Translate this page into:
Defence response of host plants for cyst nematode: A review on parasitism and defence
⁎Corresponding author. ahmad_nematol@yahoo.com (Faheem Ahmad) faheem.bt@amu.ac.in (Faheem Ahmad)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The Cyst nematodes (CN), such as Heterodera spp. and Globodera spp. are key biotrophic pathogens inflicting high levels of damage to agricultural and horticultural crops. This review sheds light on the parasitism of the CN and molecular defence responses of infected plants. We highlight the role of effector proteins secreted from the oesophageal gland cells of the CN, hormone-signalling pathway, and miRNA regulation of gene expression that modulate the differentiation of the feeding site. In addition, we speak of the role of pattern-triggered immunity (PTI), effector-triggered immunity (ETI), resistance genes (R genes), and pathogenesis-related proteins in the immune defence responses of the CN. We conclude this review by discussing recent progress in genomic studies and molecular mechanisms involved in the recognition process of the infesting CN that provides scope for future investigations and the discovery of novel strategies to manage these biotrophic pathogens.
Keywords
Cyst nematode
Parasitism
Host response
Defence gene
1 Introduction
Soil-borne pathogens are the important biotic constraints of agricultural and horticultural crops. Among biotic pathogens, plant-parasitic nematodes (PPN) cause significant damage to crop production worldwide. According to Nicol et al. (2011), the damage is estimated at more than US$80 billion annually. The Heteroderidae group of nematodes, known as cyst nematodes (CN), is an important pest of crops that leads to economic yield losses worldwide. The CN is the most complex group of sedentary endoparasitic nematodes because they spend most of their active lives within the roots of the host crops. The female CN is non-motile, globular (Globodera spp.) and lemon (Heterodera spp.) shaped, and it contains many embryonated eggs male is motile, which causes the stunting of the plants and leads to complete crop failure.
During infection, the CN first interacts with the roots as infective second-stage juveniles (J2). The J2 is motile that invades the root tips using stylet (Fig. 1). Cyst juveniles move intracellularly towards the vasculature. They then target a competent/spherical cell to initiate a feeding site to form a multinucleate syncytium (Moens et al., 2018). The syncytium is formed by fusing the neighbouring cells from an initial syncytial cell targeted by the CN after the invasion. It provides nutrients for developing CN (Sobczak and Golinowski, 2011). CN are biotrophic parasites because they feed on syncytia until complete reproduction. It can be controlled by cultural practice, crop rotation, sanitation, use of nematicides, and resistant cultivars. However, these management strategies are low output, expensive, and require a careful handling system. As a result, new techniques and resources to control CN are necessary that are non-toxic and suitable for the environment. Therefore, it is necessary to understand how plants interact with CN and how they respond against parasitism.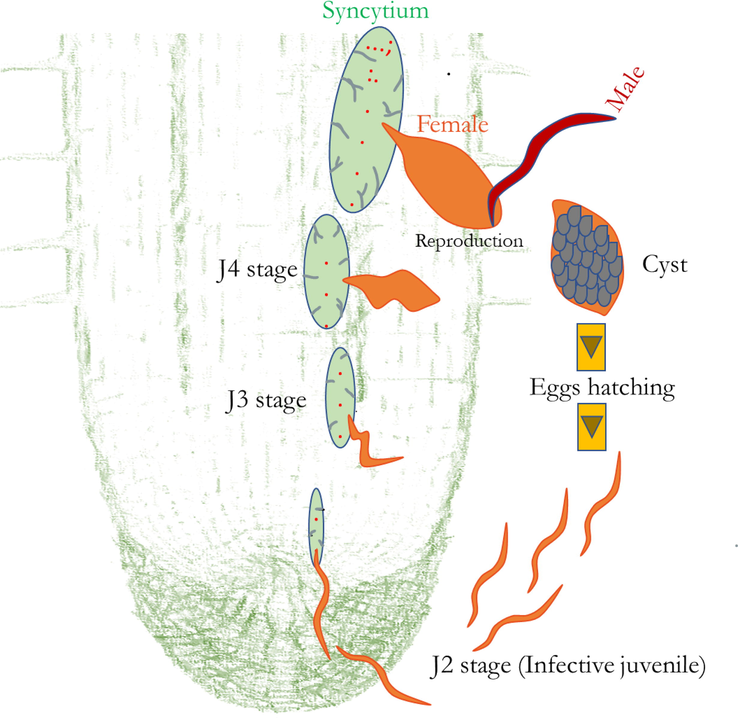
An illustrated diagrammatic representation of infective juveniles (J2) of cyst nematode (CN) enters the root system, migrate through plant tissues, and develop a feeding site called syncytium. The juveniles (J2) become sedentary within the vascular tissues and CN, including Heterodera spp. and Globodera spp. acquire nutrients from the host-derived feeding sites, syncytia. Feeding sites lead to root swellings blocking water and nutrient supply within the plant body, thereby affecting the growth of the host plant. Adult CN females retain eggs inside the body, which form a cyst after death.
