Translate this page into:
Determination of toxic effects of lead acetate on different sizes of zebra fish (Danio rerio) in soft and hard water
⁎Corresponding author. hak3962@sch.ac.kr (Hak-Jae Kim)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The main objective of the present study is to determine lead toxicity on various sizes of zebra fish in soft and hard water. Lead toxicity analysis was carried out in the laboratory trials for four days at various concentrations for the determination of LC50 value. Zebra fish was grouped into various sizes (G1-G4) and treated with lead for 96 h. Lead induced toxicity and induced various behavioural changes in Zebra fish. In the case of G1 (1.5 ± 0.2 cm length) fish the LC50 values ranged from 27.2 ± 1.8 mg/L to 9.7 ± 1.3 mg/L. In G2 (2.0 ± 0.2 cm length) fish, the LC50 value was 37.01 ± 2.3 mg/L and it reduced as 32.03 ± 3.4 mg/L after 48 h. In G3 group (2.5 ± 0.2 cm length fish), LC50 value was 38.78 ± 2.4 mg/L after 24 h treatment and this value decreased as 33.18 ± 1.1 mg/L after 48 h. After 72 h, LC50 value was 27.2 ± 2.1 mg/L and this value decreased considerably after 96 h (18.3 ± 2.1 mg/L) in G4 (3.0 ± 0.2 cm length) fish. Further, G4 fish was treated with lead in hard water and LC50 value was analyzed. LC50 value was observed as 28.62 ± 2.7 mg/L in hard water and the same fish group (G4) showed 18.3 ± 2.1 mg/L after 96 h. Toxicity analysis revealed that lead is less toxic in hard water than in soft water.
Keywords
Lead
Toxicity
Water hardness
Zebra fish
1 Introduction
Heavy metals are highly toxic to the environment, and the main sources are coal burning, mining, agriculture, sewage, domestic and industrial runoff. In aquatic environment, metal toxicity affects fishes and these toxic heavy metals affected ion regulation in fishes. Heavy metal poses severe threat to the aquatic organisms and has various sources to enter aquatic system. Aquatic pollution causes stress to the aquatic organism and induce negative effect. Lead is the one of the metals have no nutritive value and cause pollution in aquatic environment (Vasanthi et al., 2019). These metals easily enter into the food chain and subsequently involved in bioaccumulation process (Azaman et al., 2015). Toxicity of heavy metals to the organism mainly based on the route of exposure, absorbed dose and duration of exposure (Chaurasia et al., 2016a; Kumaresan et al., 2016; Chaurasia et al., 2016b; Kumaresan et al., 2015a). This can lead to many disorders and also cause excessive damage due to oxidative stress mainly induced by the formation of free radicals (Jaishankar et al., 2014). Some heavy metals are very essential for routine physiological function of fish however become toxic when heavy metals accumulate in the muscle and body tissues (Pandey and Madhuri, 2014). Also, elevated level of heavy metals can effectively change its physiological function that leads to mortality in fishes (Avenant-Oldewage and Marx, 2000). The river is mainly contaminated by heavy metals due to the outcome of industrial waste, mining, domestic wastes including battery and agricultural wastes. Contamination of heavy metals in aquatic environment may affect the diversity and food web of aquatic organisms (Varol, 2011). Like other animals, fishes from the pond, lake or river water cannot escape from the harmful effects from these heavy metals (Ravichandran et al., 2017; Arasu et al., 2017a,b,2016; Ravichandran et al., 2016).
