Translate this page into:
Determination of polyphenolic content, HPLC analyses and DNA cleavage activity of Malaysian Averrhoa carambola L. fruit extracts
⁎Corresponding author. Tel.: +60 9 947 7114x2114; fax: +60 9 947 7022. zakiakhanam09@gmail.com (Zakia Khanam)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
In developing countries, the increasing gap between population growth and food supply has created renewed interest in finding reliable and cheap natural resources of nutraceutical value and health promoting properties. Therefore, the present study deals with the phytochemical analyses and DNA cleavage activity of Averrhoa carambola L. fruit (starfruit) extracts. The phytochemical studies involve colour tests and quantification of phenolics and flavonoids of the prepared ethanolic and aqueous extracts. Identification of phenolic acids and flavonoids present in the extracts were conducted by high performance liquid chromatography (HPLC) equipped with diode array detector (DAD). DNA cleavage activity of the extracts was evaluated through gel electrophoresis against plasmid Escherichia coli DNA at different concentrations (0.125–0.60 μg/μl). The results of the study exhibited that the starfruit is a rich source of polyphenols and all the extracts exhibited a dose dependent DNA cleavage activity, whereas ethanolic extract induced more cleavage as compared to the aqueous extract. In conclusion, the present study provides preliminary evidence with regard to nutraceutical value of the fruit. So, further extensive study is a prerequisite to exploit DNA cleaving properties of the fruit extracts for therapeutic application.
Keywords
Averrhoa carambola L.
Starfruit
DNA cleavage activity
Polyphenols
Flavonoids
1 Introduction
The most plentiful antioxidant in the diet are polyphenols, mainly obtained from fruits, vegetables, cereals, legumes and other plant derived products such as fruit juices, tea, coffee, chocolate and red wine. The total dietary intake of polyphenols is considered much higher as compared to other classes of phytochemicals and known dietary antioxidants (Scalbert et al., 2005). Among polyphenols there has been increasing interest in the research of flavonoids from dietary sources due to growing evidence of the versatile health benefits through epidemiological studies (Yao et al., 2004). Polyphenols demonstrated various pharmacological properties and recent findings strongly support its contribution in the prevention of neurodegenerative diseases, diabetes mellitus, cardiovascular disease and most importantly incurable cancer (Lambert et al., 2005; Manach et al., 2004). Diet rich in polyphenols act as antioxidant or prooxidant which may improve cell survival, induce apoptosis and prevent tumour growth (Lambert et al., 2005). Thus, the present information on protective effects of polyphenols against diseases has generated our interest to identify polyphenols and evaluate DNA cleavage activity of Malaysian tropical fruit, Averrhoa carambola L. (starfruit). Designing drugs from natural resources by DNA cleavage may serve as a lead for the development of novel anti cancer agent like bleomycin which works by causing breaks in DNA (Akhand et al., 1998; Moseley, 1989). Recently various researchers have evaluated the DNA cleaving potential of polyphenol rich extracts including Aloe vera, Citrus maxima and some brown algae (Blunden et al., 1994; Gopalakrishnan and Vadivel, 2011; Khanam et al., 2014; Naqvi et al., 2010). A. carambola is a unique tropical fruit from oxalidaceae family originated from Sri Lanka and Indonesia but has been cultivated in Southeast Asia and Malaysia for many centuries (Morton, 1987). It is low in calories and rich in vitamin C, vitamin A, calcium and potassium. It is a potential source of both primary and secondary polyphenolic antioxidants such as alkaloids, flavonoids, saponins, and tannins (Avinash et al., 2012; Gheewala et al., 2012). Traditionally, star fruit is used to treat eye afflictions, throat pain, toothache, dysuria, scurvy, eczema, fever, hyperdipsia, haemorrhoids, hepatodynia, vomiting, scabies and various kinds of poisoning and general debility (Gheewala et al., 2012; Sheth, 2005). The in vitro and in vivo studies conducted on various parts of the plant exhibited impeccable biological activities (Gheewala et al., 2012; Sripanidkulchai et al., 2002; Thomas et al., 2008). To the best of our knowledge no report is available pertaining to DNA cleavage activity and phytochemical analysis of Malaysian A. carambola fruit of variety ‘belimbing manis’. Thus, the present paper deals with the quantitation and identification of polyphenols; phenolic acids and flavonoids as well as evaluation of pathogenic Escherichia coli DNA cleavage potential of aqueous and ethanolic extract of A. carambola fruit. The results of the present study will provide information regarding health promoting factors of A. carambola fruit commonly consumed in Malaysia and other neighbouring countries.
2 Materials and methods
2.1 Plant materials and extraction
The fresh fruits of A. carambola L. of variety ‘belimbing manis’ (sweet variety) were purchased from local market of Jeli, Kelantan, Malaysia. Samples of uniform shape, size, colour and ripening stage were selected with no apparent physical and microbial damages. The fruits were thoroughly washed, peeled manually and the edible part (100 g) was cut into small pieces. The homogenized sample was immersed in 200 ml of ethanol on water bath at 40 °C. During the extraction conical flask was covered with aluminium foil to prevent direct sunlight exposure. After 24 h, the fruit extract was filtered on whatman filter paper and the filtrate was evaporated to dryness under reduced pressure at 40 °C using rotary evaporator. The crude extract was collected and kept in the dark glass bottle at −4 °C for further study. The same procedure was followed for the preparation of aqueous extract of the fruit.