2 Role of effectors in host parasitism
The interactions between the host and pathogen are mediated by effectors, i.e., secreted proteins which play roles in parasitism. During the CN parasitism, these effectors initiate a signalling process that suppresses the host defence responses and facilitates infection. The increasing availability of genome sequences and genetic analyses has greatly encouraged scientists to understand plant-nematode interaction better. The effector proteins are CLC (cadiotrophin-lycytokine) signalling peptides, expressed in the oesophageal gland cell during infection in a plant with H. glycines encoded in the gene. A specific feature of these nematode CLEs is their processing from proteins containing single or multiple CLE motifs. CN acquires CLE peptides to change its host’s behaviour, which favours syncytium formation (Wang et al., 2010).
Smant et al. (1998) identified β-1,4-Endoglucanases (cellulase) effector from the CN e.g., Globodera rostochiensis and Heterodera glycines. They found that effectors are involved in forming the feeding site, defence suppression signalling pathway, and alteration of phytohormones. Yang et al. (2019a and 2019b) identified the two effectors, Ha18764 and HaGland5, expressed in dorsal oesophageal gland cells of H. avenae. The effector ‘HaGland5′ promotes nematode parasitism and represses the defence-related genes; it reduces cell wall callose deposition and the burst of reactive oxygen species (ROS). On the other hand, the effector molecule ‘Ha18764′ promotes parasitism by suppressing the plant pathogen-associated molecular pattern-triggered immunity (PTI) and effector-triggered immunity (ETI) during infection. The CN induces morphological and physiological changes within host plants through the secretion of an effector, which is involved in parasitism and promotes interaction with the host plant (Aharen et al., 2020). Hu et al. (2019) reported the effector ‘Hg16B09′ expression in H. glycines using in-situ hybridization. They indicated that the upregulation of effector proteins in the parasitic stage juvenile establishes a metabolically hyperactive feeding site in the host plant by suppressing basal plant defences. Verma et al. (2022) investigated a cytoplasmic effector ‘Hs2D01′ interaction with AtHAE, which is a leucine-rich repeat (LRR) receptor-like kinase found on the surface of cells that plays a crucial role in the parasitism by H. schachtii. They demonstrated that Hs2D01-AtHAE activates the cell wall enzymes that are essential for cell separation during abscission and lateral root emergence. Identifying the effector molecule is necessary to understand better the relationship between nematode effector and host cell protein. Verma et al. (2018) firstly reported one of the novel effectors, ‘30D08′ contributes to nematode parasitism by interacting with the nucleus and a host auxiliary spliceosomal protein (SMU2) to alter gene expression in feeding sites. Pogorelko et al. (2020) screened 51 effectors of the soybean cyst nematode (SCN) Heterodera glycines. They identified three effectors inhibiting effector-triggered immunity (ETI) and seven effectors as pattern-triggered immunity (PTI) suppressors. The effector proteins altered the host gene’s expression and created specialized infection sites. In plants, autophagy is crucial for homeostasis, growth, senescence, and resistance to biotic and abiotic stressors. Autophagy aided in plant defences against bacterial, viral, and filamentous pathogens and emerged as a key target for microbial effectors. However, how plants engage in autophagy in nematode parasitism is mostly unknown. Recently, Chen et al. (2022) identified NMAS1 (Nematode Manipulator of Autophagy System 1), a novel and conserved effector from CN, using molecular and genetic analyses. They demonstrated that NMAS1, a virulence effector, can promote disease by targeting a crucial component of host autophagy and inhibiting PTI and defence-related cell death, which is mediated by membrane-associated and intracellular immune receptors. The CN effector that causes changes in the host is enlisted in Table 1.
Effector Gene
Nematode
Function
Reference
Ha18764
Heterodera avenae
Immune suppression by triggering PTI and ETI
Yang et al., 2019a
HaGland5
H. avenae
Repression of defence-related genes, the burst of ROS
Yang et al., 2019b
HsPGx
H. schachtii
Feeding site formation via parasitism protein
Aharen et al., 2020
Hg16B09
H.glycines
Suppression of plant innate immunity
Hu et al., (2019)
Hs30D08
H. schachtii
Feeding site formation via interaction with nucleus and host SMU2 protein
Verma et al., 2018
HsGLAND4
H. schachtii
Feeding site formation via interaction with the promoter of LTP genes
Barnes et al., 2018
HsPDI
H. schachtii
Syncytial feeding site formation via redox homeostasis
Habash et al., 2017
Hs32E03
H. schachtii
Alter the histone acetylation and depression of host rRNA genes.
Vijayapalani et al., 2018
GpIA7
Globodera pallida
Target the function of host EBP1 and hinder the plant cell cycle.
Coke et al., 2021
HaVAP1, HaVAP2
H. avenae
Knocking down and silencing both proteins hamper the parasitism.