The impact of these heavy metals and other various pollutants, on fishes can be tested by toxicity analysis, which is mainly used to evaluate and to detect the toxic effects of heavy metals on aquatic organisms (Fu et al., 2013). Fishes are commonly applied to validate the nature of aquatic ecosystems because heavy metals and other pollutants involved in food chain mechanism and are highly responsible for severe impacts and mortality in the aquatic eco-system. Fishes get trace elements through food, gill, or through body surface (Arockiaraj et al., 2015a; Palanisamy et al., 2015; Arockiaraj et al., 2015b; Chaurasia et al., 2015; Kumaresan et al., 2015a,b; Rao et al., 2015). Heavy metals such as, Zn, Cu Mn, Co and Cr are very important for the growing organisms, however As, Pb, Cd and Hg are not required for aquatic organisms for their metabolism (Sathyamoorthi et al., 2019;Kumaresan et al., 2019; Sathyamoorthi et al., 2018; Ravichandran et al., 2018;Sathyamoorthi et al., 2017). These heavy metals at higher concentrations in the aquatic environment cause hazardous and highly toxic. Once heavy metals enter into the aquatic environment, these heavy metals are absorbed on solid surface, and suspended in water or absorbed by fauna. Also, animal tissues accumulate heavy metals from the environment. Heavy metals such as cobalt is a highly toxic substance, fertilizers are the importance source of cobalt contamination in the running water and cause heavy risk to the environment (Mansouri et al., 2011, 2012).
Lead causes severe impact to fishes and cause mortality at lethal concentrations and at sub-lethal concentrations cause impotency, behaviour and growth performance changes (Afshan et al., 2014). In aquatic environment Pb2+ is highly stable form of lead and has been accumulated in fish organs such as, liver, gills, kidney, scales, muscles and skin. Exposure of lead in the aquatic environment causes mortality, growth inhibition properties, and abnormalities in the muscle and changes in reproductive performance (Srivastav et al., 2013). The contaminated water by heavy metals easily entered into the food chain in freshwater ecosystem. Based on absorption and excretion pattern, bioaccumulation rate differs. Many factors such as, chemical and physical including pH of the environment directly influence bioaccumulation in various fish tissues. Heavy metals affect reproduction, cause mortality, physiological functions and also affect growth rate and feed conversion rate in fishes (Abascal et al., 2007). In aquatic environment, heavy metals enter into the fish body through body surface, digestive system and gills. However, accumulation of heavy metals through body surface is limited. The objective of the present analysis is to determine lead toxicity on various sizes of zebra fish in soft and hard water.
2 Materials and methods
2.1 Experimental fish
In our study, the experimental animal, Zebrafish (Danio rerio) was collected from the aquarium and stocked in a rectangular glass aquarium tank (75 × 60 × 35 cm, 20 L capacity) for about two weeks. About 14 h light and 10 h dark cycle was maintained throughout the experimental period. The aquarium tank was connected with air compressor and ensured uninterrupted aeration. Initially 250 animals were collected from the aquarium and maintained. Zebra fish was grouped into experimental and control. The fish was graded based on the length. A total of four experimental groups were maintained strictly based on the length, viz, 1.5 ± 0.2 cm (G1), 2.0 ± 0.2 cm (G2), 2.5 ± 0.2 cm (G3) and 3.0 ± 0.2 cm (G4), respectively. In each group, twenty two experimental animals were incorporated. Control and experimental animals were fed with brine shrimp (Artemia), tubifexworm and pellet feed daily.
2.2 Chemicals
For toxicity analysis lead acetate was purchased from Sigma, U.S.A and stock was prepared in double distilled water. All other reagents, chemical used were analytical grade (Himedia, Mumbi, India).
2.3 Toxicity analysis in soft water
Lead toxicity analysis was carried out in the laboratory trial in the above conditions for four days. Lead acetate was prepared at eight different concentrations, 5, 10, 15, 20, 25, 30, 35 and 40 mg/L. Heavy metal stock was prepared in double distilled water. Three experiment analyseswere performed and an average was considered for this study. Exchange of water was performed daily and fresh metal was added daily. Finally, mortality rate of fish was determined after 24–96 h. Neurotoxicity was also monitored during experimental trials. Dead animals were separated from the tank and stored at −20 °C up to the completion of all experiments.
2.4 Toxicity analysis in hard water
The approximate CaCO3 hard water level of tap water was 20 mg/L. To this tap water Ca (NO3)2 was added to increase the hardness of water (upto250 mg/L). Lead was prepared at various concentrations in double distilled water. The concentration of lead was maintained as described previously. Zebra fish (3.0 cm long) was introduced into the glass aquarium which was previously acclimatized and fed with live and pellet feed. LC50 value was determined for 24–96 h.
