Translate this page into:
Cytotoxicity and apoptosis response of hexagonal zinc oxide nanorods against human hepatocellular liver carcinoma cell line
⁎Corresponding author. javedahmad@ksu.edu.sa (Javed Ahmad)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Hexagonal zinc oxide nanorods (ZnO-NRs) were prepared via solution process using zinc nitrate hexahydrate (Zn(NO3)2·6H2O, 0.25 M), hexamethylenetetramine (HMT) and sodium hydroxide at low concentration (4 × 10-3 M in 100 ml of distilled water) and refluxed at 100 °C for an hour. The HMT was used because its act as a template for the nucleation and growth of zinc oxide nanorods. The X-ray diffraction patterns (XRD) clearly reveal that the grown product is pure zinc oxide. The diameters and lengths of the synthesized nanorods lie between 200 and 250 nm and 2–3 μm respectively as observed from the field emission scanning electron microscopy (FESEM). The chemical functional properties was analyzed by Fourier Transform infra-red (FTIR) spectroscopy. This study was designed to show the possible effect of ZnO-NRs in human liver cancer (HepG2) cells. It induces cytotoxicity via reactive oxygen species (ROS), mitochondrial membrane potential. For gene expression analysis, it induces apoptotic gene marker P53, Bax, caspase3, Bcl2 genes. Based on analysis and observations, it concludes that the ZnO-NRs were utilized for toxicity at dose dependent via ROS generation against human hepato cellular liver carcinoma cell line.
Keywords
Zinc oxide nanorods
X-ray diffraction pattern
FTIR spectroscopy
Human cell line
ROS
RT-PCR
1 Introduction
Investigation of the toxicity of nanomaterials (NMs) is increasing due to the large production and incorporation of these materials in many products. The increase of these consumer products causes NMs release and bioavailability in the environment. Zinc oxide (ZnO) NMs are widely used in the manufacture of anti-fouling paints as well as skincare products. ZnONMs have excellent ultraviolet (UV)-absorbing properties and transparency to visible light, making these NMs excellent sunscreen agents (Vijayakumar et al., 2016; Chen et al., 2007). Additionally, the biocidal activity of ZnO NMs gives high potential for the applications in products such as foods, packaging, cosmetics, medicines and healthcare formulations (Kumar et al., 2017). Zinc oxide nanorods (ZnO-NRs) show wurtzite crystal structure, which is formed due to interpenetrating hexagonal closely packed sub-lattices consisting of one specific kind of an atom. Wurtzite structure of ZnO results in higher chemical and mechanical stability (Gojova et al., 2006).
The ZnO-NRs shows a band-gap of about 3.37 eV with 60 meV of binding energy. This material showed anticancer activity in the non-viable cell and bactericidal property as well, so in increased use of ZnO NMs as an anticancer and antibacterial agent as well as biomedical image contrasting agent (Pande et al., 2007). Therefore, due of increasing application of ZnO-NRs day by day the demand is also increased. Hence, owing to which different approaches have come for the easy and large scale synthesis of ZnO-NRs.