Luo et al., 2019
GLAND5,
GLAND6H. glycine
Suppress the Pattern trigger immunity (PTI)
Pogorelko et al., 2020
RHA1B
G. pallida
Facilitates nematode parasitism by suppressing the ETI and hypersensitive response.
Kud et al., 2019
TUBG1, TUBG2
H. schachtii
Down-regulation of both genes is essential for successful parasitism and syncytium development
Różańska et al., 2018
NMAS1
Globodera spp.
Promoting disease by targeting a crucial component of host autophagy and inhibit PTI
Chen et al., 2022
3 Role of phytohormones and microRNA in nematode infestation
The involvement of phytohormones such as auxin, ethylene, salicylic acid, and jasmonic acid regulates the process in plants. These phytohormones also regulate the plant-nematode interaction, including controlling the plant defense system and developing nematode diseases. The phytohormones are key players in the formation of nematode-feeding sites. During the formation of feeding sites, nematodes interact with hormone homeostasis, giving complex results. Siddique et al. (2015) showed that the cytokinin hormone manipulates the host functions and establishes a long-term parasitic interaction by activating cytokinin signalling, cell cycle and progression. Cytokinin facilitates nematode development and infection during feeding site initiation and promotes syncytium expansion. Escudero Martinez et al. (2019) analyzed the effect of strigolactones (SLs), a novel class of phytohormones, during H. schachtii parasitism on Arabidopsis. They observed that SLs don’t promote the hatching of CN but participate in host attraction and invasion. Dowd et al. (2017) have identified different expressions of cytokinin biosynthesis, catabolism, and signalling genes in response to infection developed by CN. They observed that CN manipulates the cytokinin signalling pathway and is responsible for cell cycle activation, which induces feeding site formation. Hu et al. (2017) demonstrated the ethylene signalling pathway's role in regulating root attractiveness in response to sugar beet cyst nematode. Ethylene and auxin are also involved in syncytia formation and control the nematode-induced regulatory networks in the host (Goverse and Bird, 2011).
Researchers have also studied the role of miRNA in plant nematode interaction. MicroRNAs (miRNAs) are non-coding RNAs that regulate the gene expression of the host plant and modulate the large-scale changes in the nematode feeding sites. Hewezi et al. (2016) reported the overexpression of ‘miR827′ in the Arabidopsis to suppress the immune responses necessary to establish infection and cause disease (Table 2). The nematode-activated ‘miR827′ targets the nitrogen limitation adaptation (NLA) gene, enhancing susceptibility to the CN. Piya et al. (2017) discovered that ‘miR858′ contributes to transcriptome reprogramming during syncytia formation. The ‘miR858′ regulates the target transcription factor MYB83, and constitutive overexpression reduces susceptibility to CN (Table 2). The miRNA targets the growth-regulating factor (GRF) genes for productive CN infections (Noon et al., 2019). Thus, the miRNA plays a diverse role in the biological process, especially in antagonistic responses against pests.
miRNA for parasitism
miRNA
CystNematode
(CN)Function
Reference
miR858
Heterodera schachti
Formation of syncytium by the regulating transcription factor, MYB83
Piya et al., 2017
miR827
H. schachti
Activation of nitrogen limitation adaptation gene enhances the CNs infection
Hewezi et al., 2016
miRNA396
H. glycines
Target the growth-regulating factor genes that are essential for CNs infection
Noon et al., 2019
miRNA for defence
miR159-GmMYB33
Heterodera glycines
Modulate the GA-signalling and metabolism in the plant for defence
Lei et al., 2021
miR398
Heterodera glycines
Facilitate the plant resistance against CN
Tian et al., 2017
miR5032
Heterodera glycines
Overexpression of gene provide robust resistance against CN
Rambani et al., 2020
4 The host immune response against the CN infection
