Translate this page into:
Development and validation of anodic stripping voltammetry method for the determination of tretinoin in human urine and plasma using glassy carbon electrode
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Oxidation behavior of tretinoin (TRN) was evaluated using anodic stripping voltammetry (ASV), differential pulse voltammetry (DPV) and cyclic voltammetry (CV) techniques in Britton-Robinson (B-R) pH 7. The glassy carbon (GC) electrode was used to deposit the compound (TRN) into its surface to record a well-defined oxidation peak at 0.75 V in the presence of Ag/AgCl reference electrode and a Pt counter electrode. Some analytical parameters were studied to obtain the best oxidation signal, for example; buffer solution, pH, accumulation potential and time, amplitude, frequency, scan rate and convection rate. The high sensitivity anodic peak was obtained using B-R, pH 7, 40 s accumulation time, −0.6 V accumulation potential, 50 mV amplitude, 20 Hz frequency, 350 mV s−1 scan rate, and 1000 rpm. These parameters were chosen as optimum conditions for the continued experiments. The analytical performance of the used technique was evaluated using the study of repeatability, stability, recovery, calibration curve and detection limit. Repeatability and stability was monitored for 5 × 10−6 mol L−1 of TRN to give 0.24% relative standard deviation (RSD%) for eighth anodic measurements, while the stability was been very well for 80 min. The calibration curve was studied over the range of 1 × 10−6–1 × 10−5 mol L−1 (n = 6), yielded, a linear relationship between concentrations and anodic current for TRN. A detection limit (LOD) was calculated to be 7.5 × 10−9 mol L−1 (2.25 ppb), while a quantification limit (LOQ) was yielded 2.49 × 10−8 mol L−1 (7.47 ppb). Anodic stripping voltammetry method was developed and applied for the determination of TRN in the human urine and plasma samples.
Keywords
Anodic stripping voltammetry
Oxidation
Tretinoin
Human fluids
GC electrode
B-R buffer
1 Introduction
Tretinoin drug is a widely used retinoid in the topical treatment of various dermatological diseases, especially acne and photoaging. It is called all-trans retinoic acid that also used to treat a certain kind of cancer that destroyed the white blood cells. This type of disease is used to stop the function property of white blood cells. Tretinoin takes care to work on the growth of normal mature cells in the bone and blood. It belongs to the drugs which are known as retinoids, which are related to vitamin A (James et al., 2012, Tivnan, 2017). The IUPAC name of tretinoin is (2E,4E,6E,8E)-3,7-Dimethyl-9- (2,6,6-trimethylcyclohexen-1-yl) nona-2,4,6,8-tetraenoic acid. The molar mass of tretinoin is 300.44 g mol−1, and its chemical structure is C20H28O2. Several published articles related to the determination of tretinoin at the medical creams and other pharmaceuticals such as chromatography methods (Kril et al., 1990; Tashtoush et al., 2007; Yuerong et al., 2004; Zarghi et al., 1998; Ibrahim et al., 2019) and spectrophotometry were reported (Tehrani et al., 2013; Gupta et al., 2009; Al-Jamal, 2019; Zayed and Abdel-Basset, 2018). Tretinoin was also determined in cosmetic, pharmaceuticals and human serum respectively, using electrochemical methods (Wang, 2000; Simona et al., 2018; Machini et al., 2016) and HPLC, RP-LC, LC-MS with UV–visible spectrophotometric and electrochemical detections (Bryan et al., 1991; Wang and Wang, 2001; Karami et al., 2019).
Anodic stripping voltammetry (ASV) has many advantages that included the capability for simultaneous organic, inorganic compounds and metals determination and with relatively the cheapest instruments compared to that required for the chromatographic and spectrophotometric techniques. Anodic stripping voltammetry is the most sensitive of all commonly used electrochemical techniques and widely applied to determine some metals (Sari et al., 2017; Lan et al., 2012; Marcolino-Junior et al., 2007; Zinoubi et al., 2017; Guo et al., 2011; Yang et al., 2019; Grabarczyk and Wardak, 2016) and pharmaceutical compounds (Alghamdi, 2018; Alghamdia, 2014; Rosolina et al., 2016).
The purpose of this research is to develop a rapid, selective and sensitive anodic stripping voltammetry method containing an efficient and reproducible sample clean-up step for quantitative and qualification analysis mixture of tretinoin in human urine and plasma. Anodic stripping voltammetry technique was applied to determine the trace levels of tretinoin onto the surface of the glassy carbon electrode (GC). To the best of our knowledge there is no published article that used the same analytical conditions as reported in my research.