2.2 Phytochemical screening
The extracts were analysed for the presence of phenolic compounds, flavonoids, terpenoids, saponins, alkaloids, cardiac glycosides and chalcones following the method of Raaman (2006).
2.3 Determination of total phenolic content (TPC)
The total phenolic content of the extracts was determined spectrophotometrically by using Folin–Ciocalteu (FC) reagent described by Singleton et al. (1999) with slight modifications. Briefly, 0.5 ml of methanolic solution of extract (1 mg/ml) was added into 2.5 ml of 10% FC reagent, previously diluted with distilled water. After 8 min, 2.5 ml of 7.5% sodium hydrogen carbonate (NaHCO3) was added. Blank sample was prepared by adding 0.5 ml of methanol, 2.5 ml of 10% FC reagent and 2.5 ml of 7.5% NaHCO3. The mixture was covered with aluminium foil, vortexed for 10 s and allowed to stand for 1 h in dark at 45 °C. The absorbance was measured at 765 nm, using a UV–vis spectrophotometer. A calibration curve was prepared with gallic acid as standard at various concentrations (20, 40, 60, 80 and 100 mg/l, y = 0.0099x–0.0478, R2 = 0.995). The results were expressed as mg gallic acid equivalents (GAE)/g of extract on dry weight (dw) basis. All the measurements were taken in triplicate and the mean values were calculated.
2.4 Determination of total flavonoidic content (TFC)
The total flavonoidic content of the extracts was determined spectrophotometrically using the aluminium trichloride colorimetric method with slight modification (Marinova et al., 2005). Briefly, methanolic solution of the extract (1 mg/ml) was mixed with 0.3 ml of 5% (w/v) sodium nitrite (NaNO2) solution. After 5 min, 0.3 ml of 10% aluminium trichloride (AlCl3) solution was added to the mixture. Then, after 6 min, 2 ml of 1 M sodium hydroxide (NaOH) solution was added followed by which the volume of reaction mixture was made up to 10 ml with distilled water and vortexed for 10 s. The absorbance was measured at 510 nm after pink colour was developed using UV–vis spectrophotometer. A calibration curve was prepared with quercetin as standard at various concentrations (20, 40, 60, 80 and 100 mg/l, y = 0.0099x–0.0478, R2 = 0.965). The results were expressed as mg quercetin equivalents (QE)/g of extract on dry weight (dw) basis. All the measurements were taken in triplicate and the mean values were recorded.
2.5 HPLC analyses of extracts
HPLC analyses of the fruit extracts were performed on Shimadzu HPLC system equipped with a Diode Array Detector (DAD). About 5 μl of analyte solution was injected into HPLC valve using a Puradisc 25 mm (Sterile and Endotoxin Free 0.2 μm PES Filter Media) and Terumo Syringe (5 cc/ml) at room temperature. Phenolic compounds were separated on a Thermo Scientific Hypersil Gold reverse phase (RP-18) column, 250 mm × 4.6 mm (Merk) packed with C18 stationary phase with particle size of 5 μ. The gradient was maintained at a flow rate of 1 ml/min and the time of HPLC run was over 10 min. The binary mobile phase consisted of a solvent A (water: acetic acid; 99:1; v/v) and solvent B (acetonitrile). The gradient elution from the column was achieved with 13% of solvent B until 10 min. The phenolic acids and flavonoids were detected by a UV detector at wavelength 200–500 nm. The chromatographic peaks were identified by comparing retention time of analytes with that of reference compounds.
2.6 Plasmid DNA extraction from E. coli
Plasmid DNA was isolated from E. coli culture by the conventional method reported elsewhere (Yadav et al., 2011). The E. coli solution was added in a test tube containing detergent. The test tube was capped and slowly inverted several times to mix the solution without foaming. The test tube was placed in a hot water bath at 55–60 °C for 10 min and about two drops of contact lens cleaning solution were mixed gently. The test tube was reheated on water bath at 55–60 °C for 20 min. Later, plasmid DNA was precipitated by adding cold absolute ethanol into the test tube. The solution was left stand for 2–3 min and the DNA precipitate was spool on to a glass rod from alcohol layer. The DNA was air dried and suspended in 300 μl TAE buffer (10 mM Tris, pH 7.5, 1 mM EDTA).
2.7 DNA cleavage activity
The DNA cleavage of supercoiled E. coli plasmid DNA was carried out using agarose gel electrophoresis, according to the method by Dhanaraj and Nair (2008) with slight modifications. About 1.0 g of powdered agarose was mixed in 100 mL of 1× TAE buffer (working) and heated to dissolve agarose completely. The ethidium bromide (1.0 μg/ml) was added to the above solution and mixed well. The molten agarose was cooled to 60 °C before pouring into cassette and clamped immediately with comb to form sample wells. After setting of gel, the comb was removed and the gel was mounted into electrophoretic tank filled with working buffer. The samples at various concentrations (0.125–0.600 μg/μl) along with plasmid DNA (15 μl) and loading dye were filled into the wells (40 μl) of the submerged gel using a micropipette. The plasmid DNA without extracts is taken as control. The gel electrophoresis was carried out at 60 V and finally, gel was photographed under a UV transilluminator.