Nanotechnology has tremendous innovation over the last decades. ZnO, which possesses unique properties like optical, semiconducting etc. ZnO used in biomedical applications, drug delivery, gene delivery, and biosensing of a wide array of molecules of interest. (Zhang et al., 2013). Although applications of ZnONMs in biomedical field are increasing, however, preparation of biocompatible of ZnO-NRs is still challenging (Riggio et al., 2010). ZnO-NRs also used as an adhesion-resistant inducing death in anchorage-dependent cells (Lee et al., 2008). ZnO-NPs showed cytotoxicity on multi-type of tumor cells A549, MCF-7, and EMT-6 (Dang et al., 2021). ZnO-NRs biocompatibility studies on LE cells showed that ZnO-NRs could be a non-toxic NM for biological applications (Gopikrishnan et al., 2010; 2011). Toxicological assessment of ZnO-NRs with green microalgae, D.subspicatus and Tetraselmis sp effects of oxidative stress induction through CAT activity (Almeida et al., 2021). ZnO-NRs does not affect any mortality in zebrafish embryos but it exhibited cytotoxicity of cell-death by exposed to MCF-7 breast cancer cell lines (Ali et al., 2021). On the Immunosensing platform built on ZnO-NRs for finding cancer cell (B-lympho- blastoid cell line IM9) revel strong near-band-edge (NBE) emission (Tamashevskia et al., 2019). Neuro-toxicity of ZnO-NMs shows comparatively lower toxicity but ZnO-NRs prompted developemental neurotixciy that subsequently developed to PD-like symptoms at high doses (Jin et al., 2021). The cellular uptake of gold NPs in Human liver cancer HepG2 cells was measured, and the results monitored that the order nanospheres > nanorods > nano stars. A study by ZnO-NRs on human alveolar adenocarcinoma cells induced oxidative stress and apoptosis (Ahamed et al., 2011). In this connection, Gold NRs exposed by the A549 cells are localized in membranous vesicles with mitochondrial swelling were also observed. High Quantity of ROS production proposed that the oxidative stress will be the main reason causing cytotoxicity (Tang et al., 2015). Cellulose mesh, deposited with ZnO-NRs clusters demonstrated excellent antimicrobial activity for gram negative (E. coli, Pseudomonas aeruginous) and gram positive (Staphylococcus aureus, Bacillus ceresus, Streptococcus thermophilis) bacteria (Bhutia et al., 2018). ZnO-NRs decorated with graphene nanoplateles have proven it a promising agent against C. albicans infections (Ficociello et al., 2018). ZnO-NR exposed to MCF7 cells exhibited a decrease in proliferation with the surge of apoptosis compared to HaCaT cells. (Zanni et al., 2016). Nanorod (ZnO-FA in vitro efficacy of DOX loaded ZnO-FA (ZnO-FA-DOX) has been evaluated against breast cancer cells MDA-MB231, (Mitra et al 2012) showed the potential of ZnO-NRs to induce complement stimulation and inflammatory response, changes in expression of genes involved in apoptosis and oxidative stress. (Aula et al., 2018).
The current study aimed to synthesize ZnO-NRs using zinc nitrate hexahydrate (Zn(NO3)2·6H2O) and hexamethylenetetramine (HMT) via solution process and used as an anticancer material and also to assess the toxicity against Hepatocellular liver carcinoma cell lines (HepG2), testing cytotoxicity, intracellular ROS and apoptotic gene markers were discussed.
2 Materials and methods
2.1 Chemicals and reagents
The Zinc nitrate hexahydrate (Zn(NO3)2·6H2O), hexamethylenetetramine (HMT) and Sodium Hydroxide (NaOH) were purchased for this experiment from Aldrich chemical corporation. Fetal bovine serum, penicillin–streptomycin and LTR (LysoTracker™ Red DND-99) were purchased from Invitrogen Co. USA. DMEM F-12, MTT [3-(4,5-dimethyl thiazol-2-yl)-2,5-diphenyl tetrazolium bromide], pyruvic acid, perchloric acid, DHE (dihydroethidium), DAR-1 (4,5-Diamino-N,N,N′,N′-tetraethylrhodamine), JC-1 (5,5′,6,6′- Tetrachloro −1,1′, 3,3′-tetraethyl-imidacarbocyanine iodide), were obtained from Sigma–Aldrich (Sigma–Aldrich, MO, USA). Ultrapure water was used from a Milli-Q system .All other chemicals used were of reagent grade.