2 Experimental part
2.1 Apparatus
The anodic stripping voltammetry, differential pulse voltammetry and cyclic voltammetry techniques were carried out using 797 VA instrument (Switzerland made) to analyze tretinoin under optimum conditions. The VA apparatus is connected with three electrodes system which included GC working electrode, Ag/AgCl (3.0 mol L−1 KCl) reference electrode and the platinum counter electrode. A digital pH meter (model pH 211) was used for controlling the pH values. A Millie-Q Plus water purification system (USA made) was used for obtaining the distilled water. A Labofuge 200 instrument (Germany made) was used for centrifuging the human urine and plasma.
2.2 Chemicals
Tretinoin (TRN) standard material was obtained from Jamjoom pharmaceutical Company, Jeddah -KSA. The stock solution of TRN was prepared in a 50 mL volumetric flask by dissolving this drug in methanol to obtain a yellow color solution. The other requested solutions of TRN were prepared by diluting the stock of TRN with methanol solvent for further investigation. The buffer solutions such as Britton-Robinson (B-R), phosphate, acetate and carbonate were prepared by using boric acid, acetic acid, ortho-phosphoric acid, sodium carbonate and other materials to give a well oxidation signal (Alghamdi et al., 2014).
2.3 Procedures
The voltammetric cell was cleaned many times by distilled water and alcohol then a 10 mL of buffer solution was injected in the cell. Electrochemical oxidation was carried out by scanning the potential from 0.0 to 1.0 V. The buffer solutions should be stirred and purged by nitrogen gas for 100 s to remove interference gases such as oxygen gas. The anodic stripping, differential pulse and cyclic voltammograms were obtained under the optimum conditions. The parameters of B-R pH 7, 40 s accumulation time, − 0.6 V accumulation potential, 20 Hz, 350 mVs−1 scan rate, 50 mV amplitude, and 1000 rpm were selected to record high sensitivity and good selectivity for the determination of TRN by ASV technique.
2.3.1 Preparation of human urine and plasma
A 2 × 10−6 mol L−1 of TRN was added to a half milliliter of human urine or plasma. The mixture was added to 1.0 mL of 5.0% ZnSO4·7H2O, 1.0 mL of ethanol and 0.1 mL of NaOH, (Alghamdib, 2014) in a centrifuge tube. This mixture was centrifuged with 5000 rpm for 8 min by centrifuge instrument, then the resulted solution was filtered. A 1.00 mL of the filtrate was injected in the voltammetric cell to directly determine TRN content at the human samples by recovery, using ASV technique. The anodic measurement was repeated five times for every TRN determination.
3 Results and discussion
3.1 Voltammetric observations
The differential pulse voltammetry (DPV) and cyclic voltammetry (CV) techniques were used to initially investigate the voltammetric behavior for the tretinoin determination. They were applied to determine 5 × 10−6 and 8 × 10−6 mol L−1 of TRN respectively, as shown in Fig. 1(a, b) under optimum conditions. A well-defined DPV oxidation peak was obtained at 0.75 V with 305nA. The obtained voltammetric peaks are probably due to the electrochemical oxidation of alkene bond (⚌) to alklyne bond (≡) as found in the hydrocarbonic chain (Morrison and Boyd, 1992; Graham Solomons, 1988). The cyclic voltammetry was confirmed the suggested mechanism for the electrochemical oxidation of the electroactive alkene group in TRN drug as illustrated in Scheme 1. As can be seen from CV voltammogram (Fig. 1b), no cathodic peak was observed by the measured cyclic voltammogram for 8 × 10−6 mol L−1 of TRN, indicating the irreversibility nature of the anodic oxidation process.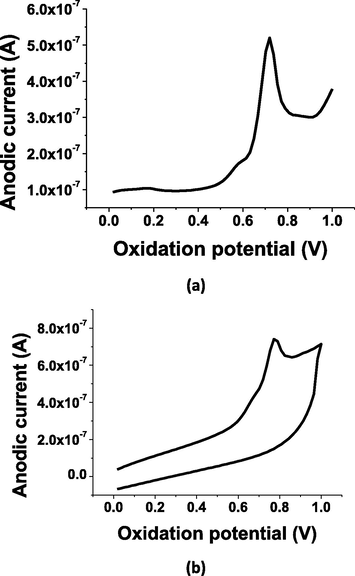
Oxidation behavior of 5 × 10−6 and 8 × 10−6 mol L−1 of TRN, respectively using; (a) differential pulse voltammetry and (b) cyclic voltammetry; under optimum conditions.