2.8 Statistical analyses
All data were expressed as means ± standard deviations (SD) of triplicate measurements. All data were analysed by a one-way analysis of variance (ANOVA) using the Statistical Package for the Social Sciences (SPSS) software. ANOVA was used to determine the significant differences (P < 0.01) between the means.
3 Results and discussion
3.1 Phytochemical screening, total phenolic content (TPC) and total flavonoidic content (TFC)
There are several thousands of polyphenols that have been identified in higher plants among which several hundreds are present in edible plants (Manach et al., 2004). It consists of a wide variety of compounds and may be classified into different groups on the basis of the number of phenol rings and the structural elements that bind these rings to one another; such as phenolic acids, flavonoids, stilbenes, and lignans (Manach et al., 2004). Phenolic compounds give unique taste, flavour, colour and health-promoting properties to vegetables and fruits which have been reportedly linked to their antioxidant capacity (Wang, 2006). Today there are several well established reports that evidenced that polyphenols could be administered as supplements or with food, to improve the health status of an individual (Scalbert et al., 2005). The preliminary phytochemical screening (colour test) of ethanolic and aqueous extracts of A. carambola fruit showed the presence of phenolic acids, flavonoids, terpenoids and saponins (Table 1). The total phenolic content (TPC) of the fruit extracts was measured using the Folin–Ciocalteu colorimetric method, based on the principle of reduction of phosphomolybdic acid by phenols in the presence of aqueous alkali (Varadharajan et al., 2012). TPC in aqueous and ethanol extracts ranged from 74.93 to 80.31 mg GAE/g and 90.92 to 99.55 mg GAE/g (dw), respectively (Table 2). The average TPC of aqueous A. carambola fruit extract was calculated as 77.00 ± 2.89 mg GAE/g, while ethanolic extract exhibited 97.16 ± 4.29 mg GAE/g of extract (dw). Flavonoids are secondary metabolite ubiquitous in fruits and vegetables, which protect cell from degradation, stress, act as signalling molecules, phytoalexins, detoxifying agents, reduce toxic effects and stimulants (Bala et al., 2010; Jones and Vogt, 2001). Recent researches indicated that flavonoids can be nutritionally helpful by triggering the production of natural enzymes that fight disease, hence reduce the risk of certain cancers, heart disease, and age-related degenerative diseases. It has chemopreventive role in cancer by means of their effect in signal transduction on cell proliferation and angiogenesis (Bala et al., 2010). The total flavonoidic content (TFC) of the fruit extracts was determined by using the aluminium chloride colorimetric method, based on the reaction of aluminium ion with flavonoids at alkaline medium forming red chelates (Zhu et al., 2009). The TFC ranged from 17.04 to 18.92 mg QE/g and 41.00 to 43.56 mg QE/g in aqueous and ethanolic extracts, respectively (Table 2). The average TFC of A. carambola ethanol and aqueous fruit extract was found to be 42.70 ± 1.47 mg QE/g and 18.18 ± 1.00 mg QE/g of dry extract, respectively. According to Ayub et al. (2010), the TPC of A. carambola from Mizoram, India was found to be 54 ± 0.43 mg of GAE/100 g of fresh fruit. Lately, the study conducted by Das (2012) demonstrated that ripe A. carambola fruit has lesser amount of phenolics (98 ± 12 mg GAE/100 g) as compared to unripe stage (112 ± 20 mg GAE/100 g dw) procured from West Bengal, India. Okwu and Emenike (2006) reported that the starfruit contains 0.26 mg flavonoids/100 g on dry weight basis which is much lower than the present findings. The results of this study are dissimilar from earlier observations on similar fruit with respect to TPC and TFC which was much higher in both the extracts due to different varieties. Thus, the present finding supports that the composition, content and distribution of phenolics are highly dependent on cultivar specificities, geographical origin, fruit ripeness, growing season cultural practices, as well as postharvest storage conditions (Miletić et al., 2012). The ethanolic extract showed greater amount of TPC and TFC as compared to aqueous extract and consequently ethanol performed as a better solvent with respect to water for flavonoid extraction. This can be explained probably because of the treatment with ethanol may have resulted in the precipitation of non-phenol compounds or presence of higher concentration of less polar phenolics (ethanol selective), and hence contributing to the higher flavonoidic content (Rispail et al., 2005). The statistical analysis (ANOVA) showed the significant (P < 0.01) variation in the TPC and TFC with respect to different solvents used for the extraction. The increase in TPC resulted in increased TFC and hence is positively correlated. + = present, − = absent. Results are presented as the mean of triplicate measurements (n = 3 ± SD).