A successful pathogen can protect itself from plant-associated molecules that trigger defence signalling to avoid pathogen invasion. An elicitor is a diverse group of signal molecules recognized by the plant and induces defence against the pathogen. The immune response of plants can undergo two pathways to protect them from nematode invasion. First, the immune system is triggered by the pathogen-associated molecular patterns (PAMPs) to activate the PAMP-triggered immunity (PTI), and the second is the effector-triggered immunity (ETI) initiated by the effector protein produced by the nematode. Host plant cells have membrane-bound pattern recognition receptors (PRRs). It can recognize pathogen-associated molecules produced by a pathogen that activate the different types of immune responses. The first line of inducible defence, such as the burst of ROS triggers callose deposition. The first plant immune receptor, ‘NILR1′ a leucine-rich repeat receptor-like kinase essential for the induction of basal immunity or PTI against the CN (Mendy et al., 2017). The role of mitogen-activated protein kinases (MAPKs) has been investigated as central components of cell signalling cascade against the H. schachtii parasitism in Arabidopsis spp. The MAPKs, such as MPK3 and MPK6 play a key role in the plant nematode interaction and activate the plant defence (Sidonskaya et al., 2016). McNeece et al. (2019) analyzed 32 MAPKs, of which nine have a defensive role and induced defence MAPK gene expression. The effector-triggered immunity is activated by R Proteins or resistance proteins present in the plant cell and recognizes the effectors of the pathogen and activate the hypersensitive response. In the case of CN, nematode-associated molecular patterns (NAMPs) play a wide role, initiating the defence signalling and modulating the plant immune responses. NAMPs role and its defence signalling initiation are represented in (Fig. 2). Manosalva et al. (2015) showed that nematode-conserved signalling molecules called ascarosides (ascr#18) activate plant immune response. Ascarosides are NAMPs specific to nematodes and perceived by plants, activating conserved immune reactions. The plant cells have some receptor proteins, such as polygalacturonase-inhibiting proteins (PGIPs), which regulate camalexin and indole-glucosinolate biosynthetic pathways during the nematode parasitism (Shah et al., 2017).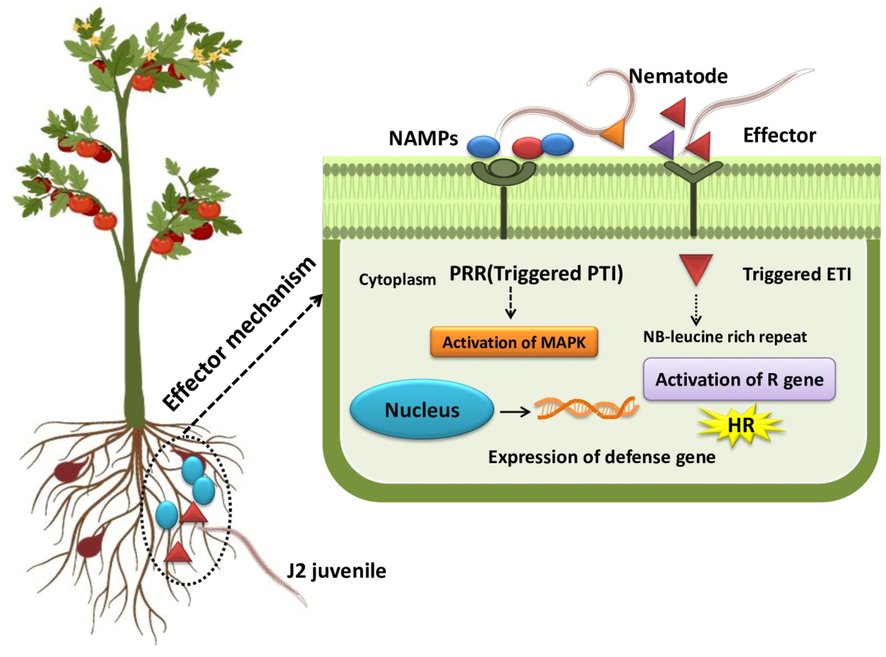
Interaction between plant immune receptor and defence signalling components. Nematode associated molecular patterns (NAMPs) recognized by the plant membrane PRR (Pattern-Recognition Receptors) that triggers PTI (Pathogen Triggered Immunity), and activate the production of effector protein triggers the ETI (Effector-Triggered Immunity) that started the hypersensitive (HR) against the nematode.
5 Systemic acquired resistance and defence marker genes
The local host responses are activated at the local infestation site, causing the hypersensitive response that prevents subsequent infection. Host plants develop resistance against various pathogens through the hormone-mediated signals known as systemic acquired resistance (SAR). SAR enhances the defence potential of host plants and activates the pathogenesis-related genes in other host tissues, which are not exposed to infection and provide protection against future attacks. Several signalling molecules are involved in SAR. In the case of a necrotrophic pathogen, jasmonic acid (JA)-mediated signalling pathways play a significant role in plant resistance against the pathogen. Salicylate (SA) also plays an important regulatory role in biotrophic pathogens like cyst nematodes (Fig. 3). SA signalling synthesis specific function in defence response against the CN is not well known. Matthews et al. (2014) investigated thirty-one Arabidopsis genes involved in the SA and JA synthesis and signalling against the cyst nematodes CN. They found that some Arabidopsis genes, such as AtNPR1, AtTGA2, and AtPR-5, primarily associated with SA