Translate this page into:
Development of an immunochromatographic test strip to detect pneumolysin by monoclonal antibody capture method
⁎Corresponding authors. shafiul.haque@hotmail.com (Shafiul Haque), bikashsahu74@yahoo.com (Bikash Ranjan Sahu)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background
The currently available methods for the detection of Streptococcus pneumoniae are labour-intensive, and involve time-consuming steps needing skilled technicians to perform the assays. Therefore, we developed a rapid lateral flow based immunochromatographic test strip for detection of S. pneumoniae using pneumolysin using monoclonal antibody capture approach. Methodology − The strip was designed by assembling a sample pad, conjugation pad, nitrocellulose membrane and adsorbent pad supported by a plastic backing card. Optimization of control spot was done by using three different concentrations of monoclonal antibody (0.1 µg, 0.25 g and 0.5 µg). Monoclonal antibody coupled with gold nanoparticles was immobilized on conjugate pad. Recombinant pneumolysin at three different concentrations (10 ng/ml, 50 ng/ml and 100 ng/ml) was used. Diluted pneumolysin was poured onto the sample pad which migrated through the conjugate pad containing gold antibody conjugate and the antigen–antibody complex migrated to the test spot to bind to the polyclonal antibody. The remaining free antibody bound to the control spot to visualize a control spot. Results: Control spot was optimized at 0.5 µg of mouse IgG. Of three different concentrations of Pneumolysin, the spot was best visualized at 50 ng/ml Pneumolysin concentration. Conclusion: Our ICT based device could detect a commercially available recombinant Pneumolysin of S. pneumoniae. Future study is directed to validate the strip for detection of Pneumolysin using biological fluids of patients (particularly children) infected with S. pneumoniae.
Keywords
Pneumolysin
Lateral Flow Assay
Immunochromatographic Test Strip
Streptococcus pneumoniae
- LFIA
-
Lateral Flow-based Immunochromatographic Assay
- ICT
-
Immunochromatographic Test
- CAP
-
Community-acquired pneumonia
- WHO
-
World Health Organization
- ELISA
-
Enzyme Linked Immunosorbent Assay
- PCR
-
Polymerase Chain Reaction
- LFA
-
Lateral Flow Assay
- CDC
-
Cholesterol Dependent Cytolysin
- PLY
-
Pneumolysin
- GNPs
-
Gold Nanoparticles
- PBS
-
Phosphate Buffered Saline
- BSA
-
Bovine Serum Albumin
- Mab
-
Monoclonal antibody
- Pab
-
Polyclonal antibody
- IgG
-
Immunoglobulin G
- NC membrane
-
Nitrocellulose membrane
Abbreviations
1 Introduction
Streptococcus pneumoniae is a leading cause of community-acquired pneumonia (CAP), accounting for up to 70 % of hospital-acquired illnesses (Macfarlane et al., 1982). Pneumonia is more common in children than adults as previous data reveal pneumonia to be responsible for 369,000 deaths (28 % of all deaths) in children aged < 5 years in India (Million Death Study Collaborator et al., 2010). Pneumococci are well-adapted commensals, and their principal reservoir is on the mucosal surface of carriers' upper airways, where they can spread. Bacteria can cause serious sickness when they invade sterile areas such as the middle ear spaces, lungs, bloodstream, and meninges due to host factors. S. pneumoniae's incredible ability to avoid or take advantage of the host's inflammatory and immunological responses is critical for transmission, colonisation, and invasion (Ortigoza et al., 2018). S. pneumoniae is considered as one of the 12 priority pathogens listed by the WHO in 2017. Detection and identification of this bacteria can be studied under two heads; direct detection of parasites and/or detection of specific parasite-derived factors released into host body fluid. Direct detection methods basically include identification of the pathogens bedside on culture-based method (Dube et al., 2013), molecular methods such as whole blood PCR (Zhang et al., 1995) immunohistochemical analysis (Guarner et al., 2007), magnetoimmunosensor (Campuzano et al., 2010), and ELISA (Harding et al., 1979). Several assays are available for detection of parasite-derived factors of S. pneumoniae such as autolysin, streptolysin, and pneumolysin released into host are the techniques are PCR, and Western blot (Ubukata et al., 1996). Although, both the above described detection approaches (whole bacteria and bacteria derived factors) are well accepted in terms of both specificities and sensitivities; most of these are labour-intensive, time-consuming, not user-friendly and there is a need for technical experts to perform the assays.
Rapid detection of pathogens has become widely accepted approach among researchers that enables quick diagnosis of diseases. Among several rapid detection approaches, lateral flow immunoassays (LFIA) are one of the most convenient, user friendly approaches which can be used as bed side procedure for rapid detection of pathogens (Sohrabi et al., 2022). Among several rapid detection formats, lateral-flow immunoassay is a simple, rapid, and on-site detection method and has gained popularity recently in many fields majorly in diagnostics in human health (Posthuma-Trumpie et al., 2009; Bu et al., 2018). A number of such detection formats is now commercially available for detecting several parasites like bacteria, viruses and fungi enabling appropriate diagnosis of diseases prompting the health professionals for proper treatment options (Hu et al., 2016).