Phytochemicals
Tests
Aqueous extract
Ethanol extract
Alkaloids
Wagner’s test
−
−
Phenolic acids
Ferric chloride test
+
+
Flavonoids
Alkaline reagent test
+
+
Saponins
Froth test
−
+
Terpenoids
Salkowski’s test
+
+
Cardiac glycosides
Keller–Killani’s test
+
+
Chalcones
Ammonium hydroxide test
−
−
Extract
Sample
TPC (mg GAE/g dw)
TPC (mg GAE/g dw)
TFC (mg QE/g dw)
TFC (mg QE/g dw)
Aqueous
Sample 1
74.93
77.00 ± 2.89⁎
17.04
18.18 ± 1.00⁎
Sample 2
80.31
18.92
Sample 3
75.77
18.58
Ethanol
Sample 1
99.55
97.16 ± 4.29⁎
41.00
42.70 ± 1.47⁎
Sample 2
95.70
43.56
Sample 3
90.92
43.54
3.2 HPLC analyses of A. carambola extracts
The phytochemical studies on A. carambola fruits are limited and identified the presence of merely small number of phenolics from the fruits and many remain unreported (Gheewala et al., 2012; Shui and Leong, 2004, 2006). Previous investigations on A. carambola fruits showed the presence of antioxidants such as proanthocyanidin and epicatechin (Gheewala et al., 2012). There are mainly two classes of phenolic acids based on derivative of benzoic acid and cinnamic acid. Substituted derivative of hydroxycinnamic acid is the more widespread phenolic acid than the hydroxy benzoic acid. While flavonols are the most common flavonoid, among which quercetin and kaempferol are main representatives (Manach et al., 2004). The HPLC analyses conducted on A. carambola fruit demonstrated the presence of various phenolic acids and flavonoids such as gallic acid, 4-hydroxycinnamic acid, 4-hydroxy-3-methoxycinnamic acid, vanillic acid, kaempferol, luteolin, naringenin, and quercetin in both aqueous and ethanol extracts (Table 3). Trans-cinnamic acid and caffeic acid were undetectable in both extracts. As polyphenols, phenolic acids are also powerful antioxidants and demonstrated various health benefits by exhibiting antibacterial, antiviral, anticarcinogenic, anti-inflammatory and vasodilatory actions (Duthie et al., 2000). The ethanol extract exhibited the presence of myricetin and chlorogenic acid whereas, apigenin was observed just in aqueous extracts of A. carambola fruit. Flavones are less common in fruits and vegetables as compared to flavonols