Translate this page into:
Detection of flumethrin acaricide residues from honey and beeswax using high performance liquid chromatography (HPLC) technique
⁎Corresponding author at: Research Center for Advanced Materials Science (RCAMS), King Khalid University, P.O. Box 9004, Abha 61413, Saudi Arabia; Unit of Bee Research and Honey Production, Faculty of Science, King Khalid University, P.O. Box 9004, Abha 61413, Saudi Arabia. khalidtalpur@hotmail.com (Khalid Ali Khan)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Residues of acaricide, flumethrin were detected from honey and beeswax using high performance liquid chromatography (HPLC) technique. Forty samples of honey were collected before flumethrin treatment and 30, 60, and 90 days after application from five Apis mellifera colonies; one sample from the central frame containing brood and honey, while the other from the frame filled with honey from peripheral side of each colony at every sampling interval. Honey samples were taken by uncapping the cells with knife and extracting the honey with a syringe of 12 mL capacity. Twenty samples of beeswax (one sample from peripheral frames of each colony) were collected from five selected beehives. Two solvents n-hexane and dichloromethane (4:6, v/v) were used for making extracts of honey and beeswax samples, respectively which were further concentrated by rotary evaporation. All the samples were purified and detected at a wavelength of 267 nm. From the HPLC residual analysis, all the honey samples were found free of acaricide flumethrin; as it could not be detected from any of the 40 samples. However, all the beeswax samples were positive for flumethrin residues. The highest level of flumethrin (0.0759 mg/kg) was detected from the samples of beeswax samples taken from the bee colonies after 30 days of flumethrin application. Despite of the fact that flumethrin residues were detected from all the test beeswax samples, however no one was noticed to exceed the tolerance levels established by Environmental Protection Agency (EPA) and European Commission guidelines. Present method of flumethrin detection through HPLC also proved to be a promising alternative of Gas Chromatography with high sensitivity and can be used as an appropriate method to determine flumethrin residues from honey and beeswax.
Keywords
Flumethrin residues
Apis mellifera
Wax
Honey
HPLC
1 Introduction
Varroa mite is considered the worst enemy of honey bees, Apis mellifera throughout the world (Alattal et al., 2015). This mite became the major pest of A. mellifera in Pakistan soon after the introduction of these bees from Australia in 1977 (Ahmad, 1988). Due to favorable climatic conditions and diverse bee flora, beekeepers can fetch high honey harvests in the country (Khan et al., 2016) but mites are posing serious losses in terms of bee population and honey production (Ahmad et al., 2017). Previously, it was considered that this parasitic mite attacks on open brood, sealed brood and adult bees and deprives them from haemolymph (Anderson and Trueman, 2000) but recently it is found that it consumes fat bodies of the target bees (Ramsey et al., 2019). The individual bees are killed due to various physiological impairments and colony performance is severely affected (Ansari et al., 2017; Al-Ghamdi et al., 2018; Khan et al., 2019). If not controlled properly, the whole colony may die within months (Le Conte et al., 2010). This parasitic mite is an important vector of many honeybee viral diseases (Alattal et al., 2017) and a key biotic colony stressor (Husain et al., 2014; Khan et al., 2017) which may result in the mortality of the whole colony population (Boecking and Genersch, 2008) if not managed properly. Beekeepers bear heavy financial losses due to attack of Varroa mite on honey bees and face severe difficulties in its control (Aziz et al., 2015; Sajid et al., 2019).
Pyrethroids (fluvalinate and flumethrin) are synthetic derivatives of pyrethrum and are amongst the most commonly used chemicals to control mites infesting A. mellifera (Mahmood et al., 2014). Fluvalinate is being used since 1990s (Camphor et al., 2005) and flumethrin is introduced since last decade (Mahmood et al., 2012). Flumethrin remained very popular mite controlling source throughout the world for many years, but with the passage of time, researchers in different countries started to report the presence of flumethrin residues in honey, propolis and wax obtained from A. mellifera (Chauzat and Faucon, 2007; Kamel and Al-Ghamdi, 2006; Mullin et al., 2010). When the beekeepers apply flumethrin strips continuously against Varroa mites, it gets deposited in the bee combs due to lipophilic nature. If these combs are not replaced regularly, they can become the source of residual contamination during honey extraction (Wallner, 1995) and comb honey production. The European Union regulations clearly indicate that honey must be free from the all sort of chemical residues due to the fact that it is a natural product (Al-Waili et al., 2012) and is directly consumed by human beings, so the image of natural, hygienic and residue free substance should must be maintained during its production and processing chain (Chauzat and Faucon, 2007). With the advent of modern technologies to detect drug residues and recent reports of honey adulteration with synthetic chemicals in different countries, people are now more concerned about honey purity than ever before.