The basic idea behind LFA is straightforward: a fluid sample (extract) containing the analyte of interest flows across several zones of polymeric strips to which molecules (antigens and antibodies) that come into contact with the analyte are attached, without the intervention of external pressures (capillary action) (Kuczula et al., 2016). The sample, combined with the conjugated antibody linked to the target analyte, travels along the strip into the detection zone (Kuczula et al., 2016). Biological constituents (antibodies or antigens) are constrained on the reaction membrane to the test line, whereas control reagents are restrained to the control line (Gupta and Ghrera, 2021). A reaction on the test line shows that the sample component has been recognized, whilst a response on the control line indicates that the liquid flow across the strip is appropriate (Kuczula et al., 2016). The read-out, displayed through the lines with varying intensities, can be examined visually or via a dedicated reader. Lateral flow assay has been used in detection of various diagnostic proteins and also in detection of various protein biomarkers (Shen et al., 2020; Mahmoudinobar et al., 2021).
Pneumolysin is an intracellular 53-kDa protein, elaborated by S.pneumoniae. Pneumolysin (PLY) serves as a cholesterol-dependent cytolysin (CDC) and a crucial pneumococcal virulence factor that plays a role in all stages of pneumococcal illness, including transmission, infection and colonisation. PLY has various biological effects on the body, which are both diverse and numerous. It contributes bacterial penetration and inflammation, causes immediate cell damage through pore-forming cytolytic action, assists bacterial escape by inhibiting complement activation, and is a critical element in host-to-host pneumococcal transmission (Mitchell and Dalziel, 2014; Zafar et al., 2017). The Pneumolysin encoding gene has extremely little sequence diversity, making this protein an ideal immunodiagnostic target. So far, the cross-reactivity is concerned, less than 5 % cross-reaction between pneumolysin and streptolysin has been observed (Kalin et al., 1987). To enable quick diagnosis of pneumonia, a rapid, user-friendly, on-site detection procedure is quite essential to develop. Keeping the importance of lateral flow assay in mind, we fabricated and developed an immunochromatographic test strip (ICT Strip) for quick detection of pneumolysin, the secretory component of S. pneumoniae. It is pertinent to note that, we developed this assay format in the laboratory using a commercially available recombinant pneumolysin using monoclonal antibody as capture antibody.
2 Materials and methods
2.1 Materials and reagents
Recombinant pneumolysin (ab236193, Abcam) protein used as an antigen Other reagents are mouse monoclonal antibody to pneumolysin (ab71810, ply-4, Abcam), Rabbit polyclonal antibodies against pneumolysin (ab71811, Abcam) and Rabbit monoclonal antibody to mouse IgG (ab190475, Abcam), Gold antibody conjugation kit (10 nm, 20 OD, ab 188215, abcam, USA) containing three vials of Gold Nanoparticles (Gold NPs) (20 nm, ab273947), Gold reaction buffer (ab273941, abcam, USA), Gold quencher reagent (ab273942, abcam, USA) and gold antibody diluent (ab 273943, abcam, USA) were purchased from Abcam, US. Conjugation check kit (ab236554, abcam, USA) having protein A/G strips (ab 274081, abcam, USA). To fabricate the ICT strip, easy membrane kit – Dipstick, containing different components such as, the NC membrane (70x260mm, Type: CNPF-SN12-L2-H50, Lot: NHF 209098L-309), sample pad (24x260mm, Type- GFB-R7L, Lot: NR148488L), conjugate pad (70x260mm, R-1731, Lot: 017318L), and absorbent pad (27x260mm, Type- AP080, Lot: Q03958L) were purchased from MDI Membrane technologies, Advanced Microdevices Pvt. Ltd., India. Other reagents also used were Bovine serum albumin (BSA), and Phosphate Buffer Saline (PBS).