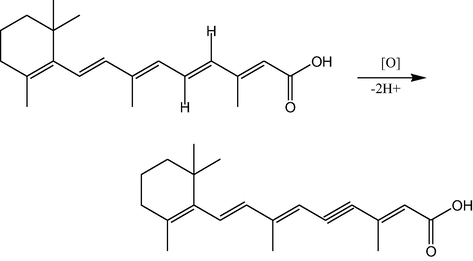
Tretinoin formula structure and the proposed mechanism for its electrochemical oxidation.
Glassy carbon working electrode was used to report the voltammetric peaks at high sensitivity for the determination of TRN. The ASV behavior of TRN was investigated using different buffer solutions, pH values and other parameters, to obtain a well-developed ASV peak corresponding to the alkene electroactive group at 0.75 V. A typical anodic stripping voltammogram for 5 × 10−6 mol L−1 of TRN in B-R buffer and pH 7 is shown in Fig. 2, that illustrated a well observed ASV signal. Moreover, ASV current was indicated an ability of GC to strongly accumulate the studied material onto its surface. Furthermore, the graphite working electrode was used for comparison with GC electrode to give a lessen current at 0.75 V for 5 × 10−6 mol L−1 of TRN under optimum conditions.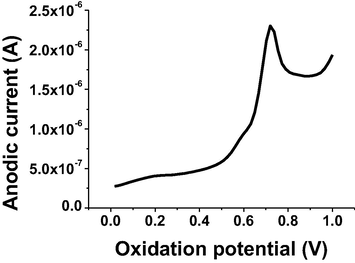
Anodic stripping voltammogram signal of 5 × 10−6 mol L−1 of TRN in B-R buffer pH 7 using GC electrode.
3.2 Study of analytical conditions
Several conditions can affect the electrochemical currents, such as buffer solutions, pH, scan rate, potential and time of accumulation, frequency, amplitude and convection rate. According to the high sensitivity current and shape of peak, the anodic stripping voltammogram was chosen and involved in this research.
3.2.1 Effect of buffer solution and pH
Different buffers were used with different pH values, such as Britton-Robinson (pH 3, 7 and 10), phosphate (pH 3), acetate (pH 3) and carbonate (pH 10), for obtaining a high anodic current for 5 × 10−6 mol L−1 of TRN drug using ASV technique. B-R buffer at pH 7 was given a high voltammetric signal, so it was selected for the next experiment. On the other hand, B-R buffer at the different pH values was used to evaluate the TRN anodic current over the range of 4–9. A high oxidation peak was obtained at pH 7 as shown in Fig. 3, and then it was selected for continued studies.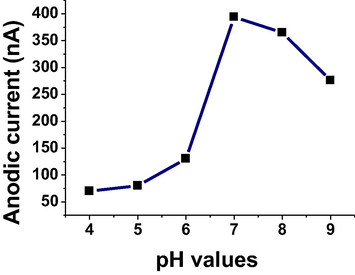
Effect of pH on oxidation current for 5 × 10−6 mol L−1 of TRN in B-R buffer.
3.2.2 Effect of potential and time accumulation
In general the accumulation parameters could be considered as important factories that affect the electrochemical reactions and signals. Herein, accumulation potential (Eacc) was studied in the range of − 0.8 to +0.4 V for the determination of 5 × 10−6 mol L−1 of TRN in B-R buffer and pH 7, resulted in a high anodic current at −0.6 V. An accumulation potential (-0.6 V) was selected as optimum parameter for the next work. Accumulation time (tacc) was monitored over the range of 0.0–100 s as shown in Fig. 4, resulted in a high anodic current at 40 s. An accumulation time (40 s) was chosen for the continues work.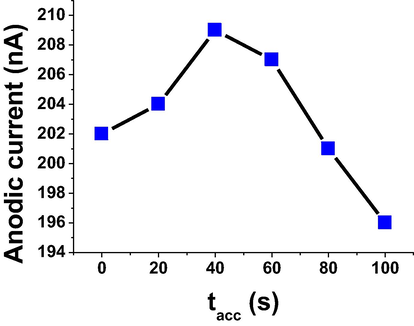
Effect of accumulation time on oxidation current for 5 × 10−6 mol L−1 of TRN in B-R pH 7 and −0.6 V Eacc.
