Translate this page into:
Development and validation of stability indicating HPLC method for determination of adrenaline tartrate
⁎Corresponding author at: Department of Emergency Medicine, Faculty of Medicine, 99 Moo 18, Phahonyothin Road, Khlong Nueng, Khlong Luang, Pathumthani 12121, Thailand. kumpona@hotmail.com (Kumpol Amnuaypattanapon)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Epinephrine (Adrenaline) is a lifesaving medication of the treatment of anaphylaxis and cardiac resuscitation. For out of hospital emergency treatment, some prefilled syringe and auto-injector device has been developed and prescribed to patients. In order to predict shelf life of the developed device, a stability-indicating high-performance liquid chromatography (HPLC) method was developed and validated for determine the amount of adrenaline tartrate. Separation was carried out using a 2.6 µm Kinetex Biphenyl column, with 50 mM sodium dihydrogen phosphate adjusted to pH 3.0 as a mobile phase and a flow rate of 0.5 mL/min, at 25 °C and detected at 279 nm. Parameters for the validation included accuracy, precision, linearity, and limit of quantitation and detection. The developed HPLC method was precise, with lower than 2% relative standard deviation. The accuracy of the method, represented by recovery studies ranged between 99.25% and 101.81%. Stress testing was carried out to demonstrate specificity of the method. The developed method could separate the potential degradation products from the adrenaline tartrate peak. This proposed method was suitable and practical for analysis the content of adrenaline tartrate in pharmaceutical products and could be of benefit for prediction shelf life of adrenaline tartrate in developed auto-injector device.
Keywords
Adrenaline
Epinephrine
HPLC
Stability-indicating method (SIM)
1 Introduction
Anaphylaxis is severe allergic reaction which affects the cutaneous, respiratory, cardiovascular, and gastrointestinal systems. It is a life threatening, under recognized, and undertreated. Hospitalization rates for anaphylaxis continue to increase year on year (Simons et al., 2015).
Epinephrine (adrenaline) is a first-line pharmacologic drug for anaphylaxis (Song and Lieberman, 2015; Simons et al., 2015). Its prompt administration is integral to prevent hospitalizations and death (Fromer, 2016). For out of hospital treatment, epinephrine auto injector has been prescribed to patients and parents of children with history of anaphylaxis. In developing countries, the cost of commercial epinephrine auto-injector, for example EpiPen®, is high and many patients are abandoned due to the financial inability. Therefore, the epinephrine prefilled-syringe was prescribed instead (Kerbdonfak et al., 2010). In order to increase the patient compliance, the low cost epinephrine prefilled auto-injector was developed for the prompt first-aid in out-of-hospital emergency treatment.
Chemical stability is of serious concern as it affects the safety and efficacy in drug product. It is a mandatory to perform stability studies and establish shelf life in a new drug product (Blessy et al., 2014). Several HPLC methods (Stepensky et al., 2004; Xie et al., 2009; Jebaraj et al., 2014) has been studied in adrenaline salts. However, a fast and simple analytical method for stability study is needed. Therefore, in this study, an analytical method was developed for determination of adrenaline (epinephrine) tartrate content using high performance liquid chromatography coupled with diode array detector (HPLC-DAD). Using the smaller particles of stationary phase, our fast LC could markedly reduce the running time. It was simple and economical compared to the existing methods. Stress testing was carried out to demonstrate specificity of the method. The developed method could be applied for prediction shelf life of adrenaline tartrate in developed auto-injector device and related pharmaceutical products.
2 Material and methods
2.1 Chemical and reagents
Deionized water was purified by Ultra Clear™ system (Siemens Water Technologies Corp.). Sodium dihydrogen orthophosphate was purchased from Loba Chemie, India. Phosphoric acid, hydrochloric acid and hydrogen peroxide were purchased from Fisher Scientific, UK. Adrenaline bitartrate (purity ≥ 98%) was purchased from TCI, Japan. All reagents were analytical grade, it they were not stated otherwise. Commercial adrenaline tartrate samples in this study were obtained from the Government Pharmaceutical Organization (GPO), Thailand.