2.2 Working principle of the strip
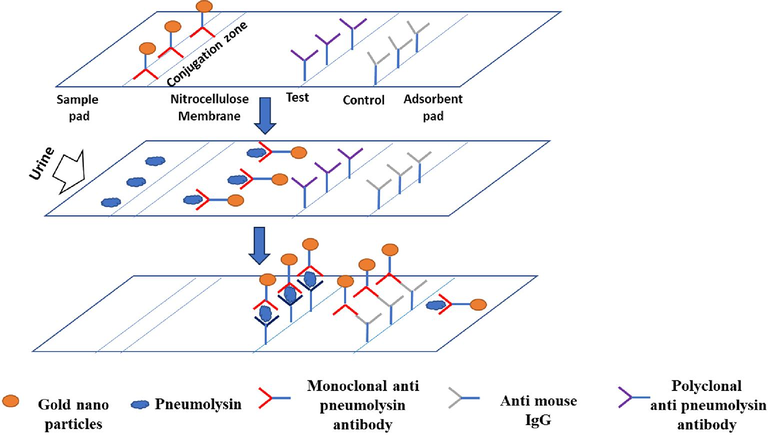
The principle underlying LFA is simple. In brief, the fluid sample containing the analyte of interest flows across several zones of polymeric strips, on which molecules (antigens and antibodies) come into contact with the analyte are attached and form antigen–antibody complexes (Kuczula et al., 2016). We fabricated the immunochromatographic test strip based on LFIA following the procedure of (Kuczula et al., 2016) that constitutes overlapping membranes such as a sample pad, conjugation pad, nitrocellulose membrane, and absorption pad supported by a backing card. As shown in supplementary data 1, Gold nanoparticles coupled to a monoclonal anti-pneumolysin antibody are loaded on the strip at conjugation zone. The recombinant pneumolysin is added on the surface of the adsorbent sample pad at one end of the strip, which is soaked with buffer salts and surfactants that render the sample appropriate for interaction with the detection system. From the sample pad, the recombinant protein moves towards the conjugate pad next to it on the strip where the labelled antibodies have been dispensed. Recombinant pneumolysin combined with the Mab- Gold NPs, travels along the strip into the detection zone. The detection zone is basically a porous membrane usually composed of nitrocellulose having two zones that is test and control zone. The complex (Pneumolysin-monoclonal anti-pneumolysin gold particles) migrates to the test zone, where a polyclonal anti-pneumolysin antibody can bind to other free epitopes of pneumolysin. The protein will be detected visually as a spot. In the control zone, spot can be visualized by reaction of anti-mouse IgG to either Pneumolysin-monoclonal anti pneumolysin- gold particles or any free monoclonal anti-pneumolysin antibody. The absorbent pad used here, absorbs the excess material from the sample and prevents the backflow of the liquid.
2.3 Methodology
2.3.1 Conjugation of monoclonal antibody to gold nanoparticles
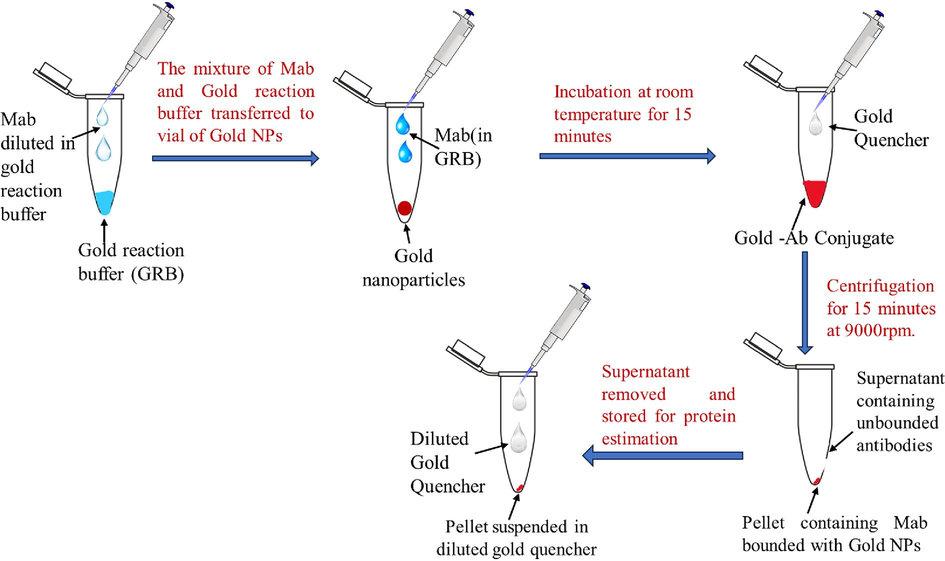
To conjugate monoclonal antibodies with gold nanoparticles, a commercially available kit was used and the procedure was used according to the manufacturer’s instructions (Supplementary data 2). Briefly, the required antibody (monoclonal anti-pneumolysin antibody. Stock – 1 mg/ml) was diluted in antibody diluent buffer at 0.2 mg/ml. 12ul of diluted antibody was added to 42ul of gold reaction buffer in a 1.5 ml tube. 45ul of this mixture was transferred to a vial of gold NPs (provided in the kit) and the mixture was thoroughly mixed. The mixture was incubated at room temperature for 15 min. 5ul of gold quencher is added to the solution to quench the reaction. 500ul of diluted quencher (1:10 ratio in milliQ) is added to the solution. The mixture was centrifuged once for 10 min at room temperature. The supernatant was removed, usually containing the unbound antibodies, and the pellet which ideally contains conjugated gold nanoparticles with monoclonal antibodies was resuspended with 50ul of the diluted quencher and stored at 4 °C till further use. The supernatant was used to calculate the conjugation efficiency by checking the concentration of unbound antibodies.
2.3.2 Fabrication and preparation of ICT strip
Lateral flow immunoassay strip is fabricated in a simple dip-stick format. First, the conjugate pad is soaked with Gold NP labelled immune antibody and was allowed to dry at 37C for 2 hrs. Sample pad, Gold NP-antibody impregnated conjugate pad, nitrocellulose membrane, and absorbent pad were assembled together in a way that the edge of each component slightly overlaps on the next component to ease lateral flow. The width of the strip was 4 mm (Li et al., 2010).