3.2.3 Effect of amplitude and frequency
An amplitude voltage step and frequency were classified as very important parameters for obtaining a high sensitivity voltammetric current. Herein, an amplitude was studied over the range of 10–80 mV for 5 × 10−6 mol L−1 of TRN in B-R buffer, pH 7, −0.6 V of Eacc and 40 s of tacc. An amplitude of 50 mV was given a high oxidation peak, so it was selected for the subsequent studies. On the other hand, the frequency was studied in the range from 5 to 50 Hz, for 5 × 10−6 mol L−1 of TRN in B-R buffer, pH 7, −0.6 V of Eacc, 40 s of tacc and 50 mV amplitude. A 20 Hz frequency was recorded a high current and it was chosen for next experiment.
3.2.4 Effect of scan and convection rates
Scan and convection rates are also considered as very important parameters for indicating the best voltammetric signals for the analyzed drug. An anodic current for 5 × 10−6 mol L−1 of TRN in B-R buffer, pH 7, −0.6 V of Eacc, 40 s of tacc 50 mV amplitude and 20 Hz, was monitored using the scan rate study over the range of 50–500 mVs−1 as shown in Fig. 5. A 350 mVs−1 scan rate was given a high oxidation current and it was chosen for next work. On the other hand, the convection rate was studied in the range from 0.0 to 3000 rpm, for 5 × 10−6 mol L−1 of TRN in B-R buffer, pH 7, −0.6 V of Eacc, 40 s of tacc, 50 mV amplitude, 20 Hz and 350 mV s−1. A 1000 rpm convection rate was reported a high oxidation current, so it was selected for next work.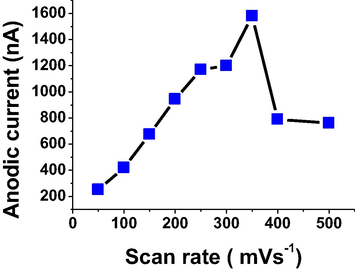
Effect of scan rate on oxidation current for 5 × 10−6 mol L−1 of TRN in B-R pH 7, 40 s tacc, −0.6 V Eacc, 50 mV amplitude and 20 Hz.
3.3 Method validation
The anodic stripping voltammetry method was validated through the studying of the calibration curve, detection and quantification limits, recovery, repeatability, and stability.
3.3.1 Calibration curve
The calibration curve was described the TRN concentrations versus voltammetric response according to the FDA guidance for bioanalytical method validation. It was studied over the range of 1 × 10−6–1 × 10−5 mol L−1 of TRN under optimum conditions. The linearity of the ASV method was observed during this concentration range to be a 0.97 r2 correlation coefficient for sex anodic measurements (see Table 1). The calibration curve was given a very good linear relationship between the oxidation currents and TRN concentrations as shown in Fig. 6.
Calibration curve
Parameters
TRN
TRN Concentrations (mol L−1)
Anodic current (nA)
1 × 10−6
400
Range (mol mL−1)
1 × 10−6–1 × 10−5
2 × 10−6
764
Correlation coefficient®2
0.97
4 × 10−6
860
6 × 10−6
1030
8 × 10−6
1240
Equation
Y = 9.63 × 107 C + 445
1 × 10−5
1360
Y
I (nA)
n (number of measurements)
6
2 × 10−5
2.003
Slope(b)
9.63 × 107
Intercept(a)
445
LOQ (ppb)a
7.47
LOD (ppb)b
2.25
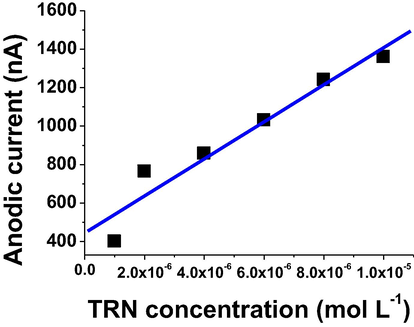
Calibration curve study over the range 1 × 10−6 − 1 × 10−5 mol L−1 of TRN in B-R pH 7, 40 s tacc, −0.6 V Eacc, 350 mVs−1 scan rate, 20 Hz, 50 mV amplitude, and 1000 rpm.
3.3.2 Detection and quantification limits
The detection and quantification limits (LOD, LOQ) were calculated by the data which reported from the calibration curve and repeatability. They became 7.5 × 10−9 mol L−1 (2.25 ppb), and 2.49 × 10−8 mol L−1 (7.47 ppb), respectively. There was no published articles that recorded the detection or quantification limits better than this study.