2.2 Formation of hexagonal zinc oxide shaped nanorods
The formation of nanopowder of ZnO-NRs were accomplished with using precursor material zinc nitrate hexahydrate (Zn(NO3)2·6H2O), hexamethylenetetramine (CH2)6N4) (HMT) and sodium hydroxide (NaOH) as per the previously described protocol with modification (Wahab et al., 2007a,b). In a typical synthesis, a small amount of zinc nitrate hexahydrate (Zn (NO3)2·6H2O, 0.25 M) was dissolved in 100 ml of distilled water, and to this mixture a small amount of HMT (4 × 10-3 M) was dissolved. A colorless solution was obtained and at this point pH was reached to 6.6. To this solution NaOH (0.1 M) was added drop by drop and again pH was observed which reaches to 12.3. The entire solution was stirred for 30 min for the complete dissolution and thereafter refluxed in a three-necked refluxing pot at 100 °C for an hour (h) for complete precipitation. Once the refluxing time completed, a white aqueous powder was settled at the bottom of refluxing pot, the pot was kept for 24 h at room temperature and the solution was washed with methanol several times and dried and was examined further.
2.3 Characterizations of ZnO-NRs
The morphological characterizations were conducted with using scanning electron microscopy (SEM). To analyze SEM, processed powder was consistently sprayed on carbon tape and transfer it into a sputtering chamber for 2–3 s with platinum coating. Once the sputtering was completed the sample holder was removed and fixed it into SEM for morphological analysis. The crystallinity and crystal phases of obtained as grown powder was determined by using X-ray powder diffractometer (XRD) CuKα radiation (λ = 1.54178 Å) Bragg angle ranging from 20° to 65° with 6°/min scanning speed. Also the Fourier transform infrared (FTIR) spectro scopy was analyzed in the range of 400–4000 cm−1.
2.4 Cell culture
Human liver cancer (HepG2) cells (ATCC, US) were maintained in DMEM culture medium supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin as an antibiotic, and 100 μg/mL streptomycin at 37 °C in a humidified 5% CO2 incubator.
2.5 Cell viability assays
MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay was carried out according to the previously described (Mosmann, 1983) protocol with some minor modifications. The cells were passaged every 3–4 days. Approximately 20,000 cells were seeded in a 96-well plate with a clean, flat base for 24 h at 37 °C with humidified environment. On the following day, cells were treated with ZnO-NRs at different concentrations. The control cells were employed as a reference with the same conditions. After the exposure period (24 h), the MTT assay was performed by measuring absorbance at 570 nm with a plate reader (Synergy HT). Cell viability is given in the percentage of control cells, assuming 100% cell viability in the control.
2.6 Detection of intracellular ROS
Detection of intracellular reactive oxygen assay (O2·- and H2O2) under physiological conditions were determined by their probe Dihydroethidium (DHE). DHE is a freely permeable probe, which react with the ROS O2·− produces red fluorescent product known as ethidium or 2-hydroxyethidium (Peshavariya et al., 2007). Although, ROS is the combined form of many reactive O2 molecules but O2·− and H2O2 are largely responsible for cellular injury and signaling pathways. Therefore, O2·− and H2O2. Due to intercalation with the DHE-derived making enhanced the nucleus bright from the rest of the cytoplasm (Nauseef, 2014). An increase in red fluorescence treated cells besides control cells suggest a more production of O2·− Cells. For the experiment DHE were labeled at a final concentration of 10 μM and incubated for 40 min. Plates were washed carefully with HBSS two to three times and imaging was accompanied by using filter in a fluorescence microscope (Leica DMi8, Germany).
2.7 Mitochondrial membrane potentials
MMP were determined by HepG2 cells in control and treated was determined by JC-1 (5,5′,6,6′-Tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine iodide). In healthy cells with high mitochondrial membrane potential, JC-1 forms complexes known as J-aggregates with intense fluorescence (Smiley et al., 1991). On the other hand, in apoptotic cells with lower membrane potential, JC-1 remains in the monomeric. Cells were seeded at appropriate densities in plate reader. After the treatment, media was aspirated and labeled with 5 μM JC-1 in HEPES buffered HBSS for 20 min. Fluorescence of JC-1 aggregate (red in color) and JC-1 monomer (green in color) in cells were taken by a fluorescence microscope (Leica DMi8, using objective 20 × and LAS core software) using green filter cube.
2.8 Real-time PCR (RT-PCR)
Total RNA was purified from HepG2 cells from untreated and treated with ZnO-NRs for 24 h using the RNeasy mini Kit (Qiagen). As the manufactural protocol RNA was isolated there purity were determined by Nanodrop 8000 spectrophotometer (Thermo Scientific, USA).