2.3.3 Immobilization of antibodies on immunochromatographic strip
To make control spot, anti- mouse IgG was immobilized on nitrocellulose membrane. Three different concentrations of this antibody (0.5 µg, 0.25 µg, and 0.125 µg) were spotted after diluting in PBS. 1ul of antibody containing the required concentrations was put nitrocellulose membrane. To make the test spot, polyclonal antibody to pneumolysin was used next to the control spot. The positions of both the control and test spot should be oner after the other with the test spot adjacent to the conjugation zone.
3 Results
3.1 Confirmation of conjugation of monoclonal antibody to gold nanoparticles
Conjugation of antibody with gold NPs was confirmed by the ability of binding of Fc receptor of Monoclonal antibody to protein A impregnated on the conjugate check and go strips. We used a commercially available conjugation check kit for this purpose. The appearance of brown–red line at the center of the nitrocellulose membrane confirmed conjugation of the two aforesaid molecules (Fig. 1).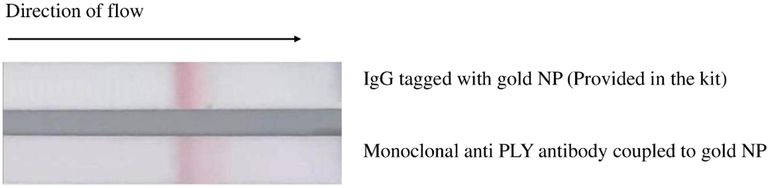
Antibody conjugation confirmation: Strip on the top – Brown line indicating the binding of Fc portion of gold NP labelled IgG (Positive control). Strip on the bottom – Brown line indicating the binding of Fc portion of gold labelled monoclonal antibody to pneumolysin (our prepared conjugate).
3.2 Efficiency of conjugation between gold nanoparticles and antibody
To ensure the conjugation between gold NPs and antibody, efficiency of conjugation was determined as follows.
Initial concentration of monoclonal anti-pneumolysin antibody (protein)- 200 µg/ml (This concentration was used according to manufacturer’s instruction as mentioned in gold conjugation kit).
Concentration of free unconjugated antibodies in supernatant − 13.2 µg/ml.
Therefore, conjugation efficiency = 100 – 13.2 µg/200 µg x 100 = 100 %- 6.6 % = 93.4 %.
3.3 Optimization of control spot
To optimize control spot, we used anti-mouse IgG at three different concentrations; 0.125 µg, 0.25 µg and 0.5 µg per spot. As shown in Fig. 2, no spot was observed at 0.1 µg, where as a spot appeared at 0.25 µg of the antibody. However, at 0.5 µg of antibody concentration, the intensity of spot was brightest. It indicates maximum antigen–antibody interaction to occur at 0.5 µg. of control antibody.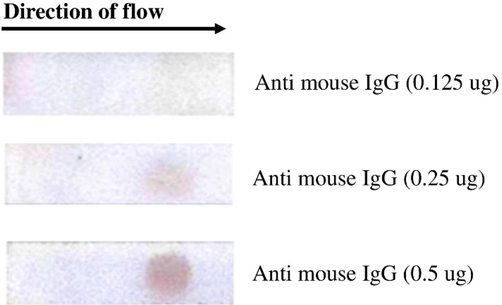
Visualization of control spot. Anti mouse IgG (0.125 µg, 0.25 µg and 0.5 µg) were spotted on the nitrocellulose membrane (8 µm size) of the strip and Gold-Ab conjugate was added at one end (left side shown in arrow marks). Mouse IgG in the monoclonal antibody coupled to Gold NP bound to anti mouse IgG and a brown spot was visible.
3.4 Selection of nitrocellulose membrane of appropriate pore size
The manufacturer of the easy membrane kit provided us four different types of nitrocellulose membrane with different pore sizes such as 8 µm, 10 µm, 12 µm and 15 µm. As mentioned in section 3.3, we considered 0.5 µg of the antibody as optimized amount (as this concentration provided maximum visibility) for the control spot and the test was performed on nitrocellulose membrane of 8 µm pore size (This pore size was selected randomly). To select the membrane of best pore size for the ICT strip, we used 0.25 µg of the antibody as control spot in all four types of membranes of varying pore sizes and observed the visibility of the spot. As observed in the Fig. 3, although the spot was visible in all 4 types of membranes; however, it is clearer in NC membrane of 8 µm size with more circular in shape. Therefore, the membrane with this pore size was considered for further work.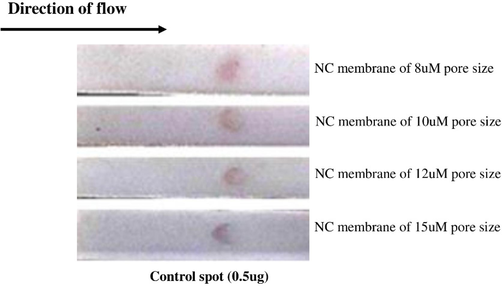
Selection of nitrocellulose membrane for spot visualization. Membranes of different pore sizes (8 µm, 10 µm, 12 µm and 15 µm) are used. 0.5 µg of monoclonal anti PLY antibody was added to spot.
