Translate this page into:
Determination of stability of cosmetic formulations incorporated with water-soluble elastin isolated from poultry
⁎Corresponding author at: Department of Food Sciences, Faculty of Science and Technology, Universiti Kebangsaan Malaysia, 43600 UKM Bangi, Selangor, Malaysia. salma_my@ukm.edu.my (Salma Mohamad Yusop)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Elastin is a flexible fibre that promotes the skin’s elasticity and flexibility. It has mainly been used in cosmetic products as a conditioning agent in many premium skincare products available globally. The present study was aimed to assess the stability of moisturiser cream containing elastin powder extracted from poultry skin as a functionally active ingredient. The elastin powder was isolated by incorporating a non-enzymatic approach and then analysed for its purity and quality by using amino acid profiling. The high content of glycine, proline, and alanine indicates a high purity of elastin was successfully extracted. Besides, the extracted elastin was also having high solubility (>80%). Subsequently, a model moisturiser system containing poultry-based water-soluble elastin powder was developed and underwent series of microbiological testing and physicochemical observation at different intervals for a maximum of 12 weeks at room temperature (RT), 40 °C, and 50 °C. The stability study on the developed moisturisers showed that the creams are physically and chemically stable throughout the 12 weeks of storage with no dramatic changes in terms of its pH, viscosity, and colour at all temperatures. In conclusion, the newly formulated moisturiser cream exhibits many promising properties and attributes that could unlock new opportunities to develop an effective, healthy, cost-competitive, high-quality product for cosmetics and pharmaceuticals use.
Keywords
Bioactive ingredient
Elastin
Moisturiser
Poultry skin
Stability study
1 Introduction
Large quantities of animal by-products come from different processing industries. Statistically, 20 and 10 million tons of discards from processing industries are produced worldwide each year from residues of fish and meat (cattle, pigs and poultry), respectively (Jayathilakan et al., 2012). In general, such by-products are disposed of or refined into fertilisers, animal feed and pet food. These practices are; however, ineffective as high management costs are required and the degradability of these by-products is slow and leads to environmental problems such as unpleasant odours and environmental pollution. This waste from poultry slaughter residues can be converted into valuable materials such as collagen (Fauzi et al., 2016), elastin (Yusop et al., 2016; Nadalian et al., 2019), keratin, and respective hydrolysates and small peptides. They have high functional and bioactive properties that can be used in various fields including food, biomedicine and pharmaceuticals as well as in highly lucrative sectors such as cosmetics.
As with other molecular components of the extracellular matrix such as collagen, elastin is a protein found in several connective tissues and responsible for elasticity (Calleja-Agius et al., 2013; Momot et al., 2013) and provides support to the blood vessels, lungs, and skin (Chen et al., 2009; Sugitani et al., 2012). Thus, elastin has been used as an ingredient in cosmetic formulations numerously by cosmetic manufactures to promote better skin elasticity. The wide variety of techniques currently available to produce cosmetic products to enhance the skin's appearance and to help preserve features such as safety, good texture, hydration and the quality of the dermal matrix with ageing.
The skin is the body’s largest and most superficial organ, capable of continuous regeneration and responsible for physiological functions (Elewa et al., 2015; Yadav et al., 2015). Compared to other biological elements of the human body, the skin is subjected to a complex lifelong ageing cycle arising from structural and physiological changes caused by factors internal to the body and external to it (Debacq-Chainiaux et al., 2012; Durai et al., 2012; Farage et al., 2013; Van Pham et al., 2014). To keep the skin moisturised, cosmetic ingredients such as elastin will help to protect against skin drying (Katayama et al., 2017). According to the market survey data, an anti-ageing product market will be the fastest-growing segment with the opportunity of $1,040.4 M by 2023.
Besides, there is a significant increase in customer consciousness about the use of ingredients in skincare and beauty products. This also led to a rise in demand for Halal and natural ingredients for cosmetics. Globally, the estimation of the demand for halal cosmetics at present is to be around $53 billion by the end of 2023. While cosmetic brands are abundant in the market, there used to be a limited choice for halal cosmetics where the Halalness of the ingredients used could also be ambiguous. Elastin and collagen, among a few essential ingredients which are often incorporated in many premium anti-ageing skincare products, could be of uncertain Halal status due to the raw material or process used to isolate them. Natural sources of elastin are obtained mostly from pig aorta, bovine neck ligament and mouse lungs (Mecham, 2008).