At present, several methods exist for detection of flumethrin residues in honey (Lin et al., 2010) established a gas chromatography method for detection of residual levels of flumethrin in honey. Wang et al. (2002) used gas chromatography method to detect the residue level of flumethrin acaricide in honey with 0.05 mg/kg limit of detection. Other scientists like Xiaofeng et al. (2005) used gas chromatography for detecting residual levels of coumaphos, fluvalinate, flumethrin in honey with a recovery of 70% to 110% and with a detection limit of 0.004 to 0.008 mg/kg while Ranganathan et al. (2018) used liquid chromatography for validation of Lenalidomide in human plasma. Although, key approach to residual flumethrin detection remained gas chromatography in most of the studies; and flumethrin detection in honey through HPLC is less reported. However, Yu et al. (2015) reported 75.7% to 82.8% recovery of flumethrin in honey with 10 µg/kg detection limit by high performance liquid chromatography and claimed this method as a propitious substitute of other chromatographic techniques. Flumethrin is resistant to heat treatment and has known UV absorption, thus it also could be detected by LC. Advantages of HPLC includes high sensitivity, high separation efficiency and fast and simple operation. The sample must be prepared as a solution without the use of vaporization (Hung et al., 1988). Residual analysis of flumethrin in honey and beeswax were made through HPLC in the current study, which proved equally good in excessive separation efficacy, easy and fast separation process for the test samples. Keeping in view the frequent application of flumethrin strips in bee hives, present research project was conducted to detect the level of flumethrin residues both from honey and wax samples up to 90 days after application with the aim to provide information to beekeepers regarding safe usage of this acaricides against Varroa mites.
2 Materials and methods
2.1 Standard, reagents, and solvents
The following equipments and agents were used in current studies: Certified analytical standard of flumethrin was obtained from sigma company (St. Louis, MO) with >95.7% purity. Waters HPLC with UV detector (Waters, Milford, MA), Merck limited C-18 column (5 µm, 4.6 by 250 mm), vortex, R series-rotating instrument, HPLC grade water (Merck Corporation), dichloromethane (Sigma-Aldrich), n-hexane (Sigma-Aldrich), acetonitrile (Sigma-Aldrich), and acetone (Merck Corporation) was purchased from local market and florisil (60–100 mesh) was used.
2.2 Preparation of standard solutions
The stock solutions of flumethrin standards of 1 mg/mL concentration were prepared individually in acetonitrile and put in storage at −18 °C in a freezer. These stock solutions were run as standards and when needed up to 3 months. The stock solution was diluted in acetonitrile to prepare the serial concentrations as 4.0, 2.0, 1.0, 0.5, 0.25 and 0.125 µg/mL. Standard concentrations in acetonitrile were prepared instantly before the preparation of samples.
2.3 Sample collection
All the samples were collected from Bee Research Unit (BRU), Pir Mehr Ali Shah (PMAS) Arid Agriculture University, Rawalpindi, Pakistan during January to June (2016). Two honey samples were taken from each colony; one from central brood comb and the other from peripheral honey comb of the colony. One wax sample was taken from each colony; from either of the peripheral honey combs (Table 1). Wax from capped honey cells was removed with knife and placed in the plastic feeders inside the hives for 24 h; the bees cleaned the wax by licking honey. These wax samples were shifted to the Toxicology Laboratory, Department of Entomology, PMAS-Arid Agriculture University and kept in freezer at low temperature (−18 °C).