regulation, signalling, and synthesis enhanced resistance towards the CN. Several changes in gene expression, including the activation of pathogenesis-related proteins, occur in potato leaves after root infection with a cyst nematode. Molecular methods are used to extract plant genes and express their up-regulated genes upon nematode infection. In Egypt, Elkobrosy et al. (2021) first reported the characterization of Globodera rostochiensis at the molecular level. In particular, they demonstrated how this nematode affected the responses of the defence genes in the infected potato. The functional transcriptome of infected and non-infected potato plants was examined, and approximately 57 up-regulated and 22 down-regulated genes were identified. These reported genes provided broad-spectrum data about the plant nematode interaction. Contradictory information exists regarding the function of PR proteins in interactions with parasitic organisms. Some studies suggest that PR proteins are activated and involved in plant defence. Temperature variation causes changes in plant transcriptome activity (activation of resistance genes H1 and Gro1-4, as well as genes PR1, PR2, PR3, and PR6, responsible for the establishment of a defensive response to infection)(Lavrova et al., 2017). Their finding reveals the expression of the genes involved in the immune reaction in potato roots to the Globodera rostochiensis. Guo et al. (2019) reported that the Rhg1 gene encodes the resistance against SCN responsible for developing resistance in soybean. They suggested that overexpression of Rhg1-GmAAT activated the jasmonic acid (JA) pathway causing resistance toward the SCN. Singh et al. (2020) highlighted the role of the ascorbate oxidase (AO) enzyme in their study. AO works as an effective systemic defence compound that protects plants against the H. schachtii. CN defence-related gene ‘GmSAMT1′ called Salicylic acid methyltransferase is involved in salicylic acid biosynthesis and signal transduction pathway and plays a defensive role against H.glycines (Lin et al., 2013). The defence related genes are enlisted in Table 3.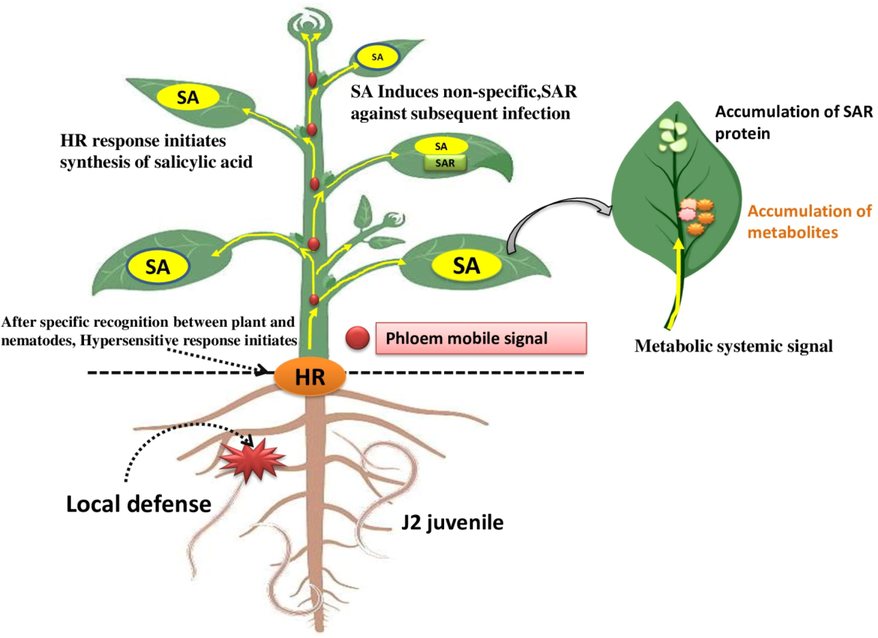
The role of SA against the nematode infection through inducing SAR (Systematic Acquired Resistance) activates the pathogenesis-related proteins in the distant tissue of the host plant.
Defence Gene
Cyst Nematode (CN)
Host Plant
Function
Reference
AtMYB59
Heterodera schachtii
Arabidopsis thaliana
Reduce the susceptibility of the host
Wiśniewska et al., 2021
WI12Rhg1
Heterodera glycines
Soybean
Interact with DELLAs proteins that contribute to hormone signalling pathways.
Dong and Hudson, 2022
AtHRS1
H. schachtii
A. thaliana
Interfere with the development of cyst-induced syncytia and reduce the number of females.
Wiśniewska et al., 2022
At3g59930
H. schachtii
A. thaliana
Overexpression of the gene enhances the resistance to CN
Hawamda et al., 2022
Hg-rps23, Hg-snb1, and Hg-cpn1
H. glycines
Soybean
Enhance the broad-spectrum resistance via host-induced silencing.
Zhang et al., 2022a, 2022b
Glyma.06g036700
H. glycines
Soybean
Expression of the cupredoxin family proteins enhances the CNs resistance.
Zhang et al., 2022a, 2022b
6 Molecular and genomics strategies for the CN
Molecular and genomic approaches improved the understanding regarding the plant nematode interaction and provided an opportunity to develop effective strategies against nematode infection. Novel genomic technologies enhanced crop sustainability by overexpressing ‘R’ genes, quantitative trait loci (QTL), and RNA interference. In the last decade, QTL played a key role in controlling CN. Jiao et al. (2015) identified the quantitative trait loci contributing to underlying resistance to CN. Swaminathan et al. (2018) detected the QTL mapping to CN resistance with the help of SNP (single nucleotide polymorphisms) markers. SNP tools provide data to study yield and disease resistance. Significant progress in RNAi-based plant protection strategies has been applied against nematode diseases.