3 Results
3.1 Lead toxicity induces bahavioral changes in Zebra fish
Lead induced toxicity cause various behavioural changes in Zebra fish. The changes like loss of equilibrium, erratic swimming was observed during the study period. The experimental animal resting at the bottom of the glass aquarium aggregated at a corner of the tank and frequently swims at the surface of the tank. The heavy metal treated fish lost control, hyper secretion of mucus from the body, heavy breathing with strong opercula movement. In the control fish, behavioural response is normal.
3.2 Lead toxicity based on the size of the fish
Zebra fish was grouped into various sizes and lead acetate was prepared at five different concentrations. In the case of G1 fish the LC50 values ranged from 27.2 ± 1.8 mg/L to 9.7 ± 1.3 mg/L. After 96 h incubation, LC50 value was 9.7 ± 1.3 mg/L. In G2 fish, the LC50 value was 37.01 ± 2.3 mg/L and it reduced as 32.03 ± 3.4 mg/L. After 72 h, LC50 value was 27.1 ± 4.1 mg/L and it reduced considerably after 96 h (17.8 ± 2.3 mg/L). In G3 fish, LC50 value was 38.78 ± 2.4 mg/L after 24 h treatment and this value decreased as 33.18 ± 1.1 mg/L after 48 h. After 72 h, LC50 value was 27.2 ± 2.1 mg/L and decreased considerably after 96 h (18.3 ± 2.1 mg/L). In G4 fish, LC50 value was found to be 18.62 mg/L after 4 days incubation in soft water (Fig. 1).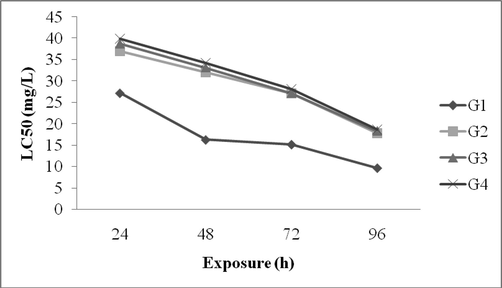
LC50 value for Zebra fish exposed to lead at various experimental groups. G1 = 1.5 ± 0.2 cm length fish, G2 = 2.0 ± 0.2 cm length fish, G3 = 2.5 ± 0.2 cm length fish and G4 = 3.0 ± 0.2 cm length fish.
3.3 Lead toxicity in hard water
The average LC50 value for tested hard water for Zebra fish is described in Fig. 2. The survival rate of Zebra fish was found to be high in hard water than soft water. The LC 50 values were, 48.91 ± 2.2, 37.28 ± 2.8, 32.13 ± 3.1 and 28.62 ± 2.7 mg/L after 24, 48, 72 and 96 h, respectively (Fig. 2).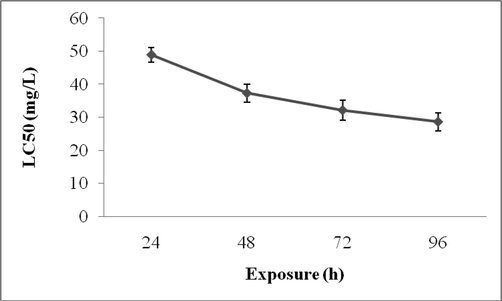
Influence of hard water on toxicity for Zebra fish exposed to lead for G4 (3.0 ± 0.2 cm length) fish.
4 Discussion
In this study, lead was exposed to Zebra fish at various concentrations and at various sizes (G1 – G4) to analyze the impact of lead towards size. Initially behavioural changes were observed in the experimental animal. Large fishes (G4) were less susceptible and small sized fishes (G1) were largely affected by lead toxicity. Experimental fishes lost equilibrium and rested at the bottom of the tank. Some fishes were found near the corner of the tank and erratic swimming was also noticed. It could be noted that heavy metals contamination is very common in aquatic environment. Heavy metals are mainly considered as hazardous material to the aquatic system because of high toxicity, ability to bioaccumulate by living organisms (Pandey and Madhuri, 2014). Hardness and pH of the aquatic environment greatly affected the availability of heavy metals. Heavy metals affect locomotion of fishes in various ways, including avoidance, homing, alters sensory perception and reduce swimming performance (Prashanth et al., 2011). Lead also causes severe health problems in humans. Lead accumulates in the blood, bones and muscles and fat. Young children and newborns are highly susceptible to lead even at very low concentrations (Gurer-Orhan et al., 2004).