In previous years, successful attempts at isolating elastin containing bioactive compounds for antioxidant activities from poultry using various approaches have been reported (Nadalian et al., 2015, 2019; Yusop et al., 2016). However, studies on the utilisation of water-soluble elastin from poultry and its potential use in the formulation of functional foods, nutraceuticals, cosmetic and pharmaceutical industry were still in infancy. Hence, the present study aimed to evaluate the potential of elastin as an ingredient in skincare production. The physicochemical and microbiological stability of a model moisturiser system containing elastin extracted from poultry skin was investigated. The purpose of doing this stability testing is to confirm the biocompatibility of the extracted elastin with other components in a skincare formulation to meet the intended physical, chemical and microbiological quality standards as well as functionality and aesthetics when stored under certain conditions. The storage conditions used in this study are based upon the climatic zone in which the potential product containing elastin is intended to be marketed and proposed to be filed for regulatory approval.
2 Materials and methods
2.1 Raw materials
Frozen broiler skin was purchased from a slaughtering house in Pedas, Negeri Sembilan, Malaysia and immediately stored in a cooler box to be carried back to the laboratory. The skins were kept in the freezer at −18 °C and thawed approximately 1 h before the extraction process. All chemical reagents used for extraction and analysis were analytical grades.
2.2 Elastin extraction
The elastin extraction process is based on the method by Nadalian et al. (2015) with some modification. Broiler skin was suspended in 1 M NaCl for 24 h in a cold room. Then, the homogenate was centrifuged using a centrifuge (Eppendorf 5804R, Germany) at 13000 × g for 20 min. Consequently, the pellet was washed and then suspended in 0.1 N NaOH and heated with constant shaking for 1 h. The residual of NaOH-insoluble material was washed several times in water and lyophilised. Subsequently, treatment with acid was done for 40 min at 100 °C for solubilising step as water-soluble elastin. The sample was freeze-dried by using a benchtop freeze dryer (Labconco, Kansas City, MO, USA) at temperature −80 °C and vacuum pressure of 4.5 Pa to obtain elastin powder.
2.2.1 Proximate composition analysis
The proximate composition of extracted elastin powder including crude protein, crude fat, moisture and ash was analysed according to methods of the Association of Official Analytical Chemists (AOAC 1995). Crude protein content was measured by the Kjeldahl method; crude fat content was determined by the Soxhlet’s method with hexane as a solvent; moisture content was measured by the hot air oven method, and ash content was calculated by incineration in a muffle furnace at 550–600 °C. Carbohydrate was obtained by the formula of 100 – (the sum of moisture, protein, fat, and ash).
2.2.2 Amino acid composition
Elastin samples were hydrolysed in 6 mol/L HCl at 110 °C for 16 h. The hydrolysate was dissolved in deionised water and filtered. The amino acid composition was obtained using a High-Performance Liquid Chromatography (HPLC), equipped with a Waters 410 Scanning Fluorescence and AccQ Tag column (3.9 × 150 mm) (Waters, Dublin, Ireland). AccQ Tag Eluent A and AccQ Tag Eluent B or 60% acetonitrile acid was used as the mobile phase (flow rate = 1 mL/min). For amino acid quantification, measurement of the absorbance was done at 248 nm, while the associated fluorescence detector was measured at excitation and emission of 250 nm and 395 nm respectively.
2.2.3 Solubility of elastin powder
The solubility of elastin powder was according to the method by Lakka and Goswami (2012) with slight modification. The solubility was investigated by measuring the solubility of elastin powder using buffers of various pH (4.5 (sodium acetate buffer), 6.8 (phosphate buffer), 7 (control pH), 7.4 (phosphate buffer), 8.2 (coupling buffer)). A weighted test sample of about 0.5 g (W1) of elastin powder was added to 100 mL of different buffer solution in a water bath shaker at 30 °C for 4 h. Then, the sample was centrifuged at 5000 rpm for 20 min. The supernatant was discarded, and the pellet was put on the filter paper (Whatman no 1) to remove the excess water (W2). The pellet was then dried in the oven overnight and weighed (W3). The percentage of total solubility was calculated as below:
2.3 Formulation of the moisturiser cream
Moisturiser cream was prepared by containing the components shown in Table 1. The moisturiser cream was formulated by mixing the pre-heated water phase and oil phase materials. The mixture was cooled down and stirred until the desired product is obtained.