RNAi is a powerful tool that regulates silencing gene expression, such as post-transcriptional gene silencing (PTGS), and inhibits translation and transcriptional gene silencing. Tian et al. (2019) reported that RNAi gene silencing reduces the populations of SCN and enhances host resistance. RNA interference decreases the pathogenicity of the CN. The genetic basis of broad-based resistance to SCN has also been reported, and the impact of ‘rhg1′ and ‘Rhg4′ genes for interaction with nematodes is highlighted by research (Patil et al., 2019). Marker-assisted selection (MAS) has also been developed. The three functional competitive allele-specific PCR (KASP) marker assays were sufficient for high-throughput marker-assisted selection for SCN resistance (Shi et al., 2015). There is no doubt that genetic tools have facilitated the identification of resistance genes and promoted the development of resistant varieties by improving desirable traits. Identifying the ‘Rhg1′ and ‘Rhg4′ genes improved the cyst nematode susceptible lines through the gene-editing (Liu et al., 2017; Bayless et al., 2018). Therefore, genome editing tools can modify specific targeted sequences and can be used to develop resistant varieties. The RNA-seq-based identification of resistance genes against the CN revealed that breeding nematode-resistant varieties is the safest and most effective method to manage plant diseases (Jiang et al., 2021).
7 Conclusion and future perspectives
CN are regarded as the biggest hazard to crop productivity worldwide due to their extensive host range. In recent years, it has become clear that nematode secretory effector proteins play a key role in the formation of feeding sites and the control of host cell machinery. Infection sites are established using a variety of procedures. There is no question that the host cell is regulated by phytohormone signalling and a variety of transcription factors, which also play a vital role in the immune response and emergence of symptoms in host plants. Additionally, we talked about how gene expression controls the nematode pest and initiates defensive reactions. The current understanding of molecular mechanisms supports the latest discoveries of novel genes and proteins implicated in the host-specific defence against CN. The development of RNAi (RNA interference) technology has emerged as a powerful technique for controlling the nematode population through gene silencing in light of genomic studies. So, with the aid of current developments in genetic, genomic, and molecular research, researchers were inspired to develop new tools for nematode resistance and strategies to better understand plant nematode interactions.
Author contributions
All authors contributed equally to this manuscript. Authors have read, critiqued and approved it for publication.
Disclosure statement
No potential conflict of interest was reported by the author (s).
Acknowledgements
The facility and support provided by Department of Botany-AMU are gratefully acknowledged. Rehab O. Elnour and Mohamed Hashem extend their appreciation to the Deanship of Scientific Research, King Khalid University for getting financial support through research groups program under grant number R.G.P. 2/75/44.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Heterodera schachtii glutathione peroxidase (HsGPx) is a parasitism protein. J. Plant. Dis. Prot.. 2020;127:111-118.
- [CrossRef] [Google Scholar]
- The plant-parasitic cyst nematode effector GLAND4 is a DNA-binding protein. Mol. Plant. Pathol.. 2018;19:2263-2276.
- [CrossRef] [Google Scholar]
- An atypical N-ethylmaleimide sensitive factor enables the viability of nematode-resistant Rhg1 soybeans. Proc. Natl. Acad. Sci.. 2018;115:E4512-E4521.
- [CrossRef] [Google Scholar]
- A key virulence effector from cyst nematodes targets host autophagy to promote nematode parasitism. New Phytol. 2022
- [CrossRef] [Google Scholar]
- The GpIA7 effector from the potato cyst nematode Globodera pallida targets potato EBP1 and interferes with the plant cell cycle. J. Exp. Bot.. 2021;72:7301-7315.
- [CrossRef] [Google Scholar]
- WI12 Rhg1 interacts with DELLAs and mediates soybean cyst nematode resistance through hormone pathways. Plant. Biotechnol. J.. 2022;20:283-296.
- [CrossRef] [Google Scholar]
- Divergent expression of cytokinin biosynthesis, signaling and catabolism genes underlying differences in feeding sites induced by cyst and root-knot nematodes. Plant J.. 2017;92:211-228.
- [CrossRef] [Google Scholar]
- The effect of cyst nematode (Globodera rostochiensis) isolate ddh1 on gene expression in systemic leaves of potato plant. J. Micro. Biotech. Food Sci. 2021:93-97.
- [CrossRef] [Google Scholar]
- Distinct roles for strigolactones in cyst nematode parasitism of Arabidopsis roots. Eur. J. Plant Pathol.. 2019;154:129-140.
- [CrossRef] [Google Scholar]
- The role of plant hormones in nematode feeding cell formation. In: Genomics and Molecular Genetics of Plant-nematode Interactions. Springer Dordrecht; 2011. p. :325-347.
- [Google Scholar]
- The soybean Rhg1 amino acid transporter gene alters glutamate homeostasis and jasmonic acid-induced resistance to soybean cyst nematode. Mol. Plant Pathol.. 2019;20:270-286.