In this study, lead caused toxicity to zebra fish at various concentrations. At higher exposure time, severe effect was registered. Behaviour changes were observed during the study due to neruotoxic effect. In aquatic ecosystem, heavy metal contamination results from geologic weathering, atmospheric deposition, industrial, domestic and municipal waste products also wastewater plants generate various heavy metals (Bauvais et al., 2015; Demirak et al., 2006). High concentrations of heavy metals in the aquatic environment pose serious threat to living organisms. Importantly, fishes may accumulate large quantity of heavy metals and cause acute and chronic diseased to humans beings (Al-Yousuf et al., 2000). Metals are non degradable matters and can accumulate in the particular environment and cause various health issues. In fishes, accumulation of heavy metals was mainly through gastrointestinal tract and gills (van der Oost et al., 2003). Fishes are mainly confined in a particular environment and are highly vulnerable to metal toxicity. Toxicity in an aquatic environment is mainly used to validate the toxicological properties of environmental contaminants on aquatic organisms. It is very important to explore the presence of water borne heavy metals on tolerance of the highly sensitive organisms such as fish. In natural environment, the reported lead concentration in the surface water was 0.02 μg/L and lead rarely exceeds at this value. High exposure of lead in the aquatic organism critically causes alteration in blood, generative damage, alteration in nerve cells in aquatic organisms, including fishes (Kalay et al., 1999; McCoy et al., 1995).
The results obtained from the toxicity analysis of lead for Zebra fish reveals that the mortality of the fish dramatically increased with exposure time and increasing lead concentrations. In a study, Ullah et al. (2016) observed lead toxicity to Oreochromis niloticus. It was subjected to toxicity analysis and 96 h LC50 value of lead nitrate was found to be 44 mg/L. This value was found to be higher than our finding, shows Zebra fish is highly sensitivity than Oreochromis niloticus. Batool and Javed (2015) observed lead toxicity on Labeorohita, Cirrhina mrigala and Catla catla. The LC50 value was found to be 36.72 ± 0.37 mg/L, 40.54 ± 0.32 mg/L and 31.25 ± 0.22 mg/L, respectively. In a study Ferrer et al. (2006) used crab, Chasmagnathus granulate in its early stage to determine heavy metal toxicity and the reported ranges of LC50 values among the tested heavy metals such as, Zn, Cu and Pb. Chinni and Yallapragda (2000) used the heavy metals such as, Cu, Cd, Zn and Pb to determine heavy metal toxicity on post larvae (PL) of Penaeus indicus. The findings revealed that copper pose serious threat to the PL stage of P. indicus than other tested metals. However, the toxicity value may differ between fish, experimental procedure, water pH, age of fish, sex and temperature of the aquatic environment.
In our study, exposure of lead caused severe impact on small Zebra fish than larger one after 96 h exposure at sub – lethal level. It was previously reported that, long-term exposure of heavy metals to the fishes at low doses did not show any significant lose externally, moreover, the heavy metals critically affect the reproductive behaviour of fishes by reduced reproductive organ and the fish population (Kime, 1995). Previous studies reported nuclear degeneration, severe pituitary damage and reduced egg hatching rates due to metal toxicity (Popek et al., 2006). Lead has been well known to cause haematological, neurological, circulatory, reproductive and histochemical changes (Rout and Naik, 2000). The toxic heavy metals significantly affect metabolic and physiological functions, reproductive functions, growth rate and cause mortality to fishes (Woodward et al., 1994). The LC50 value of fish was 9.7 ± 1.2 mg/L in small fish which was found to be less than large fish. In a study, LC50 value for lead on Labeo rohita was analyzed ant it was ranged between 27.2 mg/l and 32.70 ± 2.23 mg/l (Javid et al., 2007; Abdullah et al., 2007).
