Translate this page into:
Development of a sensitive liquid-liquid extraction and ultra-performance liquid chromatography-tandem mass spectrometry method for the analysis of carbaryl residues in fresh vegetables sold in Riyadh
⁎Corresponding authors. naalfaris@pnu.edu.sa (Nora Abdullah AlFaris), swabaidur@ksu.edu.sa (Saikh M. Wabaidur)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
An UPLC- mass spectrometry method has been proposed for the screening of carbaryl residues in fresh vegetables. Carbaryl treated samples of lettuce, cucumber and spinach were procured and analyzed for a time period of fifteen days to find out the amount of carbaryl residue. A simple and effective liquid–liquid sample extraction technique has been implemented for extraction of carbaryl. Multiple reactions monitoring analysis mode considering two daughter transitions was used for quantitation and confirmation analysis of carbaryl. The developed method has been authenticated by establishing various validation parameters including, linear ranges (0.001–5.0 µg/mL), percent recovery, reproducibility (precision), limits of detection (0.0003 µg/mL) and limits of quantification (0.0009 µg/mL). The recoveries were obtained in the range of 96.0 and 99.5% (RSD < 3.8%, n = 3). All fresh vegetable samples have shown unlike carbaryl contamination for a certain time interval. The results were clearly designated that the residue quantities of the inspected pesticide does not cross the established maximum residue limits (MRLs).
Keywords
UPLC
Mass spectrometry
Carbaryl
Fresh vegetables
Determination
1 Introduction
Lettuce, cucumber and spinach are the most widely cultivated vegetable in the planet. Such vegetables most of the time consumed as raw by human and animals. These widely-cultivated vegetables provide unique health benefit, including vitamin C, beta-carotene, flavonoids, manganese (antioxidant), anti-inflammatory and anti-cancer properties (Yang et al., 2006; Zealand, 2008). Many chemical compounds have been used for growing the agricultural commodities due to their enormous demand in modern community. Pesticides are crucial groups of chemicals used to control various agricultural pests. The application of pesticides in agricultural productions has been found highly beneficial for crop production in terms of quality (Wang et al., 2013). However, the improper handling of pesticides application resulting contamination of fresh vegetables, fruits and crops. The cultivators as protectants of agricultural foodstuffs have applied a huge amount of organophosphorus pesticides including carbaryl (Chowdhury et al., 2014). Its uses in public health programs has also been significant route for exposure to human (Jury et al., 1987). Carbaryl pesticides inhibit strongly interfering with neural transmission of acetyl cholinesterase in other organisms and humans as well (Mostafalou and Abdollahi, 2013). The potentially hazard nature of the materials highly demands its continuous evaluation and monitoring (No, 1980). The carbaryl exposure in the human health can be neglected by proper utilization and adequate monitoring of such toxic chemicals (Mostafalou and Abdollahi, 2013; Sinha et al., 2012).
Among the pesticides, research studies have focused on the analysis of organophosphorous pesticides residues in fresh vegetables (Mansour et al., 2009). The amount of pesticide residues in food stuffs can be reduced by frying, boiling, and roasting. However, these chances are minimal when the crops are consumed raw in the forms of salads or cold soups. The safety of humans from exposure to residual pesticide in food by taking proper actions remains a major objective (Walorczyk et al., 2013). Regarding this, World Health Organization (WHO) and Food and Agriculture Organization (FAO) have been set the maximum residue limits (MRLs) of pesticides in food products (Qin et al., 2016; Xu, 2018) to prevent animal and human health from toxicological hazards (Walorczyk et al., 2013).
Various methods have been conveyed in past for carbaryl determination (Hou et al., 2013; Prasad et al., 2013; Valles et al., 2012). Comparing to others, the hyphenated techniques has shown ability to enhance the sensitivity of pesticides analysis (Qin, et al., 2016). In the current study, a fast, sensitive and reproducible UPLC-ESI-MS/MS based method has been described for the determination of carbaryl residues in the sprayed raw vegetables. The research work will provide vital information regarding food safety to limit the excessive application of carbaryl.
2 Experimental
2.1 Materials and method
All reagents were used of analytical rank. Methanol and ethyl acetate were obtained from BDH chemicals company Ltd (Poole, England). UPLC grade water was produced from Milli–Q water purification system, Millipore Corporation (Bedford, MA, USA). The stock solution of carbaryl standard (5 µg/mL) was prepared in acetonitrile and stowed at 4 °C in the refrigerator. Acetonitrile, carbaryl and florisil were procured from chemical company (Sigma-Aldrich, St. Louis, MO, USA).