The integrity of RNA was confirmed by visualized on 2% agarose gel with the documentation system (Universal Hood II, BioRad, USA). The cDNA was prepared by the MLV reverse transcriptase (GE Health Care, UK) For the quantification of apoptotic of apoptotic and anti-apoptotic gene was determined by using Roche® LightCycler®480 (96-well block), following the cycling program. Reaction mixture of 20 µl taking 75 ng of the cDNA and 5 μM of each primer with 2 × of SYBR Green I dye Roche Diagnostics. The cycling conditions at 95 °C for 10 min denaturation step, followed by 40 cycles of 95 °C for 15 s denaturation, 60 °C for 20 s annealing, and 72 °C for 20 s elongation steps. Data were normalized by GAPDH, a housekeeping control gene. All the experiment was done in triplicate.
2.9 Data analysis
ANOVA followed by Dunnett's multiple comparison tests were used for statistical analysis data. Statistical significance was attributed at p < 0.05. Each experiment was carried in triplicates. Images were captured at constant exposure time
3 Results
3.1 X-ray diffraction (XRD) analysis
The XRD reveals the crystallite size, phases and crystallinity of the processed material. The assign peaks in the spectrum (Fig. 1) are well coordinated with a single crystalline wurtzite hexagonal phase bulk ZnO (JCPDS, Card No. 36–1451). No any other peaks or impurities again affirm that the processed material is well prepared and it’s within the detection limit of the XRD. The sharp and strong intensity peaks in the diffraction spectrum displayed the good-crystallinity of synthesized powder.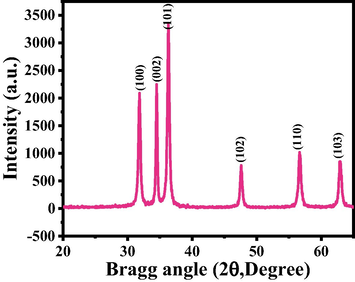
X-ray diffraction pattern of grown ZnO-NRs via solution process.
3.2 Morphological observations (FESEM result)
The morphology of the material was analyzed with FESEM and the data is represented as Fig. 2. From the obtained images, its reveals that the processed structure is full micro-flower shaped structures are seen with defied hexagonal nanorods. The average length and diameter of formed NRs are in the range of 2–4 μm (Fig. 2A and B), whereas the diameter of each NRs are varies form 200–250 nm. The individual NR surfaces are clear, smooth and hexagonal structure, which is a typical characteristic of wurtzite phase of zinc oxide (Fig. 2C). From the images it's evident that the entire rods shaped structures are originating from a center point and organize in such a way that they possess a spherical flower-like morphology. The NRs were jointed together through their wider bases in a spherical shape and form the flower-shaped structures. The full array of one flower-shaped structure is in the range of 2–3 μm.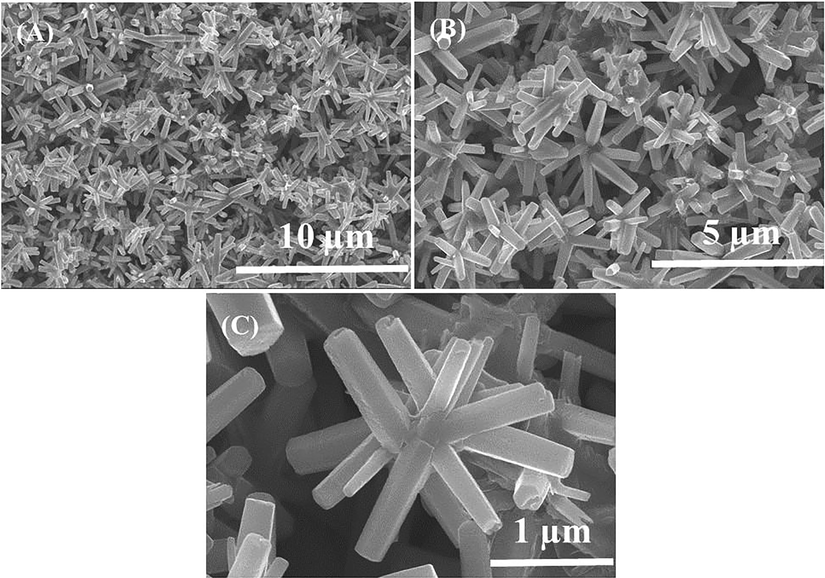
Shows the FESEM images of flowers shaped morphology of ZnO-NRs: at low magnification (a and b) whereas (c) presents the high magnification.