Honey samples
Central frame (n)
Peripheral frame (n)
Pre-Treatment
5
5
30 days after treatment
5
5
60 days after treatment
5
5
90 days after treatment
5
5
Beeswax samples
Pre-Treatment
–
5
30 days after treatment
–
5
60 days after treatment
–
5
90 days after treatment
–
5
Total Samples
20
40
2.4 Preparation of honey samples
The liquid–liquid extraction method of Rissato et al. (2004) was used to extract the residues from honey with some amendments. Fifty (50) mL centrifuge tubes (Centrifuge Tube manufacturers, China) were taken and five gram honey samples were poured in each tube. In each sample, distilled water (50 mL) was added and homogenized by using vortex for 2–3 min to reduce the thickness and to facilitate their handling. Twenty (20) mL of dichloromethane and n-hexane (4:6, v/v) was added to each sample, mixed and subjected to extraction in the separating funnel by stirring for 20 min. The organic phase of the sample from the top was removed and poured in another flask. Another 20 mL n-hexane and dichloromethane (4:6, v/v) was added to the lower phase and the solution was extracted again. The second upper phase was added with the first one. The extract was evaporated near to dryness, under reduced pressure and at 35–40 °C temperature on a rotary evaporator (B-740, Buchi Corporation). At the last stage, the residues of rotary evaporation were mixed with acetonitrile (3 mL), vortexed for one min, passed through PTFE filter (0.45 µm pore size), transferred to vials then analysed by HPLC using UV–VIS detector (Waters Corporation).
2.5 Preparation of wax samples
For liquid–liquid extraction from beeswax samples, the procedures of Bagdonov et al. (2003) were followed with some modifications. From each sample, five grams of beeswax was precisely weighed and put into a centrifuge tube (50 mL); solvent n-hexane (5 mL) was added and mixed with the help of a sonicator for 5 min. After this, the extract was subjected to freezing temperature (−10 °C) for 15 min, centrifuged at 5000 rpm and passed through PTFE filter (0.45 µm pore size) to remove most of the long chain hydrocarbons. The filtrate was again subjected to freezing temperature (−10 °C) for 30 min, centrifuged (8000 rpm), and passed through PTFE filter to refine the extract from the other unwanted hydrocarbons. The extract was evaporated near to dryness with the help of rotary evaporator (B-740, Buchi Corporation), under reduced pressure at 35–40 °C. Finally the residues were dissolved in acetonitrile (2 mL) and passed through 0.45 µm sized pore PTFE filter and analysed by HPLC using UV–VIS detector (Waters Corporation).
2.6 Optimization of extraction procedure
Flumethrin is a α-cyano-pyrethroid acaricide and n-hexane, acetone and dichloromethane are its commonly reported extraction solvents (Zhou et al., 2007). In the present study, the combination (4:6 v/v) of n-hexane with dichloromethane was used by following the Zhou et al. (2007) to extract the flumethrin from honey and beeswax samples as the highest extraction rate of acaricide has been reported by the authors using these solvents.
The most frequently reported method for purification of honey and beeswax includes supercritical fluid, solvent extraction and solid phase extraction (Sun et al., 2010). In this study, acetonitrile and the extraction solution were tested as eluents. When eluent was used for purification, the baseline was stable with less interference of impurities; so acetonitrile was used as an eluent
2.7 Optimization of chromatographic separation conditions
To conduct the analyses of standard solution, 267 nm wavelength as optimized by Yu et al. (2015) was used. Because of efficient separation and minimum interference, the same detection wavelength (267 nm) was used for further analysis of test samples.
2.8 Chromatographic conditions
The Chromatographic conditions were as follows: C-18 column (5 µm, 4.6 by 250 mm; Merck limited) was used as a solid phase and acetonitrile–water (90:10, v/v) as a mobile phase. The samples were run at flow rate of 1.4 mL/min and detected at wavelength of 267 nm. Temperature of column was kept at 30 °C and injection volume of 20 μL was used for HPLC analysis.