3.5 Optimization of test spot
As mentioned earlier, we immobilized 0.5 µg of polyclonal anti pneumolysin antibody on the NC membrane of ICT strip as test spot to detect the analyte of interest (Pneumolysin). Recombinant pneumolysin (Commercially available) was used at three different concentrations such as 10 ng/ml, 50 ng/ml and 100 ng/ml diluted in running buffer (0.1 %BSA in PBS). Pneumolysin was released on the sample pad which moved along the strip by lateral flow and bound to monoclonal anti pneumolysin antibody coupled to gold nanoparticles impregnated at the conjugation zone. When the pneumolysin-antibody complex moved through the test zone, the brown spot was visible (Fig. 4). Our results showed that, no spot was observed when 10 ng/ml of antigen was used. On the other hand, spot was prominently visible at pneumolysin concentration of 50 ng/ml and 100 ng/ml.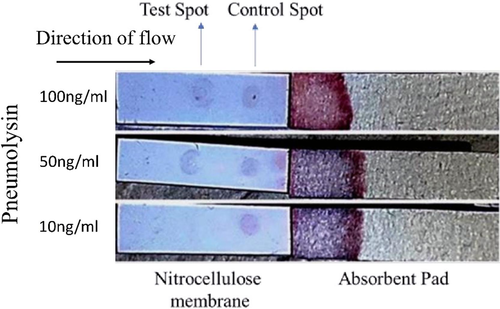
Optimization for test spot. Recombinant pneumolysin was used at various concentrations, Strip on the top – Pneumolysin used at100ng/ml. Strip in middle – 50 ng/ml and Strip on the bottom – 10 ng/ml. Test spot (polyclonal anti PLY antibody) was used at 0.5 µg/spot.
4 Discussion
LFIA based approach is considered as a quick detection strategy for pathogenic bacteria causing diseases in humans. To detect bacteria using LFIA based approaches, either whole bacteria (Scharinger et al., 2017) or any of their secretory antigenic component can also be used (Mdluli et al., 2014). Pneumolysin is considered as a secretory toxin of Streptococcus pneumoniae and its limited sequence variability has enabled it to consider it as a potential biomarker for detection of this bacteria. In our study, we made an attempt to develop an LFIA based immunochromatographic strip that can detect pneumolysin enabling a quick detection for S. pneumoniae. To design the strip, we assembled different components of the device exactly in the format described previously (Bahadir et al., 2016). Gold nanoparticles are popularly used for most of the LFIA based ICT strip to detect various biomolecules (Wang et al., 2022; Ardekani et al., 2022) and we therefore used a commercially available gold nanoparticle conjugation kit to couple monoclonal antibody against pneumolysin with gold NPs using materials and reagents provided with the kit. After conjugation, we tested the conjugation efficiency which was found as 93.4 %. Further, to examine whether the conjugation has occurred or not, we used a commercially available conjugate check strip that contains a region where Protein A/G is impregnated that specifically binds to Fc region of antibody. In our case, when the gold NP-antibody conjugate was run on the conjugate check strip, we found a brown line, at Protein A/G bound region on the strip, indicating an appropriate coupling of antibody to gold NPs (The brown colour is due to colour of gold NPs) (Fig. 2). To access the experimental validity of the conjugation check assay, we used a positive control. The reagent for positive control was provided by the manufacturer of the conjugate check assay kit. It contains gold nanoparticle-labeled immunoglobulins. The Protein A/G adsorbed on the NC membrane binds to the Fc receptor of the labelled immunoglobulin indicating appropriate functioning of the test strip.
Optimization of antibody concentrations used in control and test lines is a vital step while designing a new ICT strip (control and test spot, in our case). Previous studies have suggested a concentration range from 0.25-0.5 µg of antibodies used as control as well as test spot on ICT strip for detection of several important biomolecules (Prakashan et al., 2023). First, we optimized the amount of anti mouse IgG as control spot on the NC membrane using three concentrations of the antibody (0.1 µg, 0.25 µg and 0.5 µg) per spot. Gold nanoparticle-antibody conjugate was run on the strip and the spot was visualized. As observed in Fig. 2, the spot was clearly visible at 0.5 µg of antibody. The nitrocellulose membrane is made of cellulose fibres and is available at different pore sizes based on variation in capillary flow rates. As different pore size may influence the clarity and visibility of the spot, we used NC membranes of four different pore size (8 µm, 10 µm, 12 µm and 15 µm) in our study during optimization of control spot. It is obvious that, the NC membrane of 15 µm pore size has a better capillary flow rate as compared to membrane of 8 µm pore size. The flow rate of the liquid decides the retention capacity of the antigen on the membrane and its interaction with antibody varies accordingly. In our results, the spot was almost visible in all the membranes; however, the spot with better intensity was observed when NC membrane of 8 µm pore size was used as compared to others indicating that this pore size allows maximum interaction between antigen (monoclonal anti pneumolysin antibody) and antibody (anti mouse IgG). We, therefore, used NC membrane of 8 µm pore size to fabricate the strip. As 0.5 µg of control antibody provided the best visibility as spot, we used the similar concentration of polyclonal anti PLY antibody on NC membrane to detect the test spot. In the final experiment, recombinant pneumolysin was diluted in running buffer at concentrations of 10 ng/ml, 50 ng/ml and 100 ng/ml and was poured on the strip in sample pad region. We observed the spots in the test zone and found clear spots of almost equal intensity when 50 ng/ml and 100 ng/ml were used. However, no spot was observed when PLY was used at 10 ng/ml. We therefore assume the minimum detection level of Pneumolysin to be 50 ng/ml. The reason for appearance of spots of equal intensity for 50 ng/ml and 100 ng/ml is clear to us. During antigen–antibody interaction, when different concentrations of antigens are added to a fixed amount of antibody in a solution or any other media, maximum interaction between antigen and antibody occurs at ‘Zone of Equivalence’ and afterwards, the reaction /rate is reduced. We expect that probably, Pneumolysin (the antigen) at 50 ng and 100 ng concentrations fall in Zone of equivalence when react with polyclonal antibody causing visually equal intensity of spot. In other words, at these two concentrations of antigen (Pneumolysin), maximum interaction is observed.