Compound
Function
% W/W
Deionised water
Solvent
90.40
Glycerine
Humectant
1.00
Tetrasodium EDTA
Chelating agent
0.10
Cetyl Alcohol (and) Glyceryl Stearate (and) PEG-75 Stearate (and) Ceteth-20 (and) Steareth-20
Emulsifier
3.00
Helianthus Annuus (Sunflower) Seed Oil
Emollient
2.00
Caprylic/Capric triglyceride
Emollient
2.00
Elastin powder
Active compound
0.50
Phenoxyethanol (and) Ethylhexylglycerin
Preservative
0.80
Parfum
Fragrance
0.20
2.3.1 Storage conditions
Moisturiser cream was put in a clear, clean universal bottle with a screw-type lid ensuring proper closing avoiding any vapour or gas losses to the environment. Three replicates were prepared for each condition. The replicates were put on a laboratory bench for room temperature (RT) and the designated oven was used for storing samples at 40 °C and 50 °C. The replicates sample placed in RT and 40 °C were evaluated for physical, colour, and pH changes for 12 weeks. Samples placed in 50 °C were evaluated at 12 weeks only. The physical test was done using observation and viscometer technique. Any physical changes observed during the evaluation period were recorded.
2.3.2 Measurement of colour
Colour changes were determined using Minolta Spectrophotometer Model CM-3500d Color Spectrophotometer. Measurements were done in the CIE L*a* b colour space system. The parameters monitored as follows: a* defines green to red ratio, b* defines yellow to blue ratio, L* defines light to dark ratio. However, only L* was observed and applicable to this study.
2.3.3 Measurement of viscosity
Brookfield DV-11 + Pro Programmable, digital rotational viscometer was used to determine product viscosity using a selected spindle. The viscosity of the moisturiser cream was measured using spindle no. 29 at 6 rpm–60 rpm as recommended by the manufacturer based on the texture of the product studied which is a thick cream.
2.3.4 Measurement of pH
pH values were measured using benchtop Seven Compact pH/ion meter S220E. The instrument was calibrated before each measurement was taken with standard buffer solutions (pH 4 and pH 7). Samples’ pH value from day 0 was compared to determining changes that occurred during stability testing.
2.3.5 Microbiology stability
The test was done for every storage condition to determine the Aerobic Plate Count (APC) and Yeast and Mould Count (YMC) at 24 h and 12 weeks of storage. The test was performed according to APHA – Compendium of Methods for the Microbiological Examination of Foods 4th Edition as reference standards (Downes and Ito, 2001)
2.4 Statistical analysis
All measurements were repeated in triplicates and microbial counts were transformed into logarithms of the number of CFU (log10 CFU/g). Data were subjected to analysis of variance (ANOVA) using SPSS.
3 Results and discussion
3.1 Proximate composition of elastin powder
The results of proximate composition analyses of extracted elastin are shown in Table 2. The dry weight of the elastin powder consisted of a high percentage of protein (51%), low crude fat content (2.5%) and 13% and 7% of moisture and ash content respectively. The remaining percentage of the proximate composition which is 26.5%, represents the carbohydrate content. The data on the proximate analysis of the other sources of elastin is scarcely reported. However, a previous study showed elastin peptide extracted from Pacific yellowtail fish consists of 93% protein content (Shiratsuchi et al., 2016). High protein content can create a suitable environment for healthy skin due to protein's ability to bind water with the skin layer (Guzmán-alonso and Cortazár, 2016). The amino acids from this protein particularly hydrophilic amino acids (asparagine, glutamine, serine, threonine, lysine, arginine and histidine) may react with glycerin as a humectant to increase water retention (Arezki et al., 2017), hence aids in retaining the skin hydration.