The present findings show that lead is highly toxic to Zebra fish in both hard and soft water. In our experiment increased survival rate was registered at higher concentrations of hard water. After 96 h, the LC50 value was 18.62 ± 4.5 mg/L in soft water; however this value increased as 28.62 ± 4.5 mg/L in hard water. The increased LC50 value was reported previously with various heavy metals. The reduction of lead toxicity in hard water was mainly due to the formation of insoluble complexes in hard water. Pascoe et al. (1986) used rainbow trout to analyze cadmium toxicity after 96 h. After 96 h, LC50 value was 1.3 mg Cd/L in soft water, whereas, the LC50 value was 2.6 mg Cd/L in hard water. In hard water, heavy metal uptake significantly decreased. Jaishankar et al. (2014) stated that metal toxicity depends upon the duration of exposure, the route of exposure and adsorbed dose. Heavy metals lead to many disorders and can also results in severe damage due to severe oxidative stress. Rathore and Khangarot (2003) studied the influence of metal concentration and water hardness in Tubifex tubifex Muller. The LC50 value was reportedly higher in hard water than soft water in the case of zinc, nickel, mercury, manganese, lead, iron, copper chromium, cobalt and cadmium. These tested heavy metals enhanced mucus production and induced autonomy in the caudal region. In a study, Mansouri and Baramaki (2011) studied the influence of pH and water hardness on acute toxicity of mercury on Capoetafusca borhan. In this freshwater fish, the LC50 value was 0.180 mg/L in hard water, whereas, it was found to be low in soft water (0.118 mg/L). These findings revealed increased heavy metal toxicity in hard water than in soft water. Other toxic metal such as copper and zinc also reported as highly toxic to the organism in soft water than hard water (Ebrahimpour et al., 2010).
5 Conclusion
Lead is highly toxic to Zebra fish and caused behaviour changes at various concentrations. This behavioural response was mainly due the ill effect in nervous system cause by lead. After 24 h of exposure, LC50 value was found to be high and it decreased after 96 h of treatment. The increased exposure increases lead absorption through gill, skin and digestive system. In the present finding soft water showed high toxicity than hard water. In hard water, lead absorption may be less through body surface, gill and digestive systems. The reduction of lead toxicity in hard water was mainly due to the formation of insoluble complexes in hard water.
Acknowledgements
The authors express their sincere appreciation to the Researcher Supporting Project No. RSP-2019/93, King Saud University, Riyadh, Saudi Arabia. The authors Hak-Jae Kim thank the support received from Soochunhyang University research fund for this research work.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Characterization of sperm motility in sea bass: the effect of heavy metals and physicochemical variables on sperm motility. J. Fish Biol.. 2007;70(2):509-522.
- [Google Scholar]
- Studies on acute toxicity of metals to the fish (Labeo rohita) Int. J. Agr. Biol. 2007;9:333-337.
- [Google Scholar]
- Effect of different heavy metal pollution on fish. Res. J. Chem. Environ. Sci.. 2014;2(1):74-79.
- [Google Scholar]
- Trace metals in liver, skin and muscle of Lethrinuslentjan fish species in relation to body length and sex. Sci. Total Environ.. 2000;2000(256):87-94.
- [Google Scholar]
- Bactericidal activity of fish galectin 4 derived membrane-binding peptide tagged with oligotryptophan. Dev. Compar. Immunol.. 2017;71:37-48.
- [Google Scholar]
- Bacterial membrane binding and pore formation abilities of carbohydrate recognition domain of fish lectin. Dev. Compar. Immunol.. 2017;67:202-212.
- [Google Scholar]
- Coagulation profile, gene expression and bioinformatics characterization of coagulation factor X of striped murrelChannastriatus. Fish Shellf. Immunol.. 2016;55:149-158.
- [Google Scholar]
- Molecular and functional roles of 6C CC chemokine 19 in defense system of striped murrel Channastriatus. Fish Shellf. Immunol.. 2015;45:817-827.
- [Google Scholar]
- Fish chemokines 14, 20 and 25: a comparative statement on computational analysis and mRNA regulation upon pathogenic infection. Fish Shellf. Immunol.. 2015;47:221-230.
- [Google Scholar]
- Bioaccumulation of chromium, copper and iron in the organs and tissues of Clariasgariepinus in the Olifants River. Water SA: Kruger National Park; 2000. p. :621-640.