- [CrossRef] [Google Scholar]
- Identification and characterization of a putative protein disulfide isomerase (HsPDI) as an alleged effector of Heterodera schachtii. Sci. Rep.. 2017;7:1-14.
- [CrossRef] [Google Scholar]
- Characterization of an Arabidopsis Defensin-like Gene Conferring Resistance against Nematodes. Plants. 2022;11:280.
- [CrossRef] [Google Scholar]
- Arabidopsis miR827 mediates post-transcriptional gene silencing of its ubiquitin E3 ligase target gene in the syncytium of the cyst nematode Heterodera schachtii to enhance susceptibility. Plant J.. 2016;88:179-192.
- [CrossRef] [Google Scholar]
- Ethylene response pathway modulates attractiveness of plant roots to soybean cyst nematode Heterodera glycines. Sci. Rep.. 2017;7:1-3.
- [CrossRef] [Google Scholar]
- The Heterodera glycines effector Hg16B09 is required for nematode parasitism and suppresses plant defense response. Plant Sci.. 2019;289
- [CrossRef] [Google Scholar]
- RNA-seq-based identification of potential resistance genes against the soybean cyst nematode (Heterodera glycines) HG Type 1.2. 3.5. 7 in ‘Dongnong L-10’. Physiol. Mol. Plant Pathol.. 2021;114:101627
- [CrossRef] [Google Scholar]
- Identification of quantitative trait loci underlying resistance to southern root-knot and reniform nematodes in soybean accession PI 567516C.Mol. Breed. 2015;35:1.
- [CrossRef] [Google Scholar]
- The potato cyst nematode effector RHA1B is a ubiquitin ligase and uses two distinct mechanisms to suppress plant immune signaling. PLoS Pathog.. 2019;15:e1007720
- [CrossRef] [Google Scholar]
- Expression of genes, encoded defense proteins, in potato plants infected with the cyst-forming nematode Globodera rostochiensis (Wollenweber 1923) Behrens, 1975 and modulation of their activity during short-term exposure to low temperatures. Bio. Bull.. 2017;44:128-136.
- [CrossRef] [Google Scholar]
- Soybean miR159-GmMYB33 regulatory network involved in gibberellin-modulated resistance to Heterodera glycines. Int. J. Mol. Sci.. 2021;22:13172.
- [CrossRef] [Google Scholar]
- Overexpression of a soybean salicylic acid methyltransferase gene confers resistance to soybean cyst nematode.Plant Biotechnol. J.. 2013;11:1135-1145.
- [CrossRef] [Google Scholar]
- The soybean GmSNAP18 gene underlies two types of resistance to soybean cyst nematode. Nat. Commun.. 2017;8:1.
- [CrossRef] [Google Scholar]
- Two venom allergen-like proteins, HaVAP1 and HaVAP2, are involved in the parasitism of Heterodera avenae. Mol. Plant Pathol.. 2019;20:471-484.
- [CrossRef] [Google Scholar]
- Conserved nematode signalling molecules elicit plant defenses and pathogen resistance. Nat. commun.. 2015;6:1-8.
- [CrossRef] [Google Scholar]
- Arabidopsis genes, AtNPR1, AtTGA2 and AtPR-5, confer partial resistance to soybean cyst nematode (Heterodera glycines) when overexpressed in transgenic soybean roots. BMC Plant Biol.. 2014;14:1-9.
- [CrossRef] [Google Scholar]
- The mitogen activated protein kinase (MAPK) gene family functions as a cohort during the Glycine max defense response to Heterodera glycines. Plant Physiol. Biochem.. 2019;137:25-41.
- [CrossRef] [Google Scholar]
- Arabidopsis leucine-rich repeat receptor–like kinase NILR1 is required for induction of innate immunity to parasitic nematodes. PLoS Pathog.. 2017;13:e1006284
- [CrossRef] [Google Scholar]
- Cyst nematodes-Life cycle and economic importance. Cyst Nematodes, CABI, Wallingford.UK 2018:1-26.
- [CrossRef] [Google Scholar]
- Current nematode threats to world agriculture. In: Genomics and Molecular Genetics of Plant-nematode Interactions. Dordrecht: Springer; 2011. p. :21-43.
- [Google Scholar]
- Homeostasis in the soybean miRNA396–GRF network is essential for productive soybean cyst nematode infections. J. Exp. Bot.. 2019;70:1653-1668.
- [CrossRef] [Google Scholar]
- Whole-genome re-sequencing reveals the impact of the interaction of copy number variants of the rhg1 and Rhg4 genes on broad-based resistance to soybean cyst nematode. Plant Biotechnol. J.. 2019;17:1595-1611.
- [CrossRef] [Google Scholar]
- Cooperative regulatory functions of miR858 and MYB83 during cyst nematode parasitism. Plant Physiol.. 2017;174:1897-1912.
- [CrossRef] [Google Scholar]
- Screening soybean cyst nematode effectors for their ability to suppress plant immunity. Mol. Plant Pathol.. 2020;21:1240-1247.