3.3 Functional groups analysis (FTIR spectroscopy results)
The functional characteristic of the processed powder was examined and assigned represent the FTIR spectroscopy as Fig. 3. The peak at 405 cm−1 is related to zinc oxide whereas the bands at 3200–3600 cm−1 correspond to O–H mode of vibration. The small asymmetric stretching mode of vibration of O–H was observed between 1629 cm−1. The symmetric and asymmetric stretching vibrational peak occurs between 1385 and 887 cm−1 which designates the vibration of NO3-1 ions (Vijayakumar et al., 2016; Kumar et al., 2017).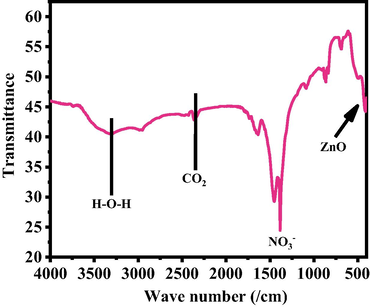
FTIR Spectra of grown NRs.
3.4 Cytotoxicity (MTT Assay) with ZnO-NRs
Cytotoxicity induction by ZnO-NRs in a concentration-dependent manner (Fig. 4A and B). Image of cell death morphology was taken only one concentration IC50 and control HepG2 cells for 24 h treatment. The cancer cells HepG2 for MTT assay as with different concentrations of material from 2 to 100 µg/ml for 24 h incubation. It shows that viability of HepG2 cells was diminished by ZnO-NRs was concentration dependent. The cancer cells viability, with MTT assay was decreased at 24 h 101.7%, 98.3 %, 94.4%, 84%, 71.3%, 65% and 55% (Fig. 4B) for the concentrations range of 1, 2, 5, 10, 25 50 and 100 μg/mL respectively (p < 0.05 for each). It showed from the obtained data at high concentration is more affected.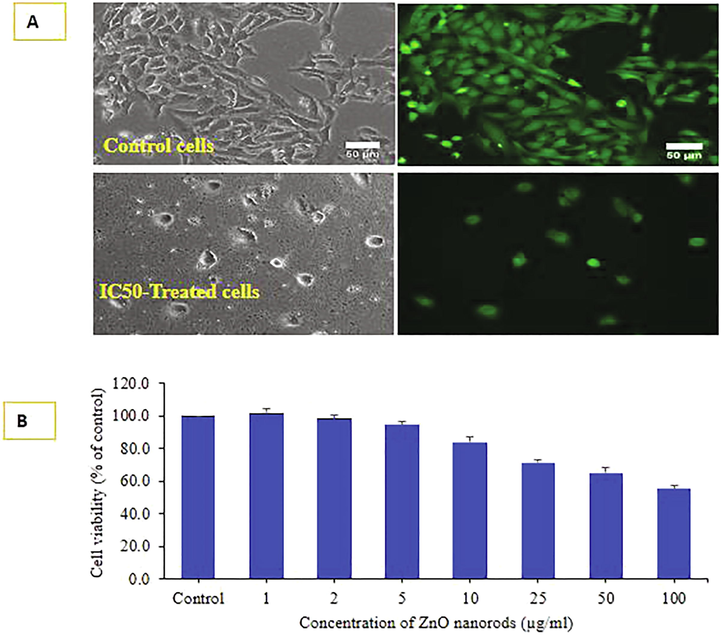
Cell viability was determined by live cell imaging (A) due to treatment with IC50 of ZnONRs under phase contrast and calceinAM live cell fluorescent probe. Phase and calceinAM images represent superimposable pairs in control and treated groups. Cell viability was also determined by MTT assay is given in figure (B).Data represented are mean ± SD of three identical experiments in triplicates.