- Heavy metal in fish: analysis and human health-a review. J. Teknol.. 2015;77(1):61-69.
- [Google Scholar]
- Synergistic effects of metals (cobalt, chromium and lead) in binary and tertiary mixture forms on Catla catla, Cirrhina mrigala and Labeo rohita. Pak. J. Zool.. 2015;47(3):617-623.
- [Google Scholar]
- Sponging up metals: bacteria associated with the marine sponge Spongia officinalis. Mar. Environ. Res.. 2015;2015(104):20-30.
- [Google Scholar]
- Molecular importance of prawn large heat shock proteins 60, 70 and 90. Fish shellfish Immunol.. 2016;48:228-238.
- [Google Scholar]
- In-silico analysis and mRNA modulation of detoxification enzymes GST delta and kappa against various biotic and abiotic oxidative stressors. Fish Shellfish Immunol.. 2016;54:353-363.
- [Google Scholar]
- Molecular profiles and pathogen-induced transcriptional responses of prawn B cell lymphoma-2 related ovarian killer protein (BOK) Fish Shellf. Immunol.. 2015;45(2):598-607.
- [CrossRef] [Google Scholar]
- Toxicity of copper, cadmium, zinc and lead to Penaeus indicus postlarvae: effects of individual metals. J. Environ. Biol.. 2000;2000(21):225-258.
- [Google Scholar]
- Heavy metals in water, sediment and tissues of Leuciscuscephalus from a stream in southwestern Turkey. Chemosphere. 2006;63(9):1451-1458.
- [Google Scholar]
- Influence of water hardness on acute toxicity of copper and zinc on fish. Toxicol. Ind. Health. 2010;26(6):361-365.
- [Google Scholar]
- Acute toxicities of four metals on the early life stages of the crab Chasmagnathus granulata from Bahia Blanca estuary, Argentina. Ecotoxicol. Environ. Safety. 2006;65(2):209-217.
- [Google Scholar]
- Risk and toxicity assessments of heavy metals in sediments and fishes from the Yangtze River and Taihu Lake, China. Chemosphere. 2013;93(9):1887-1895.
- [Google Scholar]
- Correlation between clinical indicators of lead poisoning and oxidative stress parameters in controls and lead-exposed workers. Toxicology. 2004;195(2–3):147-154.
- [Google Scholar]
- Toxicity, mechanism and health effects of some heavy metals. Interdisciplin. Toxicol.. 2014;7(2):60-72.
- [Google Scholar]
- Nickel bio-accumulation in the bodies of Catlacatla, Labeorohita and Cirrhinamrigala during 96-hr LC50 exposures. Int. J. Agric. Biol.. 2007;9:139-142.
- [Google Scholar]
- Heavy metal concentrations in fish tissues from the Northeast Mediterranean Sea. Bull. Environmen. Contamin. Toxicol.. 1999;63(5):673-681.
- [Google Scholar]
- The effects of pollution on reproduction in fish. Rev. Fish Biol. Fisheries.. 1995;5(1):52-95.
- [Google Scholar]
- Novel antimicrobial peptide derived from fish goose type lysozyme disrupts the membrane of Salmonella enterica. Mol. Immunol.. 2015;68:421-433.
- [Google Scholar]
- Comparative analysis of CsCu/ZnSOD defense role by molecular characterization: Gene expression-enzyme activity-protein level. Gene. 2015;564:53-62.
- [Google Scholar]
- A comparative transcriptome approach for identification of molecular changes in Aphanomycesinvadans infected Channastriatus. Mol. Biol. Rep.. 2019;45:2511-2523.
- [Google Scholar]
- Multifunctional murrel caspase 1, 2, 3, 8 and 9: Conservation, uniqueness and their pathogen-induced expression pattern. Fish Shellf. Immunol.. 2016;49:493-504.
- [Google Scholar]
- Acute toxicity bioassay of mercury and silver on Capoetafusca (black fish) Toxicol. Indus. Health.. 2012;28:393-398.
- [Google Scholar]
- Experimental studies on concentration and depuration of cobalt in the selected organs of fresh water fish Capoetafusca. World J. Fish Marine Sci.. 2011;3:387-392.