and to date the edible sources of flavone i.e., luteolin and apigenin are identified in parsley and celery (Manach et al., 2004; Justesen et al., 1998). The analyses of starfruit showed the presence of these flavones and could be utilized as dietary source of luteolin and apigenin. Among all the tested flavonoids, quercetin (65.66 ± 0.12%) was observed in the highest amount followed by kaempferol (3.32 ± 0.67%), luteolin (1.39 ± 0.80%), naringenin (1.38 ± 0.23%) and apigenin (0.36 ± 0.81%) in aqueous extract of A. carambola fruit (Table. 3, Fig. 1). In case of ethanolic extract luteolin (11.40 ± 0.39%) was detected in greater percentage as compared to kaempferol (4.25 ± 0.41%), naringenin (3.43 ± 0.82%), myricetin (1.77 ± 0.43%) and quercetin (0.37 ± 0.11%), among flavonoids (Table. 3, Fig. 2). The HPLC chromatogram of ethanolic and aqueous extracts exhibited gallic acid (6.47 ± 0.37%) and vanillic acid (2.41 ± 0.52%) in the highest quantity as compared to the other tested phenolic acids, respectively. Besides, chlorogenic acid (1.94 ± 0.25%) was found only in ethanol extract of the fruit. Results are presented as the mean of triplicate measurements (n = 3 ± SD), + = present, − = absent.
Standard
Aqueous extract
Ethanol extract
Present/Absent
Area (%)
Present/Absent
Area (%)
Caffeic acid
−
−
−
−
Chlorogenic acid
−
−
+
1.94 ± 0.25
Gallic acid
+
1.96 ± 0.59
+
6.47 ± 0.37
4-Hydroxycinnamic acid
+
0.50 ± 0.56
+
3.59 ± 0.43
4-Hydroxy-3-methoxycinnamic
+
1.11 ± 0.31
+
1.87 ± 0.54
Trans-cinnamic acid
−
−
−
−
Vanillic acid
+
2.41 ± 0.52
+
4.54 ± 0.99
Apigenin
+
0.36 ± 0.81
−
−
Kaempferol
+
3.32 ± 0.67
+
4.25 ± 0.41
Luteolin
+
1.39 ± 0.80
+
11.40 ± 0.39
Myricetin
−
−
+
1.77 ± 0.43
Naringenin
+
1.38 ± 0.23
+
3.43 ± 0.82
Quercetin
+
65.66 ± 0.12
+
0.37 ± 0.11
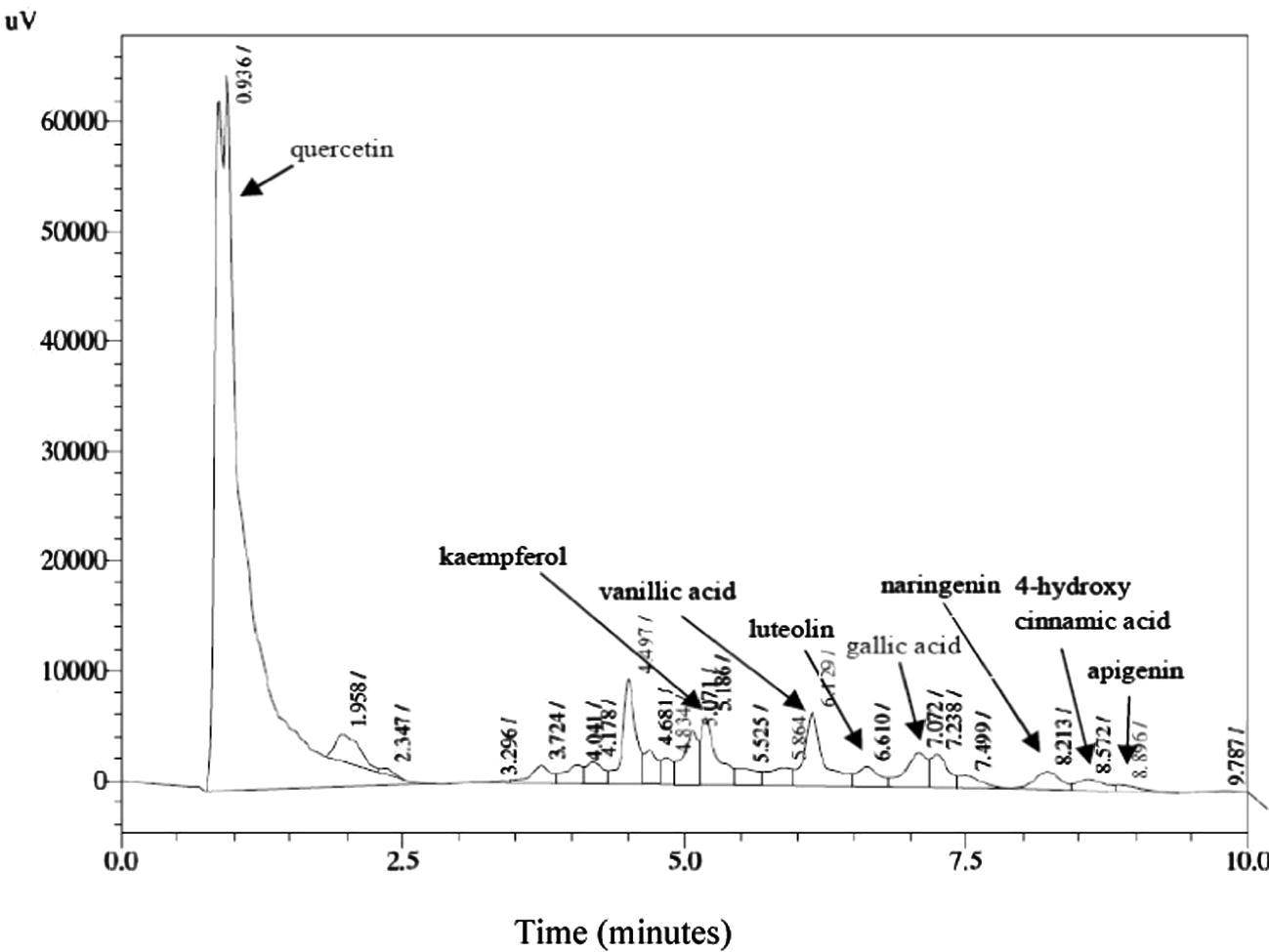
HPLC chromatogram of aqueous A. carambola fruit extract.
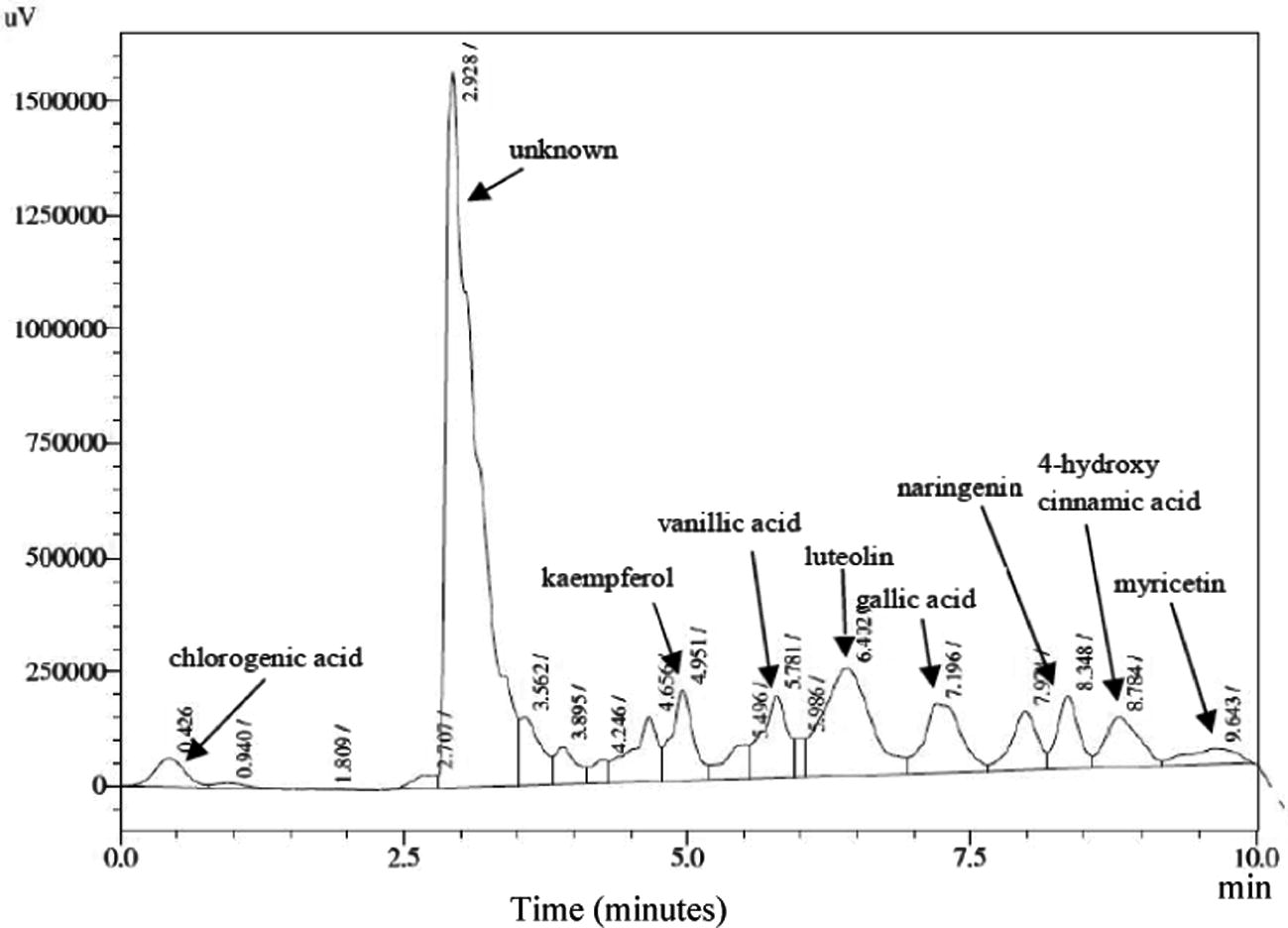
HPLC chromatogram of ethanolic A. carambola fruit extract.
3.3 DNA cleavage activity