2.2 HPLC apparatus and conditions
HPLC was achieved on an Agilent 1260 Series (Agilent Technologies) equipped with a 1260 Quat pump VL quaternary pump, 1260 ALS autosampler, 1260 TCC column thermostat, and 1260 DAD VL diode array detector. The separation was done on a Kinetex® 2.6 µm Biphenyl 100 Å size 100 × 3.0 mm i.d. (Phenomenex, USA). The elution was performed on isocratic solvent system using 50 mM sodium dihydrogen phosphate (NaH2PO4) in water adjusted to pH 3.0 with phosphoric acid. The flow rate was set at 0.5 mL/min with controlled temperature at 25 °C. DAD detector was set at the wavelength of 279 nm and injection volume was 1 µL for every samples and standard.
2.3 Stock and working solutions of standard compound
Stock solution was prepared by accurately weighed adrenaline tartrate standard 50.00 mg, dissolved in mobile phase, and adjusted to 5.00 mL with a volumetric flask. Working standard solutions were obtained by appropriate dilution of the stock solution with mobile phase.
2.4 Stress testing
The stress conditions employed for the degradation study included base hydrolysis, acid hydrolysis, oxidation, and photolytic condition. Stress testing was done by adding 50 µL of reagent to 1 mL of adrenaline tartrate sample. Concentrated hydrochloric acid (36% w/w), 5 N sodium hydroxide, and hydrogen peroxide (30% w/w) were used as reagent for acid hydrolysis, base hydrolysis, and oxidative stress, respectively. Deionized water was used as solvent. The spiked solutions were stored in our developed auto-injection device (patent pending) and incubated at 60 °C for 60 min. The photolytic stress was done by spiking the deionized water and exposed to light (4500 Lux) for 72 h. Each samples was then analyzed with the proposed HPLC method. The peak purity of stressed samples was monitored by the diode array detector in the wavelength range of 200–400 nm.
2.5 Method validation
Validation of the method was done according to the International Conference on Harmonization guideline (ICH, 1996/2005). The method was validated for linearity, precision, accuracy, limit of detection (LOD), and limit of quantification (LOQ).
2.5.1 Linearity
Linearity of the method was studied by injection of six known concentrations of analyte in the range of 0.3–10 mg/mL in triplicate. The calibration curve was obtained by plotting the peak areas versus the amounts of the standard.
2.5.2 Precision
The measurement of intra- and inter-day precisions was done by analyzing sample solution containing adrenaline tartrate of 1.8 mg/mL. The intra-day precision was determined by analyzing the seven-time injection within one day, while the inter-day precision was examined for five consecutive days by the proposed method. The precision was expressed as percentage of relative standard deviation (%RSD).
2.5.3 Accuracy
Recovery was used to evaluate the accuracy of the method. Standard addition was performed with pre-analyzed standard solution. Three different levels of standard mixtures were added to the sample extracts. Spiked samples were prepared in triplicate. The recovery was calculated as follows: recovery (%) = 100 × (detected amount – original amount)/spiked amount.
2.5.4 Limit of detection (LOD) and limit of quantitation (LOQ)