3.5 ROS generation with ZnO-NRs induced HepG2 cells
ROS generation was examined in HepG2 cells after the exposure of ZnO-NRs at a concentration of 10, 25, 50, and 100 μg/ml for 24 h (Fig. 5A) with compared to control. As it can be observed from the image that the concentration of IC50 and control (Fig. 5A) that ROS is increases with the increase of ZnO-NRs as compared to control cells. The ROS were upsurge at 10, 25, 50 and 100 μg/ml were 110, 138, 165, 185 % as compared to control (100%) (Fig. 5B). Data represented are mean ± SD of three identical experiments in triplicates. *statistically significant difference as compared to the controls (p < 0.05).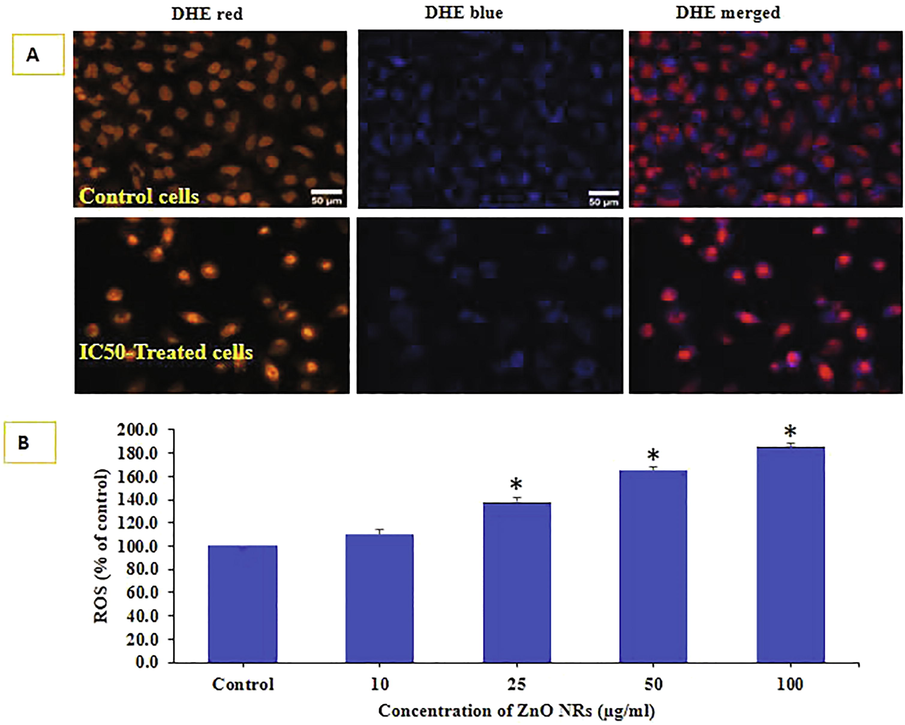
ROS was detected by DHE probe in control and IC0-treated NPs in HepG2 cells in Fig. 5 (A). Live cell images were taken in tandem for DHE red and DHE blue. Quantification of DHE red fluorescence calculated in Image J software (NIH,US) is given in Fig. 5. (B). Data represented are mean ± SD of three identical experiments in triplicates. *statistically significant difference as compared to the controls (p < 0.05).
3.6 Mitochondrial membrane potential (MMP) analysis induced ZnO-NRs
Degree of MMP induction was determined by the JC-1 imaging and JC-1 ratio of monomer/ aggregate both IC50 and control HepG2 cells treated with ZnO-NRs (Fig. 6A). MMP level of percent control was determined at different concentration of ZnO-NRs in HepG2 cells exposed to for 24 h at 10, 25, 50 and 100 µg/ml are presented in Fig. 6B. The decrease in MMP level was recorded in terms of fluorescence intensity of mitochondrial-specific dye JC-1. It is clearly presented that ZnO-NRs decreased the fluorescence intensity.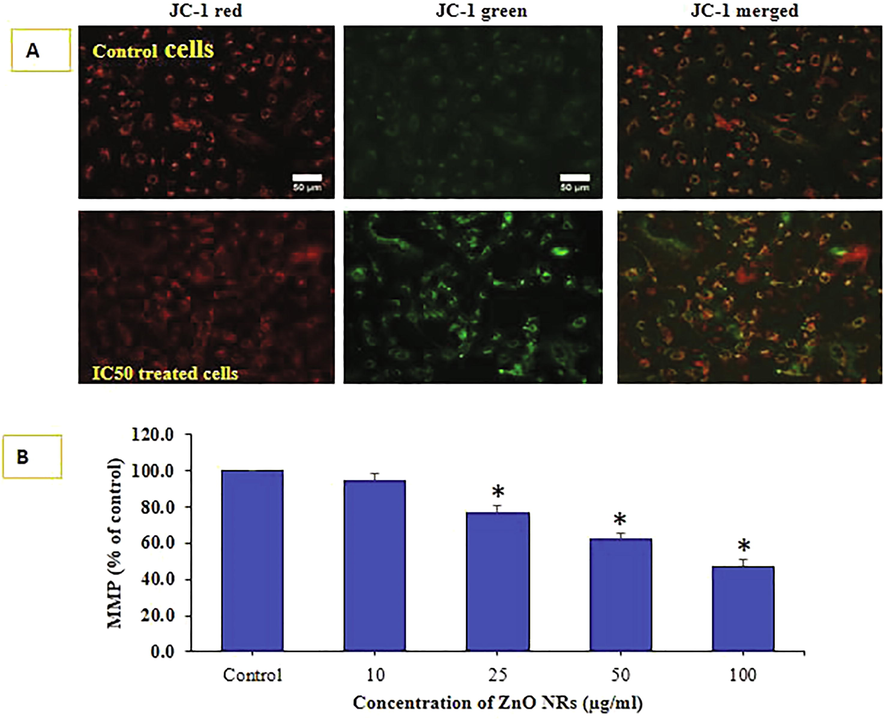
MMP was detected by JC-1 in control and treated cells. Images taken in tandem for JC-1 aggregate (red) and JC-1 monomer (green) and merged Fig. 6 A. MMP level of percent control was determined at different concentration of ZnONRs Fig. 6 B. Data represented are mean ± SD of three identical experiments (n = 3) made in triplicates. *statistically significant difference as compared to the controls (p < 0.05).
3.7 Real time PCR induced by ZnO-NRs
For the gene expression analysis mRNA level was determined by quantitative RT-PCR with HepG2 cells treated ZnO-NRs for 24 h at a concentration of 100 μg/ml. Quantitative RT-PCR was performed to calculate the change of genes at the mRNA level (bax, p53, casp3, and bcl-2). The level of gene expression apoptotic gene fold changes for bax 1.8, p53 2.2 casp3 2.9 and anti-apoptotic gene bcl2 showed 0.6 fold respectively (Fig. 7). Data represented are mean ± SD of three identical experiments in triplicates. *statistically significant difference as compared to the controls (p < 0.05).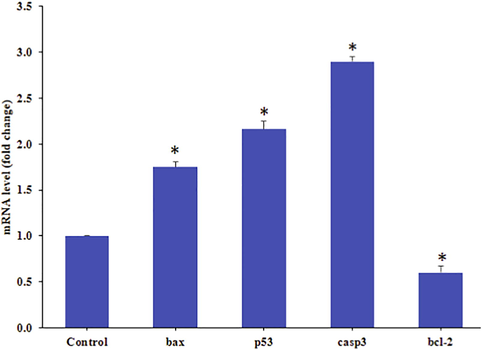
Quantification of mRNA fold change, apoptotic genes (p53, bax, and casp3) and anti-apoptotic gene (bcl-2) was evaluated. HepG2 cells treated 50 μg/mL of ZnO-NRs for 24 h. For internal control GAPDH was used. The values are mean ± SE of three independent experiments. *Statistically significant difference as compared to control (p < 0.05).
3.8 Discussion
Based on the experimental and their observations related to work, we have discussed here that the hexagonal shaped ZnO nanorods were prepared with HMT, zinc nitrate hexahydrate (Zn(NO3)2·6H2O) and alkali sodium hydroxide (NaOH). As detailed in the material and methods Zn(NO3)2·6H2O was dissolved in water and a transparent solution was formed. We have used HMT to this solution because it acts as a structure directing agent. In this solution the NaOH was added as a wise in the beaker, at this point a small flocculent was seen in the beaker for few seconds and then it was disappeared. As we may assume that these initial flocculent is an initial molecule for the formation of hexagonal shaped zinc nanorods. It’s evident that the solution method provides the bulk amount of nanorods shaped powder. The obtained liquidly product was eroded completely and dried for the purpose of biological study (Wahab et al., 2011). A number of reports published on the nanoparticles and related structures whereas a limited study is available on hexagonal shaped zinc oxide nanorods (Wahab et al., 2009), As we know that the inorganic based oxide nano or microstructures structures cytotoxicity depends on different chemical and physicochemical parameters (eg: size, shape, chemical compound, concentration, cells medium concentration, incubation periods etc) (Wahab et al., 2009). In the current case hexagonal shaped NRs, which exhibit a unique shaped structure with diameter about 200 nm and length varies to 2–3 μm, initially attach to the surface of the cancer cells. The cells provide spaces because the cells size (20 μm) is more as compared to the NRs facilitates to enter in to cells cytoplasm and react with the cells organelles and are responsible for cell death. It’s also postulated that the reactive oxygen species (ROS), which is a key factor in cell death produces in cells interaction with the foreign materials, and are responsible to form the free radicals in cells. These free radicals in cells reacted with the cells organelles and produce the enzymatic change, which are responsible for a deformation of the cells and their contents (Wahab et al., 2014, Dwivedi et al., 2014). The observed data MTT, MMP are well corroborated with the available results. The microscopic image proves that the cells viability effect with zinc oxide nanostructures. A number of studies are required to conclude the phenomena why these rods shaped structures are responsible for the cell death in cancer cells (Henderson et al., 2007).
4 Conclusion
The summary of the present work demonstrates that, we have successfully fabricated the hexagonal shaped ZnO-NRs via solution process and well characterized. The XRD pattern reveals the processed nanorods crystalline property, size with size of nanostructures, in spite of this the morphology was also evaluated via FESEM and it demonstrates that each individual size of nanorod is ∼ 250 nm in diameter and 2–3 μm length with good crystalline in nature. The chemical functional characteristics of the processed material property analyzed through FTIR spectroscopy and it reveals that the material is pure zinc oxide with good chemical property. The MTT assay was utilized to check the viability of cells at dose dependent manner, which express that initial concentration is very important for the cells reduction. The result obtained in MMP level in HepG2 cells further reveals the significance of utilized nanostructures. The apoptosis in cells with NRs were also studied in presence of P53, Bax casapse 3, and Bcl2 and their experimental results showed that the ZnO-NRs exposed with apoptotic genes, which displayed the up-regulation activity while anti apoptotic gene (Bcl2) showed down-regulation activity in cancer cells. Based on the acquired data’s and their observations related to the interaction of NRs and cancer cells are also explained. The utilized analysis and their observations, reveals that the ZnO-NRs are responsible for the cells death for ROS generation. The oxide based nanostructures opens a new phenomenon to use as a nanocarrier available at low cost and can be easily use in future.
Acknowledgement
The authors are grateful to the Deanship of Scientific Research, King Saud University for funding through Vice Deanship of Scientific Research Chairs.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Evaluation of toxicity of zinc oxide nanorods on green microalgae of freshwater and marine ecosystems. Environ