Proximate composition
%
Crude fat
2.46 ± 0.31
Crude protein
51.18 ± 0.52
Ash
7.89 ± 0.06
Moisture
13.63 ± 0.50
3.2 Amino acid composition of extracted poultry elastin
Table 3 shows the composition of amino acids for the extracted elastin powder. Elastin purity was measured by high levels of glycine, alanine, proline, and valine, and low polar amino acid content (aspartate, glutamate, lysine, histidine, and arginine) (Mecham, 2008; Starcher & Galione, 1976). Glycine (6.06% ± 0.06) and proline (3.52% ± 0.04) contents are high in this extracted elastin. Glycine and proline contents are lower when compared to the work done by Nadalian et al. (2019) due to high protein content as recorded in the study. Methionine and histidine contents are at 0.3% and 1.2%, respectively, and their low content indicates the high purity of the extracted elastin. The presence of high methionine and histidine is related to the collagen fibrils and microfibril residues which could affect the purity of the extracted elastin (Shoulders and Raines, 2009). Glycine and proline contents present in fish elastin were higher than those quantified in this extracted poultry elastin (Nakaba et al., 2006; Shiratsuchi et al., 2016). However, the amino acid composition of elastin from squid (Ramírez-Guerra et al., 2019) and tannery waste (Yoseph et al., 2020) was almost similar to poultry elastin.
Amino acid
Amino acid composition (%)
Aspartic acid
2.81 ± 0.02e
Serine
2.04 ± 0.01h
Glycine
6.06 ± 0.06a
Glutamate
2.53 ± 0.10f
Histidine
1.17 ± 0.06j
Arginine
3.09 ± 0.04d
Threonine
1.43 ± 0.01i
Alanine
3.09 ± 0.01d
Proline
3.52 ± 0.05c
Tyrosine
1.48 ± 0.04i
Valine
2.29 ± 0.04g
Methionine
0.30 ± 0.01k
Lysine
2.20 ± 0.06gh
Isoleucine
2.06 ± 0.03h
Leucine
3.70 ± 0.06b
Phenylalanine
2.19 ± 0.04gh
Hydroxyproline
0.30 ± 0.26k
The total content of Gly, Pro, Ala, and Val was 15%. Conversely, the contents of acidic amino acid residues, Asp and Glu, and basic amino acid residues, Lys, His, and Arg, were relatively lower which were 5.5% and 6.4%, respectively. High hydrophobic amino acids (Gly, Pro, Ala, Val) in water-soluble elastin extracted from the porcine aorta and edible bird’s nest hydrolysate were also shown in the study by Inoue et al. (2017) and Gan et al. (2020). Three main amino acid components of the extracted elastin (Gly, Ala, and Pro) are also present in high amounts in the human epidermis (Ohara et al., 2010). It has therefore been suggested that this extracted poultry elastin is a useful cosmetic material, which can supply the epidermis with the abovementioned beneficial amino acids. Delivering these essential amino acids to the skin is crucial to re-nourish the skin and to improve the skin's elastin content.
Previous studies carried out by Yusop et al. (2016) and Nadalian et al. (2019) showed that poultry elastin has amino acids that contain antioxidant and ACE inhibitory properties due to the presence of hydrophobic amino acids (Val and Pro) in the peptide. These bioactivity properties are beneficial to counteract the action of free radicals that can damage the skin (Aguirre-Cruz et al., 2020; Mota et al., 2014). However, the exact mechanism of peptides that function as antioxidants is not clearly understood. It is reported that certain aromatic amino acids and histidine play an important role in the action (Elias et al., 2008). The amount of proline and valine were noticeably high in this present study but the amount was lower than reported by Yusop et al. (2016) and Nadalian et al. (2019). Regardless, it still contributed to the antioxidant properties of the elastin. A slight difference in extraction method will affect the form of elastin extracted thereby contributing to the variation of the amino acid contents in both studies. Nonetheless, this extracted poultry elastin can be considered a good source of natural antioxidants to substitute for the use of synthetic antioxidant ingredients for its high nutritional and therapeutic values.
3.3 Solubility of extracted poultry elastin
Solubility is the amount of protein in a sample that dissolves into solution. There are a variety of extrinsic and intrinsic factors which may affect solubility. Extrinsic factors that influence the solubility of proteins include pH, ionic strength, temperature, and various solvent additives (Kramer et al., 2012). Elastin, a protein of the connective tissue, is insoluble in water. Incorporation of acid hydrolysis in the extraction method has been resulted in obtaining a water-soluble elastin powder in this study. The peptide bonds of the insoluble elastin are hydrolysed during the hydrolysis process, thus fragmenting the insoluble elastin and reducing its molecular weight (Daamen et al., 2001).