3.3.3 Recovery, repeatability and stability
The analytical performance of ASV method was investigated in terms of some parameters such as recovery, repeatability and stability. The recovery was studied for 4 × 10−6 mol L−1 of TRN in B-R buffer pH 7, that given 105% ± 0.63 for five anodic measurements. While the repeatability and stability were studied for 5 × 10−6 mol L−1 of TRN under optimum conditions. The repeatability was recorded at 0.24% (RSD%) for eight anodic measurements. Table 2 was summarized the recovery and repeatability results for TRN drug. Finally, the stability was monitored for 80 min, to give a very signal stable for the analyzed drug.
TRN concentration
Current (nA)
Current Average ± S.D.
RSD %
Recovery% (2 × 10−7 mol L−1)
5 × 10−6 mol L−1
1150
106
104
1142
105
1143
1146 ± 2.74
0.24
105
1145
105
1150
Mean
105
SDV
±0.63
1146
1145
1147
3.4 Application of method
The validation of the developed anodic stripping voltammetry method was successfully evaluated by the determination of TRN pharmaceutical in the human urine and plasma samples. The ASV method was applied for these human samples five times to give the current readings, then the continued voltammetric procedures were carried out using standard addition method for TRN determination in human urine and plasma; five measurements for every addition. The analytical results were obtained by the recovery of 3 × 10−6 mol L−1 of TRN in urine and plasma samples, to be given the recoveries means of 97% and 92% with standard deviations of ± 1.1% and ± 0.90% for urine and plasma, respectively as listed in Table 3.
Recovered TRN (3 × 10−6 mol L−1)
Recovery %
Urine
Plasma
98
93
97
92
98
93
97
91
95
91
Mean
97
92
Standard deviation
±1.10
±0.90
4 Conclusion
In this research, the anodic stripping voltammetry method has been developed and validated for the simultaneous determination of tretinoin (TRN) in human urine and plasma. The increased specificity of the method able to measure the analyte with a linearity range of 1 × 10−6–1 × 10−5 mol L−1 for TRN at B-R buffer pH 7. This study takes the advantage of simple procedures to prepare the biological samples that provided many benefits for the developed ASV method. The results give significant advantages, including simple, rapid, and sensitive of the used analytical method. It also demonstrated good accuracy and precision and was fully validated based on the FDA guidelines. ASV method was successfully used for the determination of TRN in the human fluids. The LOD and LOQ values have also confirmed the sensitivity of the ASV method. The used GC working electrode gave additional advantage for the determination of TRN in human urine and plasma.
Acknowledgement
The author would like to thank Jamjoom pharmaceutical Company, Jeddah –KSA and Mr. Majed AlSaleemi for their assistance to obtain the drug standard material and complete my research.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Electrochemical oxidation behavior of hydrochlorothiazide on a glassy carbon electrode and its voltammetric determination in pharmaceutical formulations and biological fluids. J. Food Drug Anal.. 2014;22:363-369.
- [Google Scholar]
- High sensitivity determination of atorvastatin calcium in pharmaceuticals and biological fluids using adsorptive anodic stripping voltammetry onto surface of ultra-trace graphite electrode. Cur. Anal. Chem.. 2018;14:92-100.
- [Google Scholar]
- Non-extractive ultra-trace determination of simvastatin in biological fluids by voltammetric method via complexation with cadmium. Dig. J. Nano. Bios.. 2014;9:355-368.
- [Google Scholar]
- Voltammetric analysis of montelukast sodium in commercial tablet and biological samples using the hanging mercury drop electrode. Portugal. Electro. Act.. 2014;32:51-64.
- [Google Scholar]
- Development And validation of analytical spectrophotometric and RP-HPLC methods for the simultaneous estimation of hydroquinone, hydrocortisone and tretinoin ternary mixture in topical formulation. Int. J. Pharm. Pharm. Sci.. 2019;11:1-7.
- [Google Scholar]
- Electrochemical detection of retinoids using normal phase HPLC. J. Liq. Chromatgr.. 1991;14:2287-2295.
- [Google Scholar]
- Rapid determination of lead in environmental water by anodic stripping voltammetry. Anal. Let.. 2016;49:1004-1014.
- [Google Scholar]
- Graham Solomons, T.W., 1988. Organic chemistry, 4th, Hohn Wiley& Sons, New york USA, 1988,pp. 334-335.