We compared our results for PLY detection with previously published reports. An investigation by Salvador et al., 2022 revealed the use of a magnetic nanocluster based lateral flow assay for detection of Pneumolysin. Authors in this communication used a lateral flow immunoassay with magnetic nanoclusters conjugated to anti-pneumolysin antibodies and used two devices such as mobile camera and inductive sensor for detection of Pneumolysin. Although, this method was rapid, sensitive and specific in terms of detection of pneumolysin concentration, detection device used by the authors such as mobile camera and inductive sensor may not satisfy a bedside procedure for PLY detection. Further, a skilled technician may be essential for examining the results in this sensor based assay. On the other hand, our proposed assay offers two advantages. 1. Clarity in visibility of the control and test spots due to use of gold nanoparticles, thus offering an easier way to detect the protein without using any detection device. 2. No skilled expert is essential to perform the experiment and the strip can be used as a bedside procedure for Pneumolysin detection indicative of presence of S. pneumoniae.
Detection of antigen in biological sample using capture monoclonal antibody followed by a polyclonal antibody is a popular choice in LFIA based ICT strip (Chiao et al., 2008; Tomás et al., 2019). This approach is considered specific as the monoclonal antibody binds to an epitope of the antigen enabling specific detection of the protein. Previous studies indicate that a self-paired monoclonal antibody has also been developed and used to detect the bacteria pathogen, Acidovorax avenae subsp. citrulli through LFIA strip (Zeng et al., 2016). A pan-serotype monoclonal antibody used combinedly with a capture ligand to develop a lateral flow strip test for detecting FMD viruses (Yang et al., 2022). Owing to the accuracy of monoclonal antibody based strategy, we followed this approach to design our test strip.
It is pertinent to note that, Rajalakshmi et al., 2002 used a novel coagulation technique to detect pneumolysin in urine samples of patients infected with S. pneumoniae. In this approach, a polyclonal antisera against purified pneumolysin was used to sensitize Cowan 1 Staphylococcus aureus and slide agglutination was performed using this antisera to agglutinate pneumolysin in urine samples. Although, this technique served as a bed side procedure for detection of the protein, and as a better alternative to traditional culture based method, a less sensitivity of the procedure was observed and authors have further discussed that, it may be due to low titre of antisera used to sensitize the Staphylococci. Our test format enables visibility of control and test spot on the NC membrane indicative of presence/absence of pneumolysin.
A novel molecular based approach termed as isothermal recombinase polymerase amplification (RPA) that normally amplifies DNA at 37 °C under isothermal conditions with high specificity, sensitivity and rapidity was used for rapid detection of S. pneumoniae using autolysin gene lytA as diagnostic target. The amplification products obtained by RPA reaction were detected with gold nanoparticle based lateral flow strip. Interestingly, this technique could detect 22 different strains of S. pneumoniae (Wang et al., 2022). In contrast to this method, we have used a monoclonal antibody based approach for quick detection of the concerned pathogen using the gold nanoparticle based lateral flow strip approach.
The major drawback of our LFIA based assay is specificity and sensitivity of the assay. Earlier report suggested the presence of Pneumolysin in few other bacterial species such as Pseudomonas aeruginosa and Streptococcus agalactiae using Pneomolysin-ELISA (Garcia-Suarez., et al., 2007) Our LFIA based approach may detect these bacterial species in human harbouring these two parasites. Similarly, we found the minimum detection limit of Pneumolysin to be 50 ng/ml and no spot was observed at 10 ng/ml. This indicates that, biological samples containing < 10 ng/ml of Pneumolysin can not be detected which challenges the sensitivity of the assay. Therefore, further investigation may be carried out to use quantum dots (QDs) as an alternative to gold NPs as detection molecules as former is reported to show higher sensitivity for protein detection on ICT strip.