Determination of signal-to-noise ratio was calculated under the proposed chromatographic condition. LOD was considered as 3:1 and LOQ as 10:1.
3 Results and discussion
A fast stability-indicating HPLC technique was developed for the analysis of adrenaline tartrate injection. Critical parameters such as pH, buffer concentration, and stationary phase have been studied. Increasing acidity and buffer concentration of mobile phase could reduce peak tailing. The mechanism was probably due to the competitive interaction of the buffer cation with residual silanols of stationary phase as described by Langmuir isotherm (Flieger and Czajkowska-Zelazko, 2011; Langmuir, 1916). Selection of stationary phases were done using common reversed-phase columns, i.e., octadecylsilane (C18), and biphenyl bonded column. From several trials, the mobile phase of 50 mM phosphate buffer adjusted to pH 3.0 with biphenyl stationary phase column was the optimal condition. It provided symmetrical peaks and has the most efficient separation and speed. The maximum absorbance 279 nm was used for wavelength detection. The chromatograms of adrenaline tartrate injection in Fig. 1. The system suitability results including theoretical plate, resolution and symmetric factor are shown in Table 1. tR = retention time; N = theoretical plate; Rs = resolution; Symm. = symmetric factor.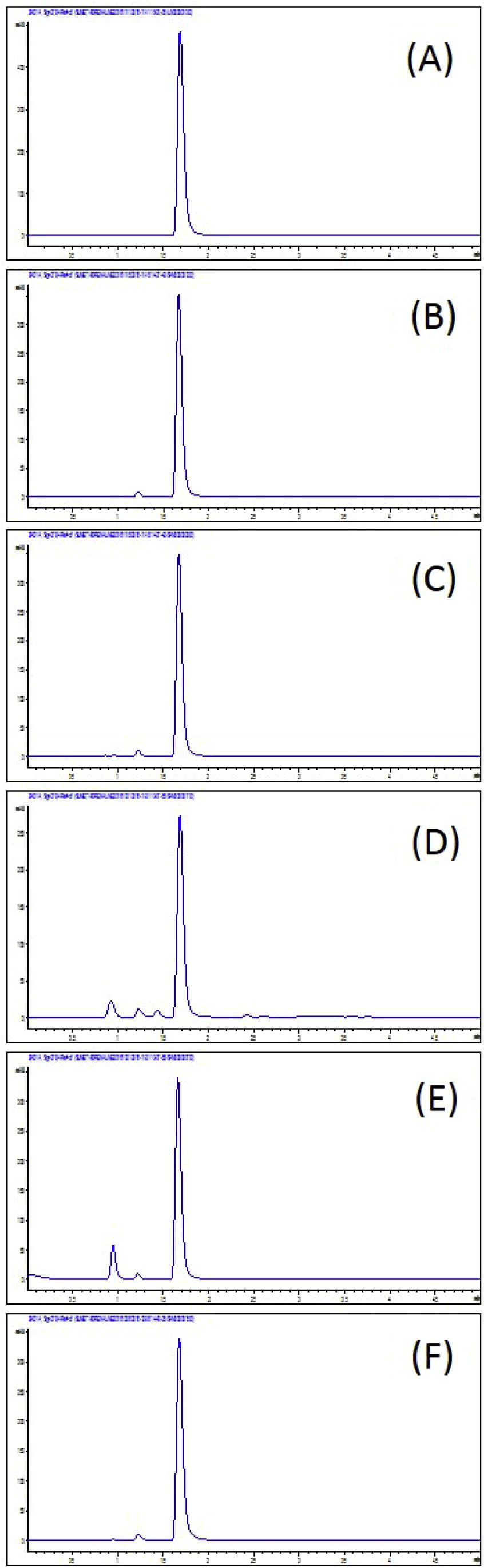
HPLC chromatogram of (A) adrenaline tartrate authentic standard, (B) adrenaline tartrate solution sample (stored in auto-injection device), (C) adrenaline tartrate solution under acid hydrolysis condition (stored in auto-injection device), (D) adrenaline tartrate solution under base hydrolysis condition (stored in auto-injection device), (E) adrenaline tartrate solution under oxidative stress (stored in auto-injection device), and (F) adrenaline tartrate solution under photolytic stress (stored in auto-injection device).
Compound
tR (min)
N
Rs
Symm.
Adrenaline tartrate
1.68
3745
1.89
0.72
In order to ensure that the method is suitable for its intended use, method validation has been performed according to the ICH guideline (ICH, 1996/2005). The method validation parameters were linearity, precision, accuracy, LOD and LOQ. The calibration curves were constructed from the pea area versus the concentration of the standards and showed that the method was linear across the range of 0.3–10 mg/mL with good correlation coefficient (r2 > 0.9999) (Table 2). Method precision was studied using the sample solution containing adrenaline tartrate of 1.8 mg/mL. Percent relative standard deviation (%RSD) values lower than 2% (Table 3) showed the acceptable precision of the method. Selectivity of the method was assessed by peak purity using UV spectrum obtained from diode array detector. The accuracy of the method, represented by recovery studies ranged between 99.3% and 101.8% (average 100.5%) (Table 4). The LOQ and LOD were found to be 0.3 and 0.1 µg/mL, indicating the high sensitivity of the method (Table 2).
Parameters
Results
Regression equationa
Y = 961.2 X + 9.4995
Correlation coefficient (r2)
0.9999
Linear range (mg/mL)
0.3–10
LOQ (µg/mL)
0.3
LOD (µg/mL)
0.1
Intra-day precision
Inter-day precision
Day 1
Day 2
Day 3
Day 4
Day 5
1.38
0.86
1.40
0.77
0.95
0.96
Serial No.