Fig. 1 shows the effect of different pH on the solubility of elastin powder. Elastin powder has high solubility (>80%) at all pH except at pH 8.2. High solubility makes handling and analysis of the elastin powder more straightforward. The solubility of the extracted elastin is important to ensure crystallisation processes do not occur during formulation making. Thus, this water-soluble elastin powder is suitable for use in water-based cosmetics, so that it easier to manipulate and more practical for formulating purposes. At neutral pH (pH 7.0) the total solubility percentage was the highest, indicating the uniqueness of elastin structure to bind with water molecules and enhance the solubility. Extracted elastin powder has a function as an active ingredient to provide benefits such as anti-wrinkle and firmness to the skin. Thus, by having high solubility, the delivery of this active compound will increase the effectiveness of the moisturiser.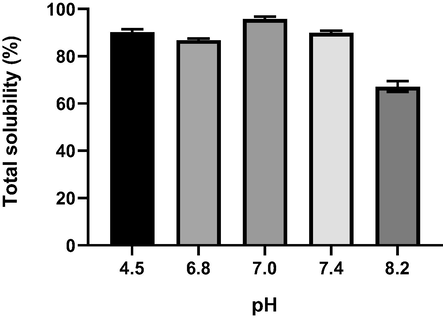
Total solubility of elastin powder extracted from poutry skin at various pH.
3.4 Observation (physical appearance) of the moisturiser cream
Moisturiser cream is off white and has a creamy texture. The odour of the product is perfume smell. Product physical characteristics did not show any changes after 4 weeks of storage at 50 ⁰C ± 0.6; the product colour maintains its original colour (white opaque) and the product smell is consistent as in day 0.
Similar observations were evident when the product was stored at RT and 40 ⁰C ± 0.7 where it did not show any physical changes (product colour and odour) after 12 weeks of study. Besides that, no differentiation of the phases was observed until the end of the experiment. This was probably due to the right homogenisation speed during emulsion formulation that could have stopped formulations from breaking during testing (Re et al., 1999).
3.5 Physical-chemical characteristics
3.5.1 Viscosity
Viscosity is a variable that characterises a system in its rheological aspect especially its flow properties. The evaluation of this parameter helps to evaluate whether a product has the correct consistency or fluidity, and may indicate whether reliability is sufficient and thus indicate the behaviour of the product over a period of time (Nasirideen et al., 1998). Viscosity readings recorded until week 12 were shown in Fig. 2. The viscosity recorded was around 7000–8000 mPa.s. Results showed that the viscosity reduced as the temperature increased. Thus, it was expecting to have to decrease in viscosity when subjected to body temperature as compared to when it was stored at room temperature. Overall, it was observed that viscosity decreased by 7% at Week 4, 15% at Week 8 and 14% at Week 12 when compared to initial viscosity (day 0) at 40 °C. The changes are much lesser at RT where the viscosity decreased by 2% at Week 4, 6% at Week 8 and 8% at Week 12 when compared to initial viscosity (day 0). However, data obtained showed that the viscosity readings are within the product's specification range which is ± 50% from the baseline (day 0) indicating product stability. Thus, the inclusion of elastin powder in the formulation has minimal effects on the emulsion viscosity throughout the testing period. The consistency of the viscosity is favourable for future testing such as sensory evaluation that provide panellists to experience attributes such as feel and flow at constant product application conditions throughout the testing period.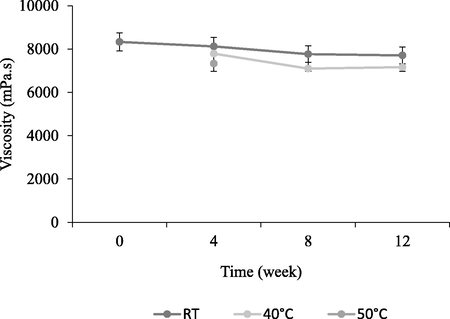
Moisturiser cream viscosity at 12 rpm from 0 day to 12 weeks of storage.