It is pertinent to note that, there is a commercially available LFIA based ICT kit (BINAX@Now Streptococcus pneumoniae detection kit). This kit is designed to detect a Pneumococcal antigen, PnC (a secretory carbohydrate antigen of S. pneumoniae) (Dominguez et al., 2003). The PnC antigen can be detected in all biological fluids of an individual infected with S. pneumoniae, thereby enabling quick diagnosis of Pneumonia (Smith et al., 2003; Gutiérrez et al., 2003). However, the limitation of this kit is that, while it particularly identifies the antigen (PnC) in pneumonic adults and children, it also detects the antigen in healthy nasopharyngeal carriers − the majority of whom are children. Vuorenoja et al. (2012) evaluated the specificity of this kit for the detection of S. pneumoniae in children and noticed cross-reactivity with healthy carriers. Although the BINAX kit was effective in detecting S. pneumoniae in adults, it was ineffective in children and this instruction is mentioned clearly in manufacturer’s protocol provided with the kit. Importantly, our protein of interest (Pneumolysin) is not detected in healthy children who are S. pneumoniae carriers (García-Suárez et al., 2007). Therefore, we anticipate that our approach for rapid detection of pneumolysin will enable specific detection of S. pneumoniae in children suffering from Pneumonia avoiding non-specific detection in nasopharyngeal carriers. Although, our approach to detect pneumolysin by LFIA based method aim to identify S. pneumoniae in pneumonic infected children, it can be successfully used for infected adults also as the protein, Pneumolysin, is present in both the infected population. We discussed the importance of this quick detection approach in the context of children due to lacuna of currently available BINAX S. pneumoniae detection kit to detect the pathogen in children as discussed earlier.
5 Conclusion
We made an attempt to develop an ICT kit for specific detection of S. Pneumoniae. Our device could detect recombinant pneumolysin at a minimum concentration of 50 ng/ml. However, it is important to further validate the device in biological fluid of persons affected with Pneumonia (particularly pneumonic children) harbouring S. pneumoniae. Therefore, we anticipate our approach as a base-level effort to detect the secretory protein of the bacteria. Further investigation is definitely essential to validate the kit in hospital/field set up to determine the specificity and sensitivity of the device.
CRediT authorship contribution statement
Somya Sephalika: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Writing – original draft. Nirmal K. Mohakud: Writing – review & editing, Data curation, Software, Visualization. Ayman K. Johargy: Resources, Software, Visualization, Writing – review & editing. Naif A. Jalal: Formal analysis, Visualization, Writing – review & editing. Farkad Bantun: Data curation, Software, Visualization, Writing – review & editing. Aditya K. Panda: Visualization, Writing – review & editing. Raju K. Mandal: Software, Validation, Writing – review & editing. Shafiul Haque: Funding acquisition, Resources, Visualization. Bikash Ranjan Sahu: Investigation, Project administration, Supervision, Writing – review & editing.
Acknowledgements
The authors extend their appreciation to the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia for funding this research work through the project number ISP23-101.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Gold nanoparticle-mediated lateral flow assays for detection of host antibodies and COVID-19 proteins. Nanomaterials. 2022;12(9):1456.
- [Google Scholar]
- Ultra technically-simple and sensitive detection for Salmonella enteritidis by immunochromatographic assay based on gold growth. Food Control. 2018;84:536-543.
- [Google Scholar]
- Disposable amperometric magnetoimmunosensors for the specific detection of Streptococcus pneumoniae. Biosens. Bioelectron.. 2010;26(4):1225-1230.
- [Google Scholar]
- Monoclonal antibody-based lateral flow assay for detection of botulinum neurotoxin type A. Hybridoma. 2008;27(1):31-35.
- [Google Scholar]
- Usefulness of urinary antigen detection by an immunochromatographic test for diagnosis of pneumococcal pneumonia in children. Journal of clinical microbiology. 2003;41(5):2161-2163.
- [Google Scholar]
- Detection of Streptococcus pneumoniae from different types of nasopharyngeal swabs in children. PLoS One. 2013;8(6):e68097.
- [Google Scholar]
- The role of pneumolysin in mediating lung damage in a lethal pneumococcal pneumonia murine model. Respir. Res.. 2007;8:1-10.
- [Google Scholar]
- Usefulness of immunohistochemical diagnosis of Streptococcus pneumoniae in formalin-fixed, paraffin-embedded specimens compared with culture and gram stain techniques. Am. J. Clin. Pathol.. 2007;127(4):612-618.
- [Google Scholar]
- Recent advances in gold nanoparticle-based lateral flow immunoassay for the detection of bacterial infection. Arch. Microbiol.. 2021;203(7):3767-3784.
- [Google Scholar]
- Evaluation of the immunochromatographic Binax NOW assay for detection of Streptococcus pneumoniae urinary antigen in a prospective study of community-acquired pneumonia in Spain. Clin. Infect. Dis.. 2003;36(3):286-292.
- [Google Scholar]
- Enzyme-linked immunosorbent assay for detection of Streptococcus pneumoniae antigen. J. Clin. Microbiol.. 1979;10(3):339-342.
- [Google Scholar]
- Sensitive and quantitative detection of C-reaction protein based on immunofluorescent nanospheres coupled with lateral flow test strip. Anal. Chem.. 2016;88(12):6577-6584.