3 Results
3.1 Linear relationship
The resultant linear regression equation for flumethrin was y = 1073274× + 67420, and the correlation coefficient (R2) was 0.9995, indicating a strong linear relationship between peak area and concentration (Fig. 1). The optimal retention time of flumethrin was approximately 7.77 min, with good peak shape and stable baseline (Fig. 2)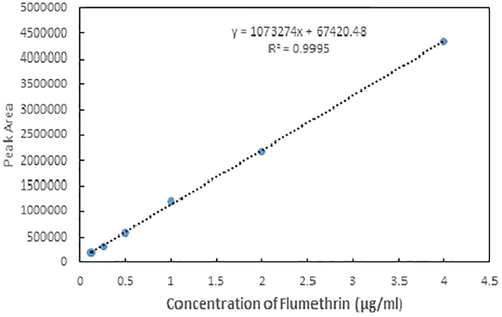
Correlation between peak area and concentration of the flumethrin standard solution.
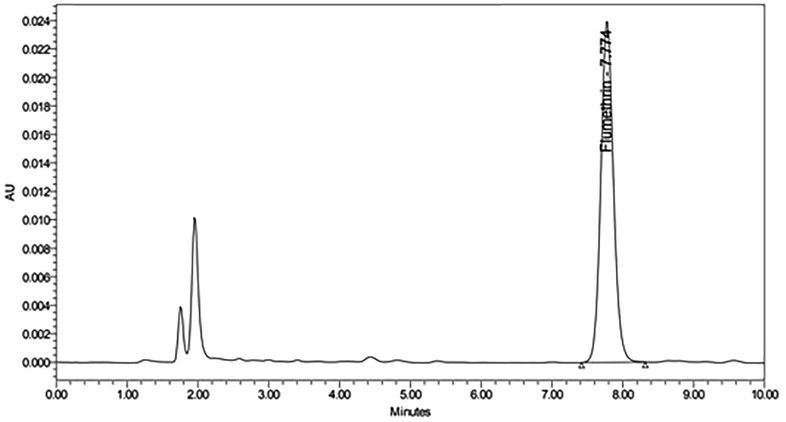
High performance liquid chromatography (HPLC) chromatogram retention time of the flumethrin standard solution.
3.2 Recovery and method validation
The spike recovery tests of flumethrin were performed by taking three different standard solution concentration levels (1.0, 0.5, and 0.25 mg/kg). The resultant recovery rates of flumethrin (Fig. 3) ranged from 84.23% to 105.26% with a relative standard deviation of <8%. Validation of method is the essential requirement for any analysis related to chromatography (Levison et al., 1995).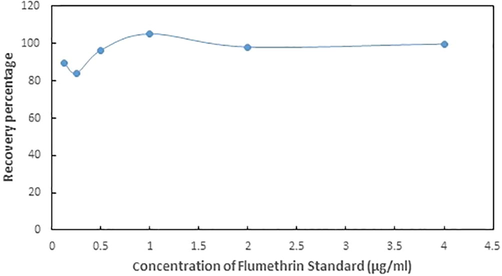
Recovery percentage of spiked concentrations of flumethrin standard solutions.
3.3 Detection limit
The detection limit is defined as the peak height of the lowest drug concentration three times of the baseline noise. The detection limit by HPLC with UV detection for flumethrin standard in honey and beeswax was 0.0053 mg/kg, lower than the maximum residues limit (MRL) (0.01 mg/kg) recommended by European-Commission (2006) regulations.
Experimental results indicated that the chromatographic conditions were reasonable as the retention time for flumethrin was approximately 7.77 min, and separation was well with the impurity at a detection limit of 0.0053 mg/kg.
3.4 Analysis of honey samples
Figs. 4A and 4B show chromatograph for honey samples before and after flumethrin strips application. As shown in Tables 2 and 3, the detection rate and contents of flumethrin in honey samples are 0% and 0.000 mg/kg. All honey samples were free of flumethrin residues. DAT = Days after treatment.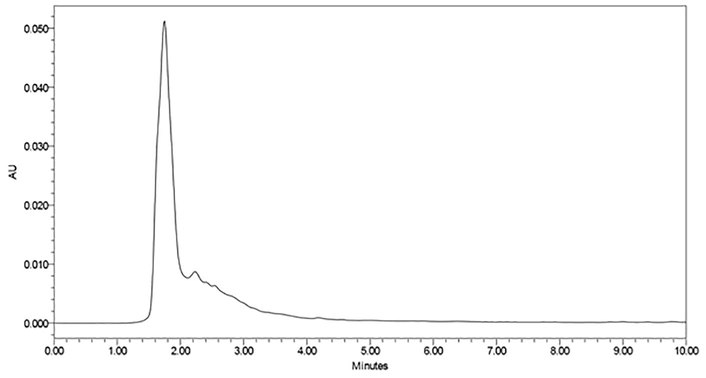
High performance liquid chromatography (HPLC) chromatogram retention time of honey sample before flumethrin application in beehive.