3.5.2 Colour
Colour changes may be caused by chemical interactions between raw materials in the formulation such as oxidation or grouping effects of ingredients used. From the data obtained, the colour is slightly decreased by 0.98% at Week 4, 0.77% at Week 8 and 0.51% at Week 12 respectively when compared to the initial colour reading, L*: 88.53 as shown in Fig. 3. Generally, it was observed that no significant changes found in the colour test for products with changes in temperature until Week 12. However, the colour changes are still within an acceptable range, which is ± 10% from the initial colour reading.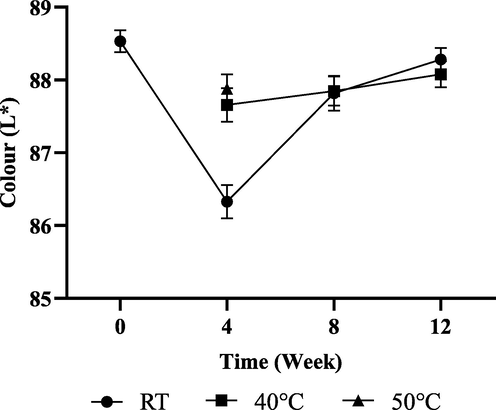
Moisturiser cream light to dark ratio (L*) from 0 day to 12 weeks of storage.
3.5.3 pH measurement
The pH of a product is very important because any changes in reading could provide information on product instability or contamination that occurred directly or indirectly during product preparation. Changes in pH values also indicate the possible chemical reaction might occur between the raw materials used in the product formulation as well as affecting the efficacy and the safety of the product (Some et al., 2000).
The pH of human skin is usually between 4.5 and 6.0. Therefore, the pH of skin care products should be included in this range to allow a formulation to be approved for industrial use (Matousek et al., 2003). A pH value of 6.04 was observed for the emulsions formulated in this work, which is close to the neutral pH. The pH of the product was gradually decreased by 3.97% at Week 4, 2.00% at Week 8 and 1.49% at Week 12 when compared to the initial pH of 6.04 at RT. On the other hand, slight changes of the samples were observed at 40 °C. The changes in the pH probably due to the growth of yeast and mold, as shown in Table 4. However, the decrement of data is within the acceptable limit which is ± 10% from the initial pH measurement. Fig. 4 showed that the product formulation is stable for all conditions tested and therefore passed the pH stability test and can be acceptable. The pH of cosmetic products should remain within a certain range, so that the colour, viscosity, skin feeling, and physicochemical stability remain intact throughout their shelf life and by the user throughout their application. APC Aerobic plate count. YMC Yeast and mold count. TNTC Too numerous to count.
Storage condition
Storage
Room temperature (RT)
24 h
12 weeks
APC
<100 cfu/g
TNTC
YMC
<100 cfu/g
<100 cfu/g
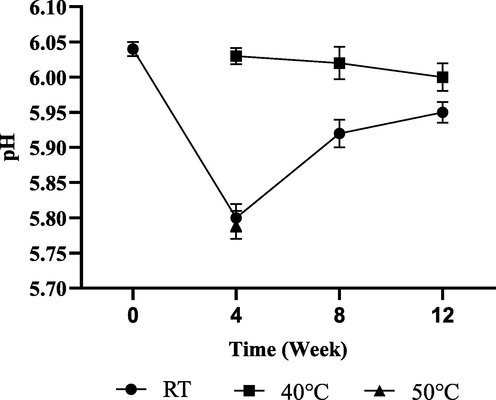
Moisturiser cream pH value from 0 day to 12 weeks of storage.
3.6 Microbiology limit testing
Moisturiser cream was evaluated at defined time intervals to evaluate the effectiveness of the preservative system necessary for the protection of the product from microbial growth or contamination. The tests were conducted at RT for 24hrs (day 0) and 12 weeks. The parameters monitored were Aerobic Plate Count (APC) and Yeast and Mould Count (YMC). Yeast and moulds have been tested for microbiological safety and product quality during processing and storage in cosmetics products. The model moisturiser was found stable until 12 weeks of storage. However, after 12 weeks, the results obtained from the APC test showed that the sample contains some microbiological activity where the results are TNTC. The result shows that the colony formation unit (cfu) is>100 cfu/g of sample for APC but<100 cfu/g for YMC as shown in Table 4. This could suggest a more efficient preservative can be used in future products' formulations to prevent aerobic growth in enrichment culture that is effective in controlling a broad spectrum of microbes while being non-toxic. The requirement for microbiology quality control limit (based on US FDA 2017) is <103 cfu/g or 103 cfu/ml.