- [Google Scholar]
- Diagnosis of pneumococcal pneumonia by enzyme-linked immunosorbent assay of antibodies to pneumococcal hemolysin (pneumolysin) J. Clin. Microbiol.. 1987;25(2):226-229.
- [Google Scholar]
- Rapid and sensitive detection of protein biomarker using a portable fluorescence biosensor based on quantum dots and a lateral flow test strip. Anal. Chem.. 2010;82(16):7008-7014.
- [Google Scholar]
- Hospital study of adult community-acquired pneumonia. Lancet. 1982;320(8292):255-258.
- [Google Scholar]
- Protein-based lateral flow assays for COVID-19 detection. Protein Eng. Des. Sel.. 2021;34:gzab010.
- [Google Scholar]
- Gold nanoparticle based Tuberculosis immunochromatographic assay: The quantitative ESE Quanti analysis of the intensity of test and control lines. Biosens. Bioelectron.. 2014;54:1-6.
- [Google Scholar]
- Causes of neonatal and child mortality in India: a nationally representative mortality survey. Lancet. 2010;376(9755):1853-1860.
- [Google Scholar]
- The biology of pneumolysin. Attack and Invasion: MACPF/CDC Proteins-Agents of Defence; 2014. p. :145-160.
- An infant mouse model of influenza virus transmission demonstrates the role of virus-specific shedding, humoral immunity, and sialidase expression by colonizing Streptococcus pneumoniae. MBio. 2018;9(6):e02359-e10418.
- [Google Scholar]
- Lateral flow (immuno) assay: its strengths, weaknesses, opportunities and threats. A literature survey. Anal. Bioanal. Chem.. 2009;393:569-582.
- [Google Scholar]
- Gold nanoparticle conjugate‐based lateral flow immunoassay (LFIA) for rapid detection of RBD antigen of SARS‐CoV‐2 in clinical samples using a smartphone‐based application. Journal of Medical Virology. 2023;95(1):e28416
- [Google Scholar]
- Pneumolysin in urine: a rapid antigen detection method to diagnose pneumococcal pneumonia in children. Indian J. Med. Microbiol.. 2002;20(4):183-186.
- [Google Scholar]
- Magnetic Nanoclusters Increase the Sensitivity of Lateral Flow Immunoassays for Protein Detection: Application to Pneumolysin as a Biomarker for Streptococcus pneumoniae. Nanomaterials. 2022;12(12):2044.
- [Google Scholar]
- Multiplexed lateral flow test for detection and differentiation of Cronobacter sakazakii serotypes O1 and O2. Front. Microbiol.. 2017;8:1826.
- [Google Scholar]
- An enhanced centrifugation-assisted lateral flow immunoassay for the point-of-care detection of protein biomarkers. Lab Chip. 2020;20(15):2626-2634.
- [Google Scholar]
- Rapid diagnosis of bacteremic pneumococcal infections in adults by using the Binax NOW Streptococcus pneumoniae urinary antigen test: a prospective, controlled clinical evaluation. J. Clin. Microbiol.. 2003;41(7):2810-2813.
- [Google Scholar]
- State of the art: Lateral flow assays toward the point-of-care foodborne pathogenic bacteria detection in food samples. Compr. Rev. Food Sci. Food Saf.. 2022;21(2):1868-1912.
- [Google Scholar]
- Development of a gold nanoparticle-based lateral-flow immunoassay for pneumocystis pneumonia serological diagnosis at point-of-care. Front. Microbiol.. 2019;10:2917.
- [Google Scholar]
- Combinational detection of autolysin and penicillin-binding protein 2B genes of Streptococcus pneumoniae by PCR. J. Clin. Microbiol.. 1996;34(3):592-596.
- [Google Scholar]
- Detection of Streptococcus pneumoniae carriage by the Binax NOW test with nasal and nasopharyngeal swabs in young children. Eur. J. Clin. Microbiol. Infect. Dis.. 2012;31:703-706.
- [Google Scholar]
- Rapid, simple, and highly specific detection of Streptococcus pneumoniae with visualized recombinase polymerase amplification. Front. Cell. Infect. Microbiol.. 2022;12:878881
- [Google Scholar]
- Combining a universal capture ligand and pan-serotype monoclonal antibody to develop a pan-serotype lateral flow strip test for foot-and-Mouth disease virus detection. Viruses. 2022;14(4):785.
- [Google Scholar]
- Host-to-host transmission of Streptococcus pneumoniae is driven by its inflammatory toxin, pneumolysin. Cell Host Microbe. 2017;21(1):73-83.
- [Google Scholar]
- Self-paired monoclonal antibody lateral flow immunoassay strip for rapid detection of Acidovorax avenae subsp. citrulli. Anal. Bioanal. Chem.. 2016;408:6071-6078.
- [Google Scholar]
- Detection of Streptococcus pneumoniae in whole blood by PCR. J. Clin. Microbiol.. 1995;33(3):596-601.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2024.103213.
Appendix A
Supplementary material
The following are the Supplementary data to this article: