0.14 nM respectively. In this study, DENV envelope protein was used as antigen instead of NS1 protein due to several reasons. NS1 remains present in the patients infected with dengue only during the early clinical phase of the disease which generally is detectable upto day 9 of the onset of symptoms in primary as well as secondary dengue infections (Parkash and Shueb, 2015; Anusha et al., 2019). Also, there are chances of cross-reactivity with the flaviviruses in both antibody assays and detection tests, although the specificity is being improved in recent studies (Matheus et al., 2016, Pereira et al., 2021; Lai et al., 2022). On the other hand, envelope protein elicits neutralizing antibody response via domain III and is being utilized for the serological detection of dengue infection by the estimation of IgM (Zhang et al., 2017; Nguyen et al., 2019; Qu et al., 2020). The detectable concentrations of IgM reaches within five days of the onset of symptoms and the peak levels are achieved after two weeks. Detection of IgM is significant in the diagnosis of patients during acute phase of the disease (Prince and Matud, 2011; Nguyen et al., 2019).
Various parameters associated with the assay procedure were optimized which included the incubation time, incubation temperature, concentration of antigen and antibodies, frequency of washing and time of detection apart from the system parameters. System parameters were optimized to achieve reduced background signals and an optimum peak shape which might lead to false positive or negative results. Incubation time also affected the detection as the functionalization and immobilization steps needed enough contact time with the electrode surface. Incubation temperature did not affect the detection as incubating the chips at room temperature or 37 °C did not change the results. Selection of proper concentration of antibodies was also important as higher concentration might have breached the blocking barrier leading to false results. Use of optimum concentration of antibodies for immobilization is necessary to achieve good results as at this concentration maximum antibody recognition sites are exposed on the electrode. Lesser concentration might result in insufficient recognition sites affecting the detection again.
4 Conclusions
A new, rapid and cost-effective immunosensor for the efficient and sensitive detection of DENV antigens was developed using SPCEs which was able to detect very low concentrations of DENV envelope protein. Use of carbon electrodes without using any expensive material reduced the cost of the developed biosensor which has mass production capabilities. The use of envelope protein as antigen has an added advantage of being specific for the DENV infection and the chances of cross-reactivity with other viruses are very less. The developed immunosensor may have application in the diagnosis of acute dengue infection in patients. However, further improvement in the sensitivity is warranted nevertheless.
Acknowledgement
The authors extend their appreciation to the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia for funding this research work through the project with number: ISP22 – 12.
Funding
This work was funded by the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia (Project no. ISP22 - 12).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Rapid detection of anti-SARS-CoV-2 antibodies with a screen-printed electrode modified with a spike glycoprotein epitope. Biosensors (Basel). 2022;12(5):272.
- [CrossRef] [Google Scholar]
- Electrochemical biosensing of mosquito-borne viral disease, dengue: a review. Biosens. Bioelectron.. 2019;142:111511
- [CrossRef] [Google Scholar]
- Electrochemical aptasensor for NS1 detection: Towards a fast dengue biosensor. Talanta. 2021;233:122527
- [CrossRef] [Google Scholar]
- Baniukevic, J.,; Kirlyte, J.,; Ramanavicius, A.,; Ramanaviciene, A., 2013. Application of oriented and random antibody immobilization methods in immunosensor design. Sens. Actuators B 2013, 189, 217–223. https://doi.org/10.1016/j.snb.2013.03.126.
- Estimating the risk of vertical transmission of dengue: a prospective study. Am. J. Trop. Med. Hyg.. 2018;98(6):1826-1832.
- [CrossRef] [Google Scholar]
- Bhatt, S., Gething , P.W., Brady, O.J., Messina, J.P., Farlow , A.W., Moyes, C.L., et alDrake, J.M., Brownstein, J.S., Hoen, A.G., Sankoh, O., Myers, M.F., George, D.B., Jaenisch, T., Wint, G.R., Simmons, C.P., Scott, T.W., Farrar, J.J., Hay, S.I.,. 2013. The global distribution and burden of dengue. Nature 496 (7446), 504–507. https://doi.org/10.1038/nature12060.