2.2 Instrumentation
An Acquity UPLC system (Waters, Manchester, UK) comprising of an autosampler, binary solvent manager and a column thermostat was exploited for the chromatographic experiment. The separations of the target analyte was accomplished with bridged ethyl hybrid C18 column (BEH) of dimension 50 mm × 2.1 mm i.d, 1.7 µm particle size (Waters, Mildford, MA, USA). All solutions to be injected were stored in the auto-sampler at 10 °C. A tandem mass spectrometer (Micromass Quattro Premier) equipped with electro spray Ionization (ESI) interface and MassLynx V4.1 software (Waters, Manchester, UK) were used during the experiment. A rotary pump by Oerlikon (Sogevac SV40 BI, France) and a nitrogen generator (NM30LA) manufactured by Peak Scientific (Inchinann, UK) were also used.
2.3 Real sample and storage
Five groups of unpeeled and unwashed carbaryl sprayed samples were harvested at a time intervals of one to fifteen days. Each harvested vegetables were properly cut into pieces followed by separated them into four subsamples of quantity 150 g each. Then stored individually in sealed polyethylene bags at −24 °C until their extraction.
2.4 Extraction procedure
A previously reported method was adopted in order to clean up and preparation of real samples (Islam, et al., 2009). The procedure briefly consisted of mixing 150 g of chopped sample of each vegetable with 300 mL acetonitrile and 15 g celite. The samples were homogenized with a grinding machine and was filtrated through Buchner funnel. The filtrate was taken in a 1000 mL separating funnel (SF) and petroleum ether of 120 mL was mixed into with shaking for 2.5 min. Then 12 mL saturated NaCl solution and Milli-Q H2O (700 Ml) were added to this mixture and was shaken (1 min). Then the mixture was allowed to stand for some time for settling the solution mixture into two clear layer. The upper organic layer that contains carbaryl was separated and repeatedly washed with 150 mL Milli-Q water and 15 g anhydrous sodium sulfate (Na2SO4) was added to it to remove any remaining aqueous portions. The pre-concentration was made using a rotary evaporator (Buchi). Florisil column was prepared by placing a piece of glass wool inside an empty chromatographic glass column and filled with 15 g of activated florisil (128–130 °C, 6–7 h). Additionally, 1.2 g of anhydrous Na2SO4 was spread on activated Florisil column and was conditioned by passing 50 mL of petroleum ether. Then the pre-concentrated extract was passed through this activated column and the elution was performed passing a mixture of 200 mL petroleum ether and diethyl ether (50:50; v/v) at flow rate 5 mL/min. Finally, the eluent was concentrated to a certain volume and injected after filtration with 0.22 µm PVDF syringe filter.
2.5 Validation study
The proposed methodology was authenticated by determine linearity, run-to-run and day-to-day precision, limits of detection (LOD), limits of quantification (LOQ) and recovery. The recovery studies were checked by spiking carbaryl solution at two fortification levels to carbaryl free (without treated) samples. Extraction of both fortification levels were performed in five replicates to validate the method. The precisions of the proposed methods were also determined at two spiking levels. The detection and quantitation limits and linearity were also determined from the standard calibration curve of carbaryl.
3 Results and discussion
3.1 UPLC method optimization
Separation of carbaryl was checked using different reversed phase column and the best results were obtained with BEH C18 column. The < 2.0 μm particle size of the column provided excellent efficiency even at the higher flow of the mobile phases (Alothman et al., 2012; Zou et al., 2013). A number of individual and binary mobile phase were tested such as methanol, water, acetonitrile, and a mixture of two eluents including water/methanol, 0.1% aqueous formic acid/methanol, acetonitrile/water, and acetonitrile/0.1% aqueous formic acid. The intense peak with higher peak symmetry was achieved with binary mobile phase mixture of acetonitrile and 0.1% aqueous formic acid (50:50, v/v). Formic acid was used to increase the ionization efficiency of the target analytes (Alothman et al., 2012). The column oven was set at room temperature during carbaryl analysis and the flow was set at 0.5 mL/min. The separation of carbaryl was achieved in less than 1 min.