In the present study, DNA cleavage activities were assessed by treating double stranded supercoiled plasmid DNA (Form I) isolated from E. coli with A. carambola fruit extract at different concentrations (0.125 μg/μl to 0.600 μg/μl). The cleavage of supercoiled plasmid DNA was observed by the increase in intensity or broadening of Form II and Form III band and simultaneous decrease in intensity of Form I band. Both extracts exhibited gradual decrease in the intensity of Form I band with a concurrent increase in intensity of Form II band in a dose dependent manner (Arianingrum, 2009; Jung and Lippard, 2007) (Figs. 3 and 4). The ethanolic extract showed more intense and broad nicked form (Form II) as compared to the aqueous extract with increasing concentration, suggesting its better efficiency of DNA cleavage (Gopalakrishnan and Vadivel, 2011; Ongeri, 2007). In case of ethanolic extract distortion of Form I appeared in lane 4 and 5 (Fig. 4) further corresponds to the conversion of Form I to Form II or cleavage of DNA (Ongeri, 2007). Also, the broadening of Form II band seems to begin merely at 0.125 μg/μl of ethanolic extract treatment (Fig. 4, lane 2), while aqueous extract showed the onset of broadening at higher concentration of 0.250 μg/μl (Fig. 3, lane 3). The appearance of nicked form at lower concentrations with respect to aqueous extract again indicates superior DNA cleavage activity of ethanol extract. The enhanced performance of ethanolic extract may be corresponded to the presence of higher amount of phenolics and flavonoids as compared to aqueous extract (Blunden et al., 1994; Gopalakrishnan and Vadivel, 2011; Khanam et al., 2014; Naqvi et al., 2010). The aqueous extract showed major changes in the bands at 0.500 μg/μl and 0.600 μg/μl (Fig. 3, lane 5 and 6), whereas ethanolic extract found most active at 0.375 μg/μl, 0.500 μg/μl and 0.600 μg/μl concentrations (Fig. 4, lane 4, 5 and 6). Furthermore, the conversion of nicked form to liner form (Form III) was not observed in both ethanolic and aqueous extracts of A. carambola fruit.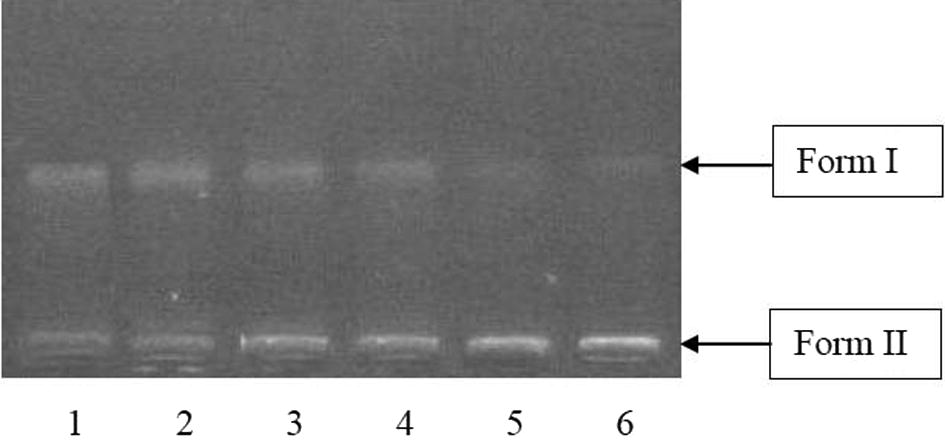
DNA cleavage activity of aqueous A. carambola fruit extract, Lane. 1: DNA (control), Lane 2: DNA + 5 μl extract, Lane 3: DNA + 10 μl extract, Lane 4: DNA + 15 μl extract, Lane 5: DNA + 20 μl extract, Lane 6: DNA + 25 μl extract.
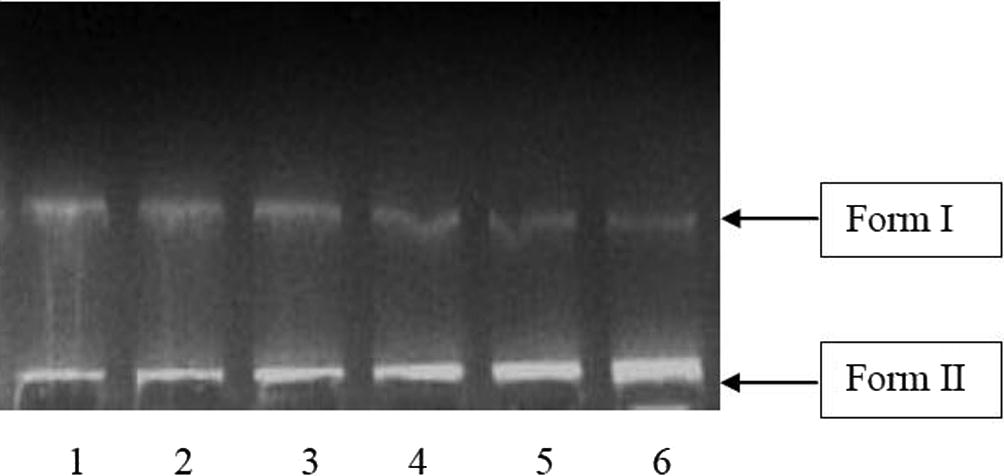
DNA cleavage activity of ethanolic A. carambola fruit extract, Lane. 1: DNA (control), Lane 2: DNA + 5 μl extract, Lane 3: DNA + 10 μl extract, Lane 4: DNA + 15 μl extract, Lane 5: DNA + 20 μl extract, Lane 6: DNA + 25 μl extract.
4 Conclusions
Quantitative and qualitative analyses of A. carambola fruit showed it to be a good source of polyphenols, where ethanol extracts contain higher TPC and TFC than the aqueous extract. HPLC analyses of both extracts identified the presence of various biologically important polyphenolic compounds which has not been reported previously in starfruit. Also, both the prepared extracts of A. carambola fruit showed good DNA cleavage activity; but ethanol extract exhibited better DNA cleaving potential as compared to the aqueous extract, which may be attributed to its high concentration of polyphenols and flavonoids. Hence, the obtained results provide preliminary evidence with regard to the nutraceutical value of star fruit and can be recommended to be incorporated into the normal diet of the general population as a dietary source of polyphenols (antioxidants). The consumption of this fruit may play a vital role in preventing human diseases or promoting general health due to the reported antioxidant potential or present findings. Therefore, further extensive study is warranted in order to exploit the potential of A. carambola fruit against certain diseases or anti-cancer drug development.
Acknowledgement
Authors would like to acknowledge Dean, Faculty of Agro Based Industry (FIAT), Universiti Malaysia Kelantan, Campus Jeli, Kelantan 17600, Malaysia for providing necessary facilities.
References
- Level of HgCl2-mediated phosphorylation of intracellular proteins determines death of thymic T-lymphocytes with or without DNA fragmentation. J. Cell. Biochem.. 1998;71:243-253.
- [Google Scholar]
- In vitro activity of supercoiled double stranded DNA cleavage by proteins extracted from sweet potato (Ipomea Batatas L.) peel. NU Sci. J.. 2009;6:73-79.
- [Google Scholar]
- A comprehensive review of an important medicinal plant – Averrhoa carambola L. Pharmacog. Commun.. 2012;2:26-39.
- [Google Scholar]
- Antioxidant activity of fruits available in Aizawl market of Mizoram, India. Int. J. Biol. Pharm. Res.. 2010;1:76-81.
- [Google Scholar]
- Evaluation of anticancer activity of Cleome gynandra on Ehrlich’s ascites carcinoma treated mice. J. Ethnopharmacol.. 2010;129:131-134.
- [Google Scholar]
- Antimicrobial and antioxidant activities of green and ripe fruits of Averrhoa carambola Linn. and Zizyphus mauritiana Lam. Asian J. Pharm. Clin. Res.. 2012;5:102-105.
- [Google Scholar]
- Electrochemical, antifungal, antibacterial and DNA cleavage studies of some Co (II), Ni (II), Cu (II) and Zn (II)-copolymer complexes. J. Mycobiol.. 2008;36:260-265.
- [Google Scholar]
- Plant polyphenols in cancer and heart disease: implications as nutritional antioxidants. Nutr. Res. Rev.. 2000;13:79-106.
- [Google Scholar]
- Phytochemical and pharmacological profile of Averrhoa carambola Linn.: an overview. Int. Res. J. Pharm.. 2012;3:88-92.
- [Google Scholar]
- DNA cleavage studies of the ethanolic extracts of the bark of Bohinia tomentosa L. and the whole plant of Mussaenda frondosa L. Int. Res. J. Pharm.. 2011;2:153-154.
- [Google Scholar]
- Glycosyltransferases in secondary plant metabolism: tranquilizers and stimulant controllers. Planta. 2001;213:164-174.
- [Google Scholar]
- Direct cellular responses to platinum-induced DNA damage. Chem. Rev.. 2007;107:1387-1407.
- [Google Scholar]
- Quantitative analysis of flavonols, flavones, and flavanones in fruits, vegetables and beverages by high performance liquid chromatography with photo-diode array and mass spectrometric detection. J. Chromatogr. A. 1998;799:101-110.
- [Google Scholar]
- Khanam Z, Ching C. H., Zakaria N. H. B. M., Sam K H., Bhat I.U.H. Phytochemical analyses and DNA cleavage activity of Citrus maxima fruit. International Conference on Chemistry and Environmental Sciences Research (ICCESR), 17-18 September, 2014. organised by Chemistry unit, Department of Applied Sciences, Universiti Technology Mara Pulau Pinang, 13500 Permatang Pauh, Penang, Malaysia.
- Inhibition of carcinogenesis by polyphenols: evidence from laboratory investigations. Am. J. Clin. Nutr.. 2005;81:284S-291S.
- [Google Scholar]
- Polyphenols: food sources and bioavailability. Am. J. Clin. Nutr.. 2004;79:727-747.
- [Google Scholar]
- Total phenolics and total flavonoids in Bulgarian fruits and vegetables. J. Univ. Chem. Technol. Metall.. 2005;40:255-260.
- [Google Scholar]
- Phenolic content and antioxidant capacity of fruits of plum cv. ‘Stanley’ (Prunus domestica L.) as influenced by maturity stage and on-tree ripening. Aust. J. Crop Sci.. 2012;6:681-687.
- [Google Scholar]
- Fruits of Warm Climates (1st ed.). Winterville, North Carolina: Creative Resources Systems Inc.; 1987.
- Augmentation of bleomycin-induced DNA damage in intact cells. Am. J. Physiol.. 1989;257:C882-C887.
- [Google Scholar]
- DNA degradation by aqueous extract of Aloe vera in the presence of copper ions. Indian J. Biochem. Biophys.. 2010;47:161-165.
- [Google Scholar]
- Evaluation of the phytonutrients and vitamins content of citrus fruits. Int. J. Mol. Med. Adv. Sc.. 2006;2:1-6.
- [Google Scholar]
- Investigation of the Mechanism of DNA Cleavage Using Ruthenium Polypyridyl Complexes. Arlington: University of Texas; 2007. M.Sc. thesis
- Phytochemical Techniques. Pitam Pura, New Delhi: New India Publishing Agency; 2006.
- Phenolic compounds: extraction and analysis. In: Marquez A.J., ed. Lotus Japonicas Handbook. Netherlands: Springer; 2005. p. :349-355.
- [Google Scholar]
- The Herbs of Ayurveda (1st eds.). India: Ashok K. Sheth Publisher; 2005.
- Analysis of polyphenolic antioxidants in star fruit using liquid chromatography and mass spectrometry. J. Chromatogr. A. 2004;1022:67-75.
- [Google Scholar]
- Residue from star fruit as valuable source for functional food ingredients and antioxidant nutraceuticals. Food Chem.. 2006;97:277-284.
- [Google Scholar]
- Analysis of total phenols and other oxidation substrates and antioxidant by means of Folin-Ciocalteu reagent. Methods Enzymol.. 1999;299:152-178.
- [Google Scholar]
- Anti-inflammatory and bactericidal properties of elected indigenous medicinal plants used for dysuria. Thai J. Pharm. Sci.. 2002;26:33-38.
- [Google Scholar]
- Pharmacognostic evaluation and physicochemical analysis of Averrhoa carambola L. fruit. J. Herbal Med. Toxicol.. 2008;2:51-54.
- [Google Scholar]
- Physicochemical, phytochemical screening and profiling of secondary metabolites of Annona squamosa leaf extract. World J. Pharm. Res.. 2012;1:1143-1164.
- [Google Scholar]
- Fruits with high antioxidant activity as functional food. In: Shi J., ed. Functional Food Ingredients and Nutraceuticals: Processing Technologies. LLC, Philadelphia: Taylor and Francis Group; 2006. p. :371-413.
- [Google Scholar]
- A novel method of plasmid isolation using laundry detergent. Indian J. Exp. Biol.. 2011;49:558-560.
- [Google Scholar]
- Flavonoids in food and their health benefits. Plant Foods Hum. Nutr.. 2004;59:113-122.
- [Google Scholar]
- Analysis of flavonoids in Portulaca oleracea L. by UV-Vis spectrophotometry with comparative study on different extraction technologies. Food Anal. Methods. 2009;3:90-97.
- [Google Scholar]