- Cavalcanti, I.T.,; Guedes, M.I.,; Sotomayor, M.D.,; Yamanaka, H.,; Dutra, R.F., 2012. A label-free immunosensor based on recordable compact disk chip for early diagnostic of the dengue virus infection. Biochem. Eng. J. 67, 225–230. https://doi.org/10.1016/j.bej.2012.06.016.
- False-positive nonstructural protein 1 antigen in a patient with Philadelphia chromosome-positive acute lymphoblastic leukemia: a case report with literature review. Am. J. Case Rep.. 2021;22:e928865.
- [CrossRef] [Google Scholar]
- Dias, A.C.,; Gomes-Filho, S.L.,; Silva, M.,; Dutra, R.F., 2013. A sensor tip based on carbon nanotube-ink printed electrode for the dengue virus NS1 protein. Biosens. Bioelectron. 44, 216–221. https://doi.org/10.1016/j.bios.2012.12.033.
- Smart immunosensors for point-of-care serological tests aimed at assessing natural or vaccine-induced SARS-CoV-2 immunity. Sensors (Basel). 2022;22(14):5463.
- [CrossRef] [Google Scholar]
- The use of nanotechnology in the fight against viruses: a critical review. Coord. Chem. Rev.. 2022;464:214559
- [CrossRef] [Google Scholar]
- A comparative study of serological diagnosis of Dengue outbreak 2019. Afr. Health Sci.. 2021;21(3):1117-1123.
- [CrossRef] [Google Scholar]
- Kassim, F.M.;, Izati, M.N.,; TgRogayah, T.A.,; Apandi, Y.M.,; Saat, Z., 2011. Use of dengue NS1 antigen for early diagnosis of dengue virus infection. Southeast Asian J. Trop. Med. Public Health 42, 562–569. PMID: 21706934.
- Development of novel dengue NS1 multiplex lateral flow immunoassay to differentiate serotypes in serum of acute phase patients and infected mosquitoes. Front. Immunol.. 2022;13:852452
- [CrossRef] [Google Scholar]
- Linares, E.M.,; Pannuti, C.S.,; Kubota, L.T.;, Thalhammer, S., 2013. Immunospot assay based on fluorescent nanoparticles for Dengue fever detection. Biosens. Bioelectron. 41, 180–185. https://doi.org/10.1016/j.bios.2012.08.005.
- A multiple-target simultaneous detection method for immunosorbent assay and immunospot assay. Anal. Chem.. 2022;94(24):8704-8714.
- [CrossRef] [Google Scholar]
- Diagnostic accuracy of commercially available immunochromatographic rapid tests for diagnosis of dengue in India. J. Vector Borne Dis.. 2021;58(2):159-164.
- [CrossRef] [Google Scholar]
- Specificity of dengue NS1 antigen antigen in differential differential diagnosis diagnosis of dengue dengue and Zika virus virus Infectioninfection. Emerg. Infect. Dis.. 2016;22(9):1691-1693.
- [CrossRef] [Google Scholar]
- The current and future global distribution and population at risk of dengue. Nat. Microbiol.. 2019;4(9):1508-1515.
- [CrossRef] [Google Scholar]
- Morales, I., Rosenberger, K.D., Magalhaes, T., Morais, C.N.L., Braga, C., Marques, E.T.A., Calvet, G.A., Damasceno, L., Brasil, P., Bispo de Filippis, A.M., Tami, A., Bethencourt, S., Alvarez, M., Martínez, P.A., Guzman, M.G., Souza Benevides, B., Caprara, A., Quyen, N.T.H., Simmons, C.P., Wills, B., de Lamballerie, X., Drexler, J.F., Jaenisch, T., IDAMS Clinical Study Group., 2021. Diagnostic performance of anti-Zika virus IgM, IgAM and IgG ELISAs during co-circulation of Zika, dengue, and chikungunya viruses in Brazil and Venezuela. PLoS Negl. Trop. Dis. 15 (4), e0009336. https://doi.org/10.1371/journal.pntd.0009336.