- Determination of trace metals by anodic stripping voltammetry using a carbon nanotube tower electrode. Electroanal. 2011;23:1252-1259.
- [Google Scholar]
- A Validated UV spectrophotometric method for simultaneous estimation of tretinoin and benzoyl peroxide in bulk and semi solid dosage form Rasa. J. Chem.. 2009;2:649-654.
- [Google Scholar]
- A new HPLC-DAD method for the concurrent determination of hydroquinone, hydrocortisone acetate and tretinoin in different pharmaceuticals for melasma treatment. J. Chromatogr. Sci.. 2019;57:495-501.
- [Google Scholar]
- Comparison of micronized tretinoin (0.05%) gel and tretinoin (0.025%) gel following exposure to ultraviolet a light. J. .Clin Aesthet. Dermatol.. 2012;5:27-29.
- [Google Scholar]
- Analytical methodologies for determination of methotrexate and its metabolites in pharmaceutical, biological and environmental samples. J. Pharm. Anal.. 2019;9:373-391.
- [Google Scholar]
- Determination of tretinoin in creams by high-performance liquid chromatography. J. Chromatogr.. 1990;28:227-234.
- [Google Scholar]
- Lan, y., Luo, H., Ren, X., Wang, Y., Liu, Y., Anodic stripping voltammetric determination of arsenic(III) using a glassy carbon electrode modified with gold-palladium bimetallic nanoparticles Microchim. Acta. 178 2012 153 161
- Isotretinoin oxidation and electroanalysis in a pharmaceutical drug using a boron-doped diamond electrode. Electroanal. 2016;28:2709-2715.
- [Google Scholar]
- Anodic stripping voltammetric determination of mercury in water using a chitosan-modified carbon paste electrode. Anal. Let.. 2007;40:3119-3128.
- [Google Scholar]
- Morrison, R.T., Boyd, R.N., 1992. Organic chemistry,6th edition, Prentice Hall, Englewood Cliffs, New Jersey, USA, pp. 425–430.
- Direct analysis of palladium in active pharmaceutical ingredients by anodic stripping voltammetry. Anal. Chim. Acta.. 2016;914:47-52.
- [Google Scholar]
- Anodic Stripping voltammetry for the determination of trace Cr(VI) with graphite/styrene-acrylonitrile copolymer composite electrodes. Anal. Sci.. 2017;33:801-806.
- [Google Scholar]
- Electrochemical study and determination of All-trans-retinol at carbon paste electrode modified by a surfactant. Food Technol. Biotechnol.. 2018;56:337-343.
- [Google Scholar]
- A rapid HPLC method for simultaneous determination of tretinoin and isotretinoin in dermatological formulations. J. Pharm. Biomed. Anal.. 2007;43:859-864.
- [Google Scholar]
- Derivative spectrophotometric method for simultaneous determination of clindamycin phosphate and tretinoin in pharmaceutical dosage forms. DARU J. Pharma. Sci.. 2013;21:29.
- [Google Scholar]
- Tivnan, A., 2017. Resistance to Targeted Therapies Against Adult Brain Cancers., 123.
- Simultaneous determination of retinal, retinol and retinoic acid (all-trans and 13-cis) in cosmetics and pharmaceuticals at electrodeposited metal electrodes. Anal. Chim. Acta. 2000;415:193-200.
- [Google Scholar]
- Determination of retinoids in human serum, tocopherol and retinyl acetate in pharmaceuticals by RP-LC with electrochemical detection. J. Pharm. Biomed. Anal.. 2001;25:785-793.
- [Google Scholar]
- Fast-scan anodic stripping voltammetry for detection of Pb(ii) at picomolar level. Rus. J. Electrochem.. 2019;55:222-228.
- [Google Scholar]
- Simultaneous determination of tretinoin and clindamycin phosphate and their degradation products in topical formulations by reverse phase HPLC. J. Separ. Sci.. 2004;27:271-277.
- [Google Scholar]
- HPLC determination of the stability of tretinoin in tretinoin–minoxidil solution. Pharm. Acta Helve.. 1998;73:163-165.
- [Google Scholar]
- Spectrophotometric micro determination of tretinoin, isotretinoin using iodine and tazarotene micro determination via reaction with rose-bengal reagent. Egpt. J. Chem.. 2018;61:143-153.
- [Google Scholar]
- Determination of trace heavy metal ions by anodic stripping voltammetry using nanofibrillated cellulose modified electrode. J. Electroanal. Chem.. 2017;799:70-77.
- [Google Scholar]