3.2 ESI-MS/MS conditions
Standards carbaryl (1 µg/mL) were infused in combined mode using both + Ve and -Ve ESI polarization. The positive ESI mode was successfully produced intense analyte signal at m/z 202.2 (precursor ion) and was selected for further analysis. The optimized MS/MS conditions for carbaryl analysis were capillary voltage, 3.1 kV; cone voltage, 21 V; source temperature, 140 ˚C; desolvation temperature, 250 ˚C; cone gas flow, 50 L/h; desolvation gas flow, 500 L/h; collision gas flow, 0.20 mL/min. The multiple reaction monitoring (MRM) was adapted for both identification and determination of carbaryl and two transitions were chosen including (202.2 > 145.1) and (202.2 > 117.2). The selected parameters for MRM acquisition are shown in Table 1, and the UPLC–MS/MS chromatogram of carbaryl standard (1 µg/mL) is illustrated in Fig. 1. Dwell time was 0.025 s in all cases; Ionization mode: ESI+
Analyte
Precursor ion [M + H]+ (m/z)
Quantification transition
Confirmation transition
Daughter ion (m/z)
Cone voltage
Collision energy
Daughter ion (m/z)
Cone voltage
Collision energy
carbaryl
202.2
145.1
25 V
15 eV
117.2
22 V
20 eV
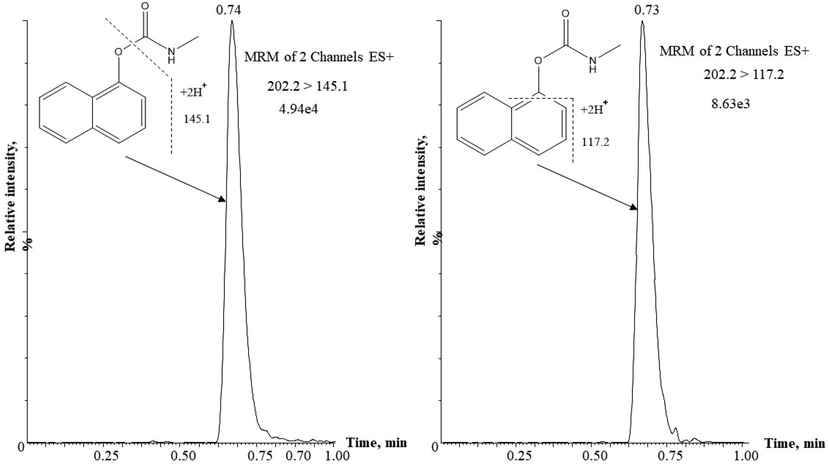
UPLC–MS/MS chromatogram of carbaryl (1 μg/mL) standards in MRM mode, injection volume 2 μL.
3.3 Method validation
The authentication of the described UPLC-MS/MS method was assessed according to ICH guidelines (Q2 R1). The crucial factors that affects the system suitability are signal response, signal stability and carry over (Briscoe et al., 2007). To check these parameters, six sets of carbaryl standard solution (1.0 µg/mL) were injected. The relative standard deviation (RSD, %) of peak area of each samples of carbaryl was found to be < 2.2% (n = 3) indicating good performance of the employed system. The carryover was checked by injecting a set of six blank solutions that reveals no carryover peak in the chromatogram.
The linearity was determined constructing a calibration curves for a set of carbaryl standard, 0.005, 0.001, 0.05, 0.01, 0.5, 0.1, 1.0, 2.0, 5.0 µg/mL. The results show that the plot was linear over the concentration range of 0.001 – 5.0 µg/mL with regression coefficient (r2) >0.993. The LOD and LOQ of the method were calculated considering a signal-to-noise ratio of 3 and 10 (Alothman et al., 2012) and were found to be 0.0003 and 0.0009 µg/mL, respectively. The very low LOQ values obviously indicates that the described method is able to determine carbaryl even at trace level.
The repeatability and reproducibility were determined at two different spiking levels of carbaryl (0.05 and 2.0 µg/mL). For repeatability, five replicates of each levels were injected to the system in the same day, while the reproducibility was evaluated by injecting fifteen replicates of each carbaryl concentration for three consecutive days (five replicates/day) and the were found to be less than 4.5% (RSD, %). The values of the validation parameters have been listed in Table 2.
To assess the usefulness of extraction and UPLC-MS/MS method, recovery studies were performed. For the same, 200 g of chopped untreated (carbaryl free) samples of fresh lettuce, cucumber and spinach were spiked with known concentration of standard carbaryl. Carbaryl fortified levels were 0.05 mg/kg and 2.0 mg/kg. After fortification, the extraction experiment was executed for five replicates of each fortified levels and elutes were analyzed under optimum experimental parameters. The percent recovery of carbaryl was found to be in the range of 94.0–98.5%. The RSD of recovery was lower than 4.2% for both concentration levels. The recovery data indicates that the applied extraction method is efficient for carbaryl extraction from the sprayed samples.
3.4 Analytical application
The proposed method was good in terms of sensitivity and selectivity for the quantitative and qualitative analysis of residual carbaryl in the extracts. The tandem quadruple mass spectrometry was provided high selectivity that enabled the detection of the carbaryl ion m/z 202.2. The quantitative diminution analysis of carbaryl residue was performed considering the higher sensitive transition (m/z 202.2 > 145.1), and the qualitative analysis was performed using the lower sensitive transition (m/z 202.2 > 117.2) of target compound (Fig. 1). The developed UPLC-MS/MS method was interference free as no detectable matrix peaks were appeared at the same retention time of carbaryl standard. To check the reliability of the method, each real samples was spiked with known amount (1 µg/mL) of carbaryl prior to the extraction process. The regression slope of the added carbaryl versus the measured concentration of carbaryl standard were used for recovery calculation. The recoveries of the fortified carbaryl were obtained in the range of 96.0–99.5% with RSD below 3.8% at certain time intervals (Table 3).
Extracted after (Days)
Carbaryl ± SD (µg/g)a
Recovery (%)
Lettuce
Cucumber
Spinach
Lettuce
Cucumber
Spinach
1
0.797 ± 0.030
0.556 ± 0.022
0.987 ± 0.051
98.0
97.2
99.5
4
0.083 ± 0.005
0.072 ± 0.002
0.099 ± 0.008
96.2
98.8
97.0
7
bd
bd
0.015 ± 0.0001
96.8
96.2
98.4
11
nd
nd
nd
96.5
98.0
96.6
15
nd
nd
nd
97.8
97.8
97.5
3.5 Diminution study of carbaryl residues
The maximum carbaryl residue in analyzed lettuce, cucumber and spinach samples after one and four days of the pesticide spray were found to be < 1.0 µg/g. A small traces of carbaryl (0.015 µg/g) was found only in spinach samples after seven days of the pesticides applications. However, in between 11 and 15 days, the total disappearances of carbaryl were noticed. Fig. 2 shows the chromatogram of spinach extracts after 1 and 4 days of carbaryl extraction. The diminution parameters of carbaryl with times are shown by column plot in Fig. 3. The obtained results indicate that the carbaryl residues present in the fresh vegetables extracts were below the reported MRLs (1 µg/g) even after one day of carbaryl spray (Qin et al., 2016; Xu, 2018).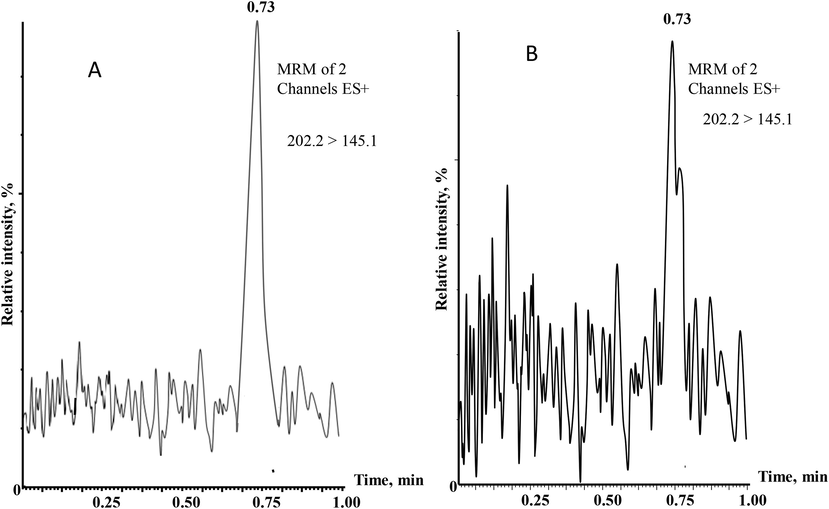
UPLC–MS/MS chromatogram of Spinach extracts extracted after (A) 1-day and (B) 4-days.
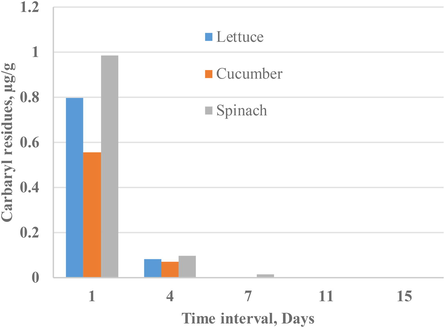
Diminution of carbaryl in lettuce, cucumber, and spinach extracts with time.