Theoreticala (mg/mL)
Foundb (mg/mL)
Recoveryb (%)
1
1.3430
1.37 ± 0.02
101.8 ± 1.1
2
1.7725
1.78 ± 0.01
100.5 ± 0.6
3
2.2008
2.18 ± 0.02
99.3 ± 0.9
Average
100.5 ± 0.1
Stress testing was carried out to demonstrate specificity of the developed method to measure the changes in concentration of adrenaline tartrate. In order to determine the specificity of the method, peak purity analysis was done on line by using diode array detection. The chromatograms of adrenaline tartrate degradation are shown in Fig. 1. The developed method could separate the potential degradation products from the adrenaline tartrate peak. The proposed HPLC method was applied for quantitative analysis of the content of the adrenaline tartrate under various stress condition. The contents of adrenaline tartrate are shown in Table 5. Degradation of adrenaline tartrate was found under basic hydrolytic and oxidative condition while the adrenaline solution was stable under acid condition. Under our developed auto-injector device, the adrenaline solution was stable in photolytic condition.
Stress type
Spiked Reagenta
Conditiona
Assay (mg/mL)
Relative Amount (%)
Control
Water
–
1.7151 ± 0.0080
100
Acid hydrolysis
36% HCl
60 °C, 60 min
1.7105 ± 0.0356
99.73 ± 2.08
Base hydrolysis
5 N NaOH
60 °C, 60 min
1.3785 ± 0.5595
80.36 ± 3.26
Oxidation
30% w/w H2O2
60 °C, 60 min
1.6715 ± 0.0144
97.46 ± 0.84
Light
Water
4500 Lx, 72 h
1.7152 ± 0.0225
100.0 ± 1.31
4 Conclusion
HPLC method for the analysis of adrenaline (epinephrine) tartrate content was developed and validated in this study. Validation parameters proved that the method was fast, sensitive, precise and accurate. Stress testing was conducted and demonstrated the specificity of the method. Despite the existing analytical methods, this proposed method could be of benefit and be applied for prediction shelf life of adrenaline tartrate in developed auto-injector device and related pharmaceutical products.
Acknowledgment
The authors gratefully acknowledged the financial support provided by Thammasat University Research Fund and the TU Innovative Scholar, Contract No. 4/2557. The research team would like to express our gratitude to Drug Discovery and Development Center, Advanced Science and Technology, Thammasat University, Thailand for laboratory facility.
References
- Development of forced degradation and stability indicating studies of drugs – a review. J. Pharm. Anal.. 2014;4:159-165.
- [Google Scholar]
- Comparison of chaotropic salt and ionic liquid as mobile phase additives in reverse-phase high-performance liquid chromatography of biogenic amines. J. Sci. Sep.. 2011;34:733-739.
- [Google Scholar]
- Prevention of anaphylaxis: the role of the epinephrine auto-injector. Am. J. Med.. 2016;129:1244-1250.
- [Google Scholar]
- ICH, 1996/2005. International Conference on Harmonization, ICH-Q2 (R1). Validation of Analytical Procedures: Text and Methodology. ICH, Geneva, Switzerland.
- Analytical method development and validation of stability indicating HPLC method for estimation of fixed dosage form of atropine sulphate, epiphephrine bitartrate and lignocaine hydrochloride injection. Int. J. Innovative Pharm. Sci. Res.. 2014;2:1337-1348.
- [Google Scholar]
- The stability and sterility of epinephrine prefilled syringe. Asian Pac. J. Allergy Immunol.. 2010;28:53-57.
- [Google Scholar]
- The constitution and fundamental properties of solids and liquids. J. Am. Chem. Soc.. 1916;38:2215-2295.
- [Google Scholar]
- 2015 update of the evidence base: World Allergy Organization anaphylaxis guidelines. World Allergy Organ. J.. 2015;8:32.
- [Google Scholar]
- Epinephrine in anaphylaxis: doubt no more. Curr. Opin. Allergy Clin. Immunol.. 2015;15:323-328.
- [Google Scholar]
- Long-term stability study of l-adrenaline injections: kinetics of sulfonation and racemization pathways of drug degradation. J. Pharm. Sci.. 2004;93:969-980.
- [Google Scholar]
- Quantitative determination of adrenaline hydrochloride injection and noradrenaline bitartrate injection by a new HPLC method via substitute for reference substance. Chem. Res. Chin. Univ.. 2009;25:433-438.
- [Google Scholar]