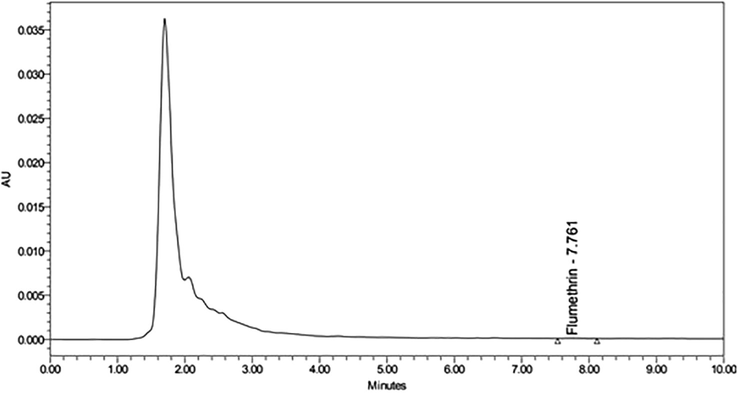
High performance liquid chromatography (HPLC) chromatogram retention time of honey sample after flumethrin application in beehive.
Experimental material
Samples
Detection
Frequency of detection
Honey
40
0
0%
Beeswax
20
20
100%
Residues in honey & beeswax samples
Range (mg/kg)
Median (mg/kg)
Honey
All samples
0.0000–0.0000
0.0000
Beeswax
Pre-Treatment
0.0025–0.1364
0.0741
30 DAT
0.0075–0.2932
0.0759
60 DAT
0.0072–0.2684
0.0721
90 DAT
0.0386–0.1017
0.0419
3.5 Analysis of beeswax samples
Figs. 5A and 5B show chromatograms for beeswax samples with and without flumethrin. As shown in Tables 2 and 3, the detection rate and contents of flumethrin in beeswax 90 days after treatment were the highest (100% and 0.0386–0.1017 mg/kg, respectively). In all beeswax samples we detected flumethrin residues but below the MRL. The lowest contents of flumethrin for beeswax samples from pre-treatment was 0.0025–0.1364 mg/kg, which is lower than MRL, whereas the flumethrin residues in beeswax samples from 90 days after treatment was as high as 0.0386–0.1017 mg/kg, but not exceeding the MRL.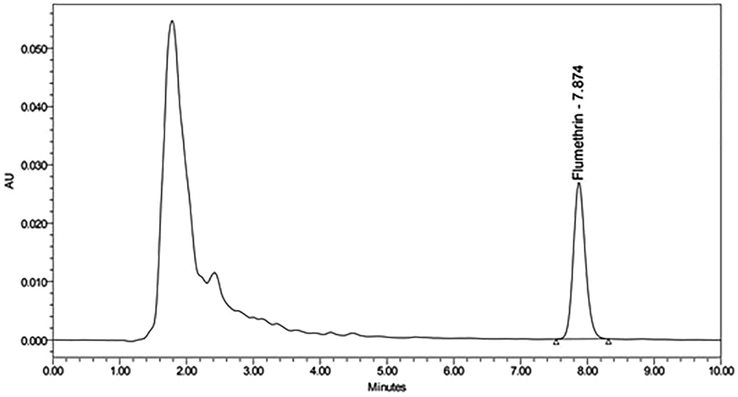
High performance liquid chromatography (HPLC) chromatogram retention time of beeswax sample before flumethrin application in beehive.