- Nguyen, N.M., Duong, B.T., Azam, M., Phuong, T.T., Park, H., Thuy, P.T.B., Yeo, S.J., 2019. Diagnostic Performance performance of Dengue dengue Virus virus Envelope envelope Domain domain III in Acute acute Dengue dengue Infectioninfection. Int. J. Mol. Sci. 20 (14), 3464. https://doi.org/doi: 10.3390/ijms20143464. .
- Diagnosis of Dengue dengue Infection infection Using using Conventional conventional and Biosensor biosensor Based based Techniquestechniques. Viruses. 2015;7(10):5410-5427.
- [CrossRef] [Google Scholar]
- NS1-based ELISA test efficiently detects dengue infections without cross-reactivity with Zika virus. Int. J. Infect. Dis.. 2021;112:202-204.
- [CrossRef] [Google Scholar]
- ASu@MNPs-based electrochemical immunosensor for vitamin D3 serum samples analysis. Talanta. 2022;251:123755
- [CrossRef] [Google Scholar]
- Estimation of dengue virus IgM persistence using regression analysis. Clin. Vaccine Immunol.. 2011;18(12):2183-2185.
- [CrossRef] [Google Scholar]
- Identification of a neutralizing monoclonal antibody that recognizes a unique epitope on domain III of the envelope protein of tembusu virus. Viruses. 2020;12(6):647.
- [CrossRef] [Google Scholar]
- Flow-cytometry detection of fluorescent magnetic nanoparticle clusters increases sensitivity of dengue immunoassay. Anal. Chim. Acta.. 2020;1107:85-91.
- [CrossRef] [Google Scholar]
- Nano-engineered screen-printed electrodes: a dynamic tool for detection of viruses. Trends Analyt. Chem.. 2021;143:116374
- [CrossRef] [Google Scholar]
- Stephen, S.,; Charles, M.,; Anitharaj, V.,; Deepa, C.,; Umadevi, S., 2014. Early dengue diagnosis by nonstructural protein 1 antigen detection: Rapid immunochromotography versus two the enzyme-linked immunosorbent assay kits. Indian J. Pathol. Microbiol. 57, 81–84. https://doi.org/10.4103/0377-4929.130905.
- Taleat, Z.,; Khoshroo, A.,; Mazloum-Ardakani, M., 2014. Screen-printed electrodes for biosensing: A review (2008–2013). Microchim. Acta 181, 1–27. https://doi.org/10.1007/s00604-014-1181-1.
- Highly sensitive detection of dengue biomarker using streptavidin-conjugated quantum dots. Sci. Rep.. 2021;11(1):15196.
- [CrossRef] [Google Scholar]
- Watthanaworawit, W.,; Turner, P.,; Turner, C.L.,; Tanganuchitcharnchai, A.,; Jarman, R.G.,; Blacksell, S.D.,; Nosten, F.H., 2011. A prospective evaluation of diagnostic methodologies for the acute diagnosis of dengue virus infection on the Thailand-Myanmar border. Trans. R. Soc. Trop. Med. Hyg. 105, 32–37. https://doi.org/10.1016/j.trstmh.2010.09.007.
- World Health Organization, 2009. Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control, Geneva: World Health Organization. 1-147. Available from: https://www.ncbi.nlm.nih.gov/books/NBK143159/.
- Ultrasensitive SARS-CoV-2 diagnosis by CRISPR-based screen-printed carbon electrode. Anal. Chim. Acta. 2022;1221:340120
- [CrossRef] [Google Scholar]
- Rapid diagnostic tests for the detection of recent dengue infections: An evaluation of six kits on clinical specimens. PLoS One. 2021;16(4):e0249602.
- [Google Scholar]
- Surface-enhanced carboxyphenyl diazonium functionalized screen-printed carbon electrode for the screening of tuberculosis in sputum samples. Nanomaterials (Basel). 2022;12(15):2551.
- [CrossRef] [Google Scholar]
- Structures and functions of the envelope glycoprotein in flavivirus infections. Viruses. 2017;9(11):338.
- [CrossRef] [Google Scholar]