4 Conclusion
Based on the results obtained, the developed model moisturiser cream containing elastin subjected to 3 different storage conditions (RT, 40 °C and 50 °C) was stable throughout the 12 weeks of storage. No apparent changes observed in viscosity and colour of the product, which is found to be within the product specification range and a slight change in pH. Chemical reactions can be occurred over time, resulting in colour shifts, odour shifts, and pH drift. Thus, minimal changes in these characteristics are important for the products to be aesthetically pleasing to use. The samples, which enriched with pure, high-quality elastin, potentially improve skins condition and elasticity as it delivers as a natural elastic biomaterial with numerous amino acids of which glycine is the most abundant. Formulation improvement to include a better preservative control can be made to prolong the shelf life and stability of the cream to be further than 12 weeks. From the results, it could be suggested that the poultry-based elastin showed good compatibility with other cosmetic ingredients in the model moisturising cream and can be used as a potential active ingredient in cosmetic formulation.
Acknowledgements
This work was supported in part by grants from FRGS/1/2018/STG05/UKM/02/8 and PRGS/1/2016/WAB01/UKM/02/2. The authors wish to thank Industrial Biotechnology Research Centre, SIRIM Berhad for the services.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Design, synthesis and characterization of linear unnatural amino acids for skin moisturization. Int. J. Cosmet. Sci.. 2017;39:72-82.
- [CrossRef] [Google Scholar]
- Collagen hydrolysates for skin protection: oral administration and topical formulation. Antioxidants. 2020;9
- [CrossRef] [Google Scholar]
- Skin connective tissue and ageing. Best Pract. Res. Clin. Obstet. Gynaecol.. 2013;27:727-740.
- [CrossRef] [Google Scholar]
- Chen, Z., Shin, M.H., Moon, Y.J., Lee, S.R., Kim, Y.K., Seo, J.E., Kim, J.E., Kim, K.H., Chung, J.H., 2009. Modulation of elastin exon 26A mRNA and protein expression in human skin in vivo. Exp. Dermatol. 10.1111/j.1600-0625.2008.00799.x.
- Comparison of five procedures for the purification of insoluble elastin. Biomaterials. 2001;22:1997-2005.
- [CrossRef] [Google Scholar]
- Compendium of Methods for the Microbiological Examination of Foods. Washington D.C.: American Public Health Association; 2001.
- Aging in elderly: chronological versus photoaging. Indian J. Dermatol.. 2012;57:343-352.
- [CrossRef] [Google Scholar]
- Age-associated skin changes in innate immunity markers reflect a complex interaction between aging mechanisms in the sebaceous gland. J. Dermatol.. 2015;42:467-476.
- [CrossRef] [Google Scholar]
- Antioxidant activity of proteins and peptides. Crit. Rev. Food Sci. Nutr.. 2008;48:430-441.
- [CrossRef] [Google Scholar]
- Ovine tendon collagen: extraction, characterisation and fabrication of thin films for tissue engineering applications. Mater. Sci. Eng. C. 2016;68:163-171.
- [CrossRef] [Google Scholar]
- Evaluation of physicochemical properties, amino acid profile and bioactivities of edible Bird's nest hydrolysate as affected by drying methods. LWT. 2020;131:109777
- [CrossRef] [Google Scholar]
- Water content at different skin depths and the influence of moisturizing formulations. Househ. Pers. Care Today. 2016;11:35-40.
- [Google Scholar]
- Investigation of water-soluble elastin as a multifunctional cosmetic material: Moisturizing and whitening effects. J. Cosmet. Sci.. 2017;68:11-23.
- [Google Scholar]
- Utilization of byproducts and waste materials from meat, poultry and fish processing industries: a review. J. Food Sci. Technol.. 2012;49:278-293.
- [CrossRef] [Google Scholar]
- Japanese guidelines for atopic dermatitis 2017. Allergol. Int.. 2017;66:230-247.
- [CrossRef] [Google Scholar]
- Toward a molecular understanding of protein solubility: Increased negative surface charge correlates with increased solubility. Biophys. J.. 2012;102:1907-1915.
- [CrossRef] [Google Scholar]
- Solubility and dissolution profile studies of gliclazide in pharmaceutical formulations By Rp-Hplc. Int. Res. J. Pharm.. 2012;3:126-129.