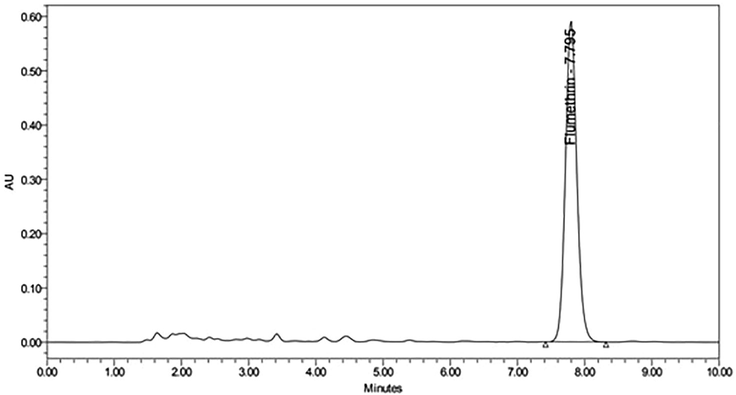
High performance liquid chromatography (HPLC) chromatogram retention time of beeswax sample after flumethrin application in beehive.
4 Discussion
Veterinary drugs of different chemical nature are being used to control V. destructor all over the world (except Australia). If these varroacides are fat soluble and non-volatile, then risk of contamination of bee products exacerbates especially with repeated applications over the years (Wallner, 1999). Since flumethrin is also a lipophilic pyrethroid; so the studies regarding its detection in honey and beeswax become enormously crucial to assess the current status of this chemical in bee products.
In the present study, 40 honey samples were investigated for flumethrin residues and all the honey samples were found free from this varroacide. These findings are in accordance with those of Kamel and Al-Ghamdi (2006), who analysed 21 honey samples and found all of them free from flumethrin residues. Another report by EMEA (1998) also confirms present results, in which a surveillance of honey samples was made from different European countries and flumethrin residues were found to be below the limit of quantification of analytical methods for these samples. However in a recent study, out of 135 honey samples collected from seven different locations of China, flumethrin residues were detected from 77 samples (Yu et al., 2015). According to the results of current study, flumethrin residues were not detected from honey samples collected up to 90 days of application, which is highly encouraging as flumethrin is being used as a main tool for Varroa management in Pakistan, however its injudicious should be avoided strictly as according to Yu et al. (2015), the flumethrin residues in Chinese honey samples may be the result of flumethrin application during nectar flow period in the apiaries and non-compliance of rules to withdraw medication during honey flow period.
The results of beeswax assays reflected various levels of flumethrin residues in all of the test samples (both pre-treatment and after treatment of flumethrin) in the hives. The reason for flumethrin residues in pre-treatment beeswax samples may be that the hives were purchased from local beekeepers, which often use the wax frames year after year without replacement. Although, flumethrin was detected in 100% of beeswax samples, however, it did not exceed the MRL of 1 mg/kg. Flumethrin is a persistent pyrethroid and may accumulate in beeswax with the passage of time. According to the report EMEA (1998), flumethrin residues were found in beeswax samples exposed to flumethrin impregnated strips in different periods of years (spring, pre-winter and honey flow season) and highest concentration 130 mg/kg was detected when beehives were treated during nectar flow period, however in all of these hives, honey samples showed flumethrin residues below the limit of quantification.
According to Wallner (1995), flumethrin dissolves five to 10 times faster in fats/lipids than fluvalinate, so it has a strong tendency to be accumulated in the beeswax as compared to the honey. In the present studies, the detection of flumethrin residues in beeswax samples may be the result of its extraordinary lipophilic character Qi et al. (2020). Flumethrin residues (between 1 and 10 mg/kg) in beeswax have been reported from Austria, where it has been in use since long against Varroa mites (Wallner, 1995). However, Bogdanov et al. (1998) reported no flumethrin residues in new beeswax. Although, the transfer of flumethrin form beeswax to honey was found negligible, however the residues, many accumulate to higher levels, if the wax is reused for several years (EMEA, 1998). According to several other studies, as degradation of miticides does not occur naturally in the beeswax, so its contamination may be a substantial cause of residues in honey. Similarly, reprocessing of used combs into foundation sheets does not modify the composition of active ingredients; therefore it may become a permanent storehouse of fat soluble ingredients (Bogdanov et al., 1998; Wallner, 1999). As beeswax is the second largest hive product after honey used in many food additives, food supplements (in tablets and capsules), even directly eaten by the people in special types of honeys called comb honey or chunk honey, so residue free wax is crucial for the safety of consumers (Wilmart et al., 2016). Moreover contaminated wax may impair the growth of brood within the hive (Nielsen et al., 2000; Qi et al., 2020). A further dedicated research is needed to examine negative implications of flumethrin residues, especially regarding the consumption of comb honey, use of contaminated wax in pharmaceutical industries, in cosmetics, and its reuse in the beekeeping field.