4 Conclusions
A simple, sensitive and rapid method has been developed for the quantitation of residual carbaryl in raw vegetables (lettuce, cucumber and spinach). All the analytical conditioning parameters confirm the reliability of the proposed UPLC-MS/MS method. The maximum amount of residual carbaryl was found in the samples analyzed after 1 day of carbaryl spraying with maximum concentration 0.987 µg/g (spinach). The validation study indicates that the method is efficient for successful analysis of carbaryl in fresh vegetables. The proposed method is precise and might be suitable for the repetitive analysis of carbaryl in various matrices.
Acknowledgement
This work was funded by the Deanship of Scientific Research at Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia through the Research Groups Program Grant no. (RGP-1440-0021).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Determination of capsaicinoids in C apsicum species using ultra performance liquid chromatography-mass spectrometry. J. Sep. Sci.. 2012;35(21):2892-2896.
- [Google Scholar]
- System suitability in bioanalytical LC/MS/MS. J. Pharm. Biomed.l Anal.. 2007;44(2):484-491.
- [Google Scholar]
- Determination of carbamate and organophosphorus pesticides in vegetable samples and the efficiency of gamma-radiation in their removal. BioMed. Res. Int.. 2014;2014:145159
- [Google Scholar]
- A multi-residue method for the determination of 124 pesticides in rice by modified QuEChERS extraction and gas chromatography–tandem mass spectrometry. Food Chem.. 2013;138(2–3):1198-1205.
- [Google Scholar]
- Application of high performance liquid chromatography to the analysis of pesticide residues in eggplants. J. App. Sci.. 2009;9(5):973-977.
- [Google Scholar]
- Jury, W.A., Winer, A.M., Spencer, W.F., Focht, D.D., 1987. Transport and transformations of organic chemicals in the soil-air-water ecosystem. In Rev. Environ. Contam. Toxicol. 119-164: Springer.
- Monitoring of pesticides and heavy metals in cucumber fruits produced from different farming systems. Chemosphere. 2009;75(5):601-609.
- [Google Scholar]
- Pesticides and human chronic diseases: evidences, mechanisms, and perspectives. Toxicol. App. Pharm.. 2013;268(2):157-177.
- [Google Scholar]
- 80/778/EEC of 15 July, 1980, relating to the quality of water intended for human consumption. Off. J. Eur. Communities L. 1980;229:11.
- [Google Scholar]
- Simultaneous determination of seven carbamate pesticide residues in gram, wheat, lentil, soybean, fenugreek leaves and apple matrices. Microchemical J.. 2013;111:91-96.
- [Google Scholar]
- Pesticide residue determination in vegetables from western China applying gas chromatography with mass spectrometry. Biomed. Chromatogr.. 2016;30(9):1430-1440.
- [Google Scholar]
- Distribution of pesticides in different commonly used vegetables from Hyderabad, India. Food Res. Int.. 2012;45(1):161-169.
- [Google Scholar]
- A sensitive and selective method for the determination of selected pesticides in fruit by gas chromatography/mass spectrometry with negative chemical ionization. J. Chromatogr. A. 2012;1264:110-116.
- [Google Scholar]
- Walorczyk, S., 2013. Dro¿ d¿ yñski D, Kowalska J, Remlein-Starosta D, Zió3kowski A, PrzewoŸniak M, et al. Food Chem. 139, 482-487.
- Pesticide residues in market foods in Shaanxi Province of China in 2010. Food Chem.. 2013;138(2–3):2016-2025.
- [Google Scholar]
- Multi-Residue Determination of Pesticides in Vegetables on Dalian Market by Gas Chromatograph, 2009–10. J. Food Nutri. Popul. Health. 2018;2(1):2.
- [Google Scholar]
- Yang, R.-Y., Chang, L.-C., Hsu, J.-C., Weng, B.B., Palada, M.C., Chadha, M., Levasseur, V., 2006. Nutritional and functional properties of Moringa leaves–From germplasm, to plant, to food, to health. Moringa leaves: Strategies, standards and markets for a better impact on nutrition in Africa. Moringanews, CDE, CTA, GFU. Paris.
- Zealand, H.N., 2008. Nutritional attributes of Indian vegetables.
- A comprehensive workflow of mass spectrometry-based untargeted metabolomics in cancer metabolic biomarker discovery using human plasma and urine. Metabolites. 2013;3(3):787-819.
- [Google Scholar]