- [Google Scholar]
- Evaluation of the effect of pH on in vitro growth of Malassezia pachydermatis. Can. J. Vet. Res.. 2003;67:56-59.
- [Google Scholar]
- Methods in elastic tissue biology: elastin isolation and purification. Methods. 2008;45:32-41.
- [CrossRef] [Google Scholar]
- Biomechanics of synthetic elastin: insights from magnetic resonance microimaging. Adv. Mater. Res.. 2013;699:457-463.
- [CrossRef] [Google Scholar]
- Antioxidant activity of cosmetic formulations based on novel extracts from seeds of Brazilian Araucaria angustifolia (Bertoll) Kuntze. J. Cosmet. Dermatological Sci. Appl.. 2014;04:190-202.
- [CrossRef] [Google Scholar]
- Effects of enzymatic hydrolysis on the antioxidant activity of water- soluble elastin extracted from broiler and spent hen skin. Int. J. Appl. Biol. Pharm. Technol.. 2015;6:1-10.
- [Google Scholar]
- Isolation, purification and characterization of antioxidative bioactive elastin peptides from poultry skin. Food Sci. Anim. Resour.. 2019;39:966-979.
- [CrossRef] [Google Scholar]
- Properties of soluble elastin peptide from bulbus arteriosus in fish species. Fish. Sci.. 2006;72:1322-1324.
- [CrossRef] [Google Scholar]
- Naproxen incorporated lipid emulsions. I. Formulation and stability studies. J. Clin. Pharm. Ther.. 1998;23:57-65.
- [CrossRef] [Google Scholar]
- Collagen-derived dipeptide, proline-hydroxyproline, stimulates cell proliferation and hyaluronic acid synthesis in cultured human dermal fibroblasts. J. Dermatol.. 2010;37:330-338.
- [CrossRef] [Google Scholar]
- Physicochemical and structural properties of recovered elastin from jumbo squid (Dosidicus gigas) by-products. J. Aquat. Food Prod. Technol.. 2019;28:275-286.
- [CrossRef] [Google Scholar]
- Antioxidant activity applying an improved ABTS radical. Free Radic. Biol. Med.. 1999;26:1231-1237.
- [Google Scholar]
- Elastin hydrolysate derived from fish enhances proliferation of human skin fibroblasts and elastin synthesis in human skin fibroblasts and improves the skin conditions. J. Sci. Food Agric.. 2016;96:1672-1677.
- [CrossRef] [Google Scholar]
- Collagen structure and stability ann rev biochemistry. Annu. Rev. Biochem.. 2009;78:929-958.
- [CrossRef] [Google Scholar]
- Improved kinetic parameter estimation in pH-profile data treatment. Int. J. Pharm.. 2000;198:39-49.
- [CrossRef] [Google Scholar]
- Purification and comparison of elastins from different animal species. Anal. Biochem.. 1976;74:441-447.
- [CrossRef] [Google Scholar]
- Sugitani, H., Hirano, E., Knutsen, R.H., Shifren, A., Wagenseil, J.E., Ciliberto, C., Kozel, B.A., Urban, Z., Davis, E.C., Broekelmann, T.J., Mecham, R.P., 2012. Alternative splicing and tissue-specific elastin misassembly act as biological modifiers of human elastin gene frameshift mutations associated with dominant cutis laxa. J. Biol. Chem. 287, 220555–22067. 10.1074/jbc.M111.327940.
- In vitro evaluation of the effects of human umbilical cord extracts on human fibroblasts, keratinocytes, and melanocytes. Vitr. Cell. Dev. Biol. - Anim.. 2014;50:321-330.
- [CrossRef] [Google Scholar]
- Anticedants and natural prevention of environmental toxicants induced accelerated aging of skin. Environ. Toxicol. Pharmacol.. 2015;39:384-391.
- [CrossRef] [Google Scholar]
- Extraction of elastin from tannery wastes: a cleaner technology for tannery waste management. J. Clean. Prod.. 2020;243:118471
- [CrossRef] [Google Scholar]
- Production of antihypertensive elastin peptides from waste poultry skin. ETP Int. J. Food Eng.. 2016;2:21-25.
- [CrossRef] [Google Scholar]