5 Conclusion
Residues were not detected through HPLC analysis in any of the honey samples collected up to 90 days duration after the application of flumethrin strips in the bee colonies. However, all the beeswax samples are found contaminated with flumethrin residues although they did not exceed the MRLs established by EPA and European Commission guidelines.
Acknowledgements
The authors are thankful to PMAS-Arid Agriculture University for providing grant under the project, “Detection of flumethrin residues from honey and beeswax of Apis mellifera” and Isra University Islamabad Campus, for providing HPLC facilities to conduct this research. Authors would like to acknowledge the support of the King Khalid University through RCAMS/KKU/09-19 under the Research Center for Advanced Materials Science (RCAMS) at King Khalid University, Kingdom of Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Effect of queen age on hygienic and grooming behavior of Apis mellifera Ligustica against Varroa destructor (Anderson and Trueman) Asian J. Agric. Biol.. 2017;5:113-118.
- [Google Scholar]
- Effect of gut bacterial isolates from Apis mellifera jemenitica on Paenibacillus larvae infected bee larvae. Saudi J. Biol. Sci.. 2018;25:383-387.
- [Google Scholar]
- Antibiotic, pesticide, and microbial contaminants of honey: human health hazards. Sci. World J.. 2012;2012:930849
- [Google Scholar]
- Fertility and reproductive rate of Varroa mite, Varroa destructor, in local and exotic honeybee, Apis mellifera L., colonies under Saudi Arabia conditions. Saudi J. Bio. Sci.. 2017;24:992-995.
- [Google Scholar]
- Surveillance and genotyping of Varroa destructor parasitizing Apis mellifera jemenitica in Saudi Arabia. Rev. Colomb. Entomol.. 2015;41:180-184.
- [Google Scholar]
- Varroa jacobsoni (Acari: Varroidae) is more than one species. Exp. Appl. Acarol.. 2000;24:165-189.
- [Google Scholar]
- Geographical distribution and molecular detection of Nosema ceranae from indigenous honey bees of Saudi Arabia. Saudi J. Biol. Sci.. 2017;24(5):983-991.
- [Google Scholar]
- Control of Varroa destructor Anderson and Trueman (Acari: Varroidae) on Apis mellifera Linguistica by using Thymol and Formic acid in Pothwar region of Punjab, Pakistan. Asian J. Agric. Biol.. 2015;3:150-154.
- [Google Scholar]
- Varroosis–the ongoing crisis in bee keeping. J. Verbrauch. Lebensm.. 2008;3:221-228.
- [Google Scholar]
- Seasonal changes in mite (Tropilaelaps clareae) and honeybee (Apis mellifera) populations in Apistan treated and untreated colonies. Apiacta. 2005;40:36-44.
- [Google Scholar]
- Pesticide residues in beeswax samples collected from honey bee colonies (Apis mellifera L.) in France. Pest Manage. Sci.. 2007;63:1100-1106.
- [Google Scholar]
- EMEA, 1998. Committe for Veterinary Medicinal Products Flumethrin- Summary Report (1) http://www.ema.europa.eu/docs/en_GB/document_library/Maximum_Residue_Limits_-_Report/2009/11/WC500014322.pdf (Accessed:02-Sep.2019).
- Commission Regulation 178/2006/EC of 1 February 2006 amending Regulation (EC) No 396/2005 of the European Parliament and of the Council to establish Annex I listing the food and feed products to which maximum levels for pesticide residues apply. Off. J.. 2006;29:3-24.
- [Google Scholar]
- Theoretical and experimental foundation for surface-coverage programming in gas-solid chromatography with an adsorbable carrier gas. Anal. Chem.. 1988;60:1090-1096.
- [Google Scholar]
- Bioassay of insecticides against three honey bee species in laboratory conditions. Cercetari Agron. Moldova. 2014;47:69-79.