- [CrossRef] [Google Scholar]
- Identification of differentially methylated miRNA genes during compatible and incompatible interactions between soybean and soybean cyst nematode. Mol. Plant-Microbe Interact.. 2020;33:1340-1352.
- [CrossRef] [Google Scholar]
- Expression of both Arabidopsis γ-tubulin genes is essential for development of a functional syncytium induced by Heterodera schachtii. Plant Cell Rep.. 2018;37:1279-1292.
- [CrossRef] [Google Scholar]
- Damage-associated responses of the host contribute to defence against cyst nematodes but not root-knot nematodes. J. Exp. Bot.. 2017;68:5949-5960.
- [CrossRef] [Google Scholar]
- SNP identification and marker assay development for high-throughput selection of soybean cyst nematode resistance. BMC Genom.. 2015;16:1-2.
- [CrossRef] [Google Scholar]
- A parasitic nematode releases cytokinin that controls cell division and orchestrates feeding site formation in host plants. Proc. Natl. Acad. Sci.. 2015;112:669-674.
- [CrossRef] [Google Scholar]
- Plant resistance against the parasitic nematode Heterodera schachtii is mediated by MPK3 and MPK6 kinases, which are controlled by the MAPK phosphatase AP2C1 in Arabidopsis. J. Exp. Bot.. 2016;67:107-118.
- [CrossRef] [Google Scholar]
- Ascorbate oxidase induces systemic resistance in sugar beet against cyst nematode Heterodera schachtii. Front. Plant Sci.. 2020;11:591715
- [CrossRef] [Google Scholar]
- Endogenous cellulases in animals: isolation of β-1, 4-endoglucanase genes from two species of plant-parasitic cyst nematodes. Proc. Natl. Acad. Sci.. 1998;95:4906-4911.
- [CrossRef] [Google Scholar]
- M. Sobczak W. Golinowski Cyst nematodes and syncytia Genomics and Molecular Genetics of Plant-nematode Interactions 2011 Springer Netherlands Dordrecht 61 82 https://doi.org/10.1007/978-94-007-0434-3_4
- Mapping of new quantitative trait loci for sudden death syndrome and soybean cyst nematode resistance in two soybean populations. Theor. Appl. Genet.. 2018;131:1047-1062.
- [CrossRef] [Google Scholar]
- Genome-wide identification of soybean microRNA responsive to soybean cyst nematodes infection by deep sequencing. BMC Genom.. 2017;18:1-3.
- [CrossRef] [Google Scholar]
- Host-derived gene silencing of parasite fitness genes improves resistance to soybean cyst nematodes in stable transgenic soybean. Theor. Appl. Genet.. 2019;132:651-662.
- [CrossRef] [Google Scholar]
- The novel cyst nematode effector protein 30D08 targets host nuclear functions to alter gene expression in feeding sites. New Phytol.. 2018;219:697-713.
- [CrossRef] [Google Scholar]
- A novel sugar beet cyst nematode effector 2D01 targets the Arabidopsis HAESA receptor-like kinase. Mol. Plant Pathol. 2022
- [CrossRef] [Google Scholar]
- An effector from the cyst nematode Heterodera schachtii derepresses host rRNA genes by altering histone acetylation. Plant Cell.. 2018;30:2795-2812.
- [CrossRef] [Google Scholar]
- Dual roles for the variable domain in protein trafficking and host-specific recognition of Heterodera glycines CLE effector proteins. New Phytol.. 2010;187:1003-1017.
- [CrossRef] [Google Scholar]
- Arabidopsis thaliana Myb59 gene is involved in the response to Heterodera schachtii infestation, and its overexpression disturbs regular development of nematode-induced syncytia. Int. J. Mol. Sci.. 2021;22:6450.
- [CrossRef] [Google Scholar]
- Arabidopsis thaliana AtHRS1 gene is involved in the response to Heterodera schachtii infection and its overexpression hampers development of syncytia and involves a jasmonic acid-dependent mechanism. J. Plant Physiol.. 2022;20:153680
- [CrossRef] [Google Scholar]
- A novel G16B09-like effector from Heterodera avenae suppresses plant defenses and promotes parasitism. Front. Plant Sci.. 2019;10:66.
- [CrossRef] [Google Scholar]
- Heterodera avenae GLAND5 effector interacts with pyruvate dehydrogenase subunit of plant to promote nematode parasitism. Front. Microbiol.. 2019;10:1241.
- [CrossRef] [Google Scholar]
- Essential roles of cupredoxin family proteins in soybean cyst nematode resistance. Phytopathol 2022
- [CrossRef] [Google Scholar]
- Enhanced resistance to soybean cyst nematode in transgenic soybean via host-induced silencing of vital Heterodera glycines genes. Transgenic Res.. 2022;8:1.
- [CrossRef] [Google Scholar]