- [Google Scholar]
- Liver and kidney concentrations of zinc, copper and cadmium in channel catfish (Ictalurus punctatus): variations due to size, season and health status. Vet. Human. Toxicol.. 1995;37(1):11-15.
- [Google Scholar]
- Functional roles and gene regulation of tumor necrosis factor receptor 1 in freshwater striped murrel. Mol. Immunol.. 2015;66:240-252.
- [Google Scholar]
- Heavy metals causing toxicity in animals and fishes. Res. J. Ani. Vet. Fishery Sci.. 2014;2(2):17-23.
- [Google Scholar]
- Heavy metal toxicity to fish and the influence of water hardness. Arch. Environ. Contamin. Toxicol.. 1986;15(5):481-487.
- [Google Scholar]
- Influence of heavy metals and 4-nonylphenol on reproductive function in fish. Reprod. Biol.. 2006;6:175-188.
- [Google Scholar]
- Effect of sodium cyanide on behaviour and respiratory surveillance in freshwater fish, Labeo rohita (Hamilton) Recent Res. Sci. Technol.. 2011;3(2):24-30.
- [Google Scholar]
- Defense properties in the epidermal mucus of different freshwater fish species. Aquacul. Aquar. Conserv. Legisl.. 2015;8:184-194.
- [Google Scholar]
- Effects of water hardness and metal concentration on a freshwater Tubifextubifex Muller. Water Air Soil Poll.. 2003;142(1–4):341-356.
- [Google Scholar]
- Pellino-1 derived cationic antimicrobial prawn peptide: bactericidal activity, toxicity and mode of action. Mol. Immunol.. 2016;78:171-182.
- [Google Scholar]
- A cumulative strategy to predict and characterize antimicrobial peptides (AMPs) from protein database. Int. J. Pept. Res. Therapeut.. 2017;23:281-290.
- [Google Scholar]
- Bactericidal and fungistatic activity of peptide derived from GH18 domain of prawn chitinase 3 and its immunological functions during biological stress. Int. J. Biol. Macromol.. 2018;106:1014-1022.
- [Google Scholar]
- Quantitative precipitation tests for anti-avidin during experimental plumbism in Clariasbatrachus Linn. J. Appl. Zool. Res.. 2000;11(1):48-53.
- [Google Scholar]
- Gene expression and in silico analysis of snakehead murrel interleukin 8 and antimicrobial activity of C-terminal derived peptide WS12. Veterin. Immunol. Immunopathol.. 2017;190:1-9.
- [Google Scholar]
- Therapeutic cationic antimicrobial peptide (CAP) derived from fish aspartic proteinase cathepsin D and its antimicrobial mechanism. Int. J. Pept. Res. Therapeut.. 2019;25:93-105.
- [Google Scholar]
- Heat Shock Cognate 70 Derived AMPs CsHSC70 A1 and CsHSC70 A2. Int. J. Pept. Res. Therapeut.. 2018;24:143-155.
- [Google Scholar]
- Effects of lead on the plasma electrolytes of a freshwater fish. Heteropneustes fossilis. Int. Aqua. Res.. 2013;5:4.
- [Google Scholar]
- Determination of 96-Hr LC50 of lead nitrate for a fish, Oreochromisniloticus. J. Entomo. Zool. Stud.. 2016;4(4):1216-1218.
- [Google Scholar]
- Fish bioaccumulation and biomarkers in environmental risk assessment: a review. Proc. Symp. Environ. Toxicol.. 2003;1989:377-380.
- [Google Scholar]
- Assessment of heavy metal contamination in sediments of the Tigris River (Turkey) using pollution indices and multivariate statistical techniques. J. Hazard. Mat.. 2011;195:355-364.
- [Google Scholar]
- Toxic effect of Mercury on the freshwater fish Oreochromis mossambicus. Res. J. Life Sci. Bioinfo. Pharmaceut. Chem. Sci.. 2019;5(3):364-376.
- [Google Scholar]
- Effects on rainbow trout fry of a metals-contaminated diet of benthic invertebrates from the Clark Fork River. Montana. Transact. Am. Fisher. Soc.. 1994;123(1):51-62.
- [Google Scholar]