- [Google Scholar]
- Determination of acaricide residues in Saudi Arabian honey and beeswax using solid phase extraction and gas chromatography. J. Environ. Sci. Health B. 2006;41:159-165.
- [Google Scholar]
- The characterization of blossom honeys from two provinces of Pakistan. Ital. J. Food Sci.. 2016;28:625-638.
- [Google Scholar]
- Structural diversity and functional variability of gut microbial communities associated with honey bee. Microb. Pathog.. 2019;138:103793
- [Google Scholar]
- Investigation of gut microbial communities associated with indigenous honey bee (Apis mellifera jemenitica) from two different eco-regions of Saudi Arabia. Saudi J. Biol. Sci.. 2017;24:1061-1068.
- [Google Scholar]
- Varroa mites and honey bee health: can Varroa explain part of the colony losses? Apidologie. 2010;41:353-363.
- [Google Scholar]
- Validation studies in the regeneration of ion-exchange celluloses. J. Chromatogr. A. 1995;702:59-68.
- [Google Scholar]
- Determination of bromopropylate, fluvalinate and flumethrin residues in honey by gas chromatography. Strait J. Prev. Med. 2010;16:13-16.
- [Google Scholar]
- Control of Varroa destructor (Acari: Varroidae) in Apis mellifera (Hymenoptera: Apidae) by using plant oils and extract. Pak. J. Zool.. 2014;46:609-615.
- [Google Scholar]
- Control of Varroa destructor using oxalic acid, formic acid and bayvarol strip in Apis mellifera (Hymenoptera: Apidae) colonies. Pak. J. Zool.. 2012;44:1473-1477.
- [Google Scholar]
- High levels of miticides and agrochemicals in North American apiaries: implications for honey bee health. PloS One. 2010;5:e9754
- [Google Scholar]
- Effects on detoxification enzymes in different life stages of honey bees (Apis mellifera L., Hymenoptera: Apidae) treated with a synthetic pyrethroid (flumethrin) Altern. Lab. Anim.. 2000;28:437-443.
- [Google Scholar]
- Flumethrin at sublethal concentrations induces stresses in adult honey bees (Apis mellifera L. Sci. Total Environ.. 2020;700:134500.
- [Google Scholar]
- Varroa destructor feeds primarily on honey bee fat body tissue and not hemolymph. Proc. Natl. Acad. Sci.. 2019;116:1792-1801.
- [Google Scholar]
- Development and validation of Lenalidomide in human plasma by LC-MS/MS. Biol. Sci. Saudi J. 2018
- [CrossRef] [Google Scholar]
- Supercritical fluid extraction for pesticide multiresidue analysis in honey: determination by gas chromatography with electron-capture and mass spectrometry detection. J. Chromat. A. 2004;1048:153-159.
- [Google Scholar]
- Efficacy assessment of soft and hard acaricides against Varroa destructor mite infesting honey bee (Apis mellifera) colonies, through sugar roll method. Biol. Sci. Saudi J. 2019
- [CrossRef] [Google Scholar]
- Progress on the research of sample pretreatment techniques in the detection of pyrethroid residues. Agrochemicals. 2010;49:11-14.
- [Google Scholar]
- Nebeneffekte bei Bekämpfung der Varroamilbe. Die Rückstandssituation in einigen Bienenprodukten. Bienenvater. 1995;116:172-177.
- [Google Scholar]
- Association of novel mutations in a sodium channel gene with fluvalinate resistance in the mite, Varroa destructor. J. Apic. Res.. 2002;41:17-25.
- [Google Scholar]
- Residues in beeswax: a health risk for the consumer of honey and beeswax? J. Agric. Food Chem.. 2016;64:8425-8434.
- [Google Scholar]
- Gas chromatographic method for simultaneous determination of bromopropylate, coumaphos, fluvalinate and flumethrin residues in honey. Apic. China 2005:6.
- [Google Scholar]
- Flumethrin residue levels in honey from apiaries of china by high-performance liquid chromatography. J. Food Protect.. 2015;78:151-156.
- [Google Scholar]
- Rapid and sensitive determination of two degradation products of flumethrin in honey by ultrasonically assisted extraction and gas chromatography with electron capture detection. J. Sep. Sci.. 2007;30:1912-1919.
- [Google Scholar]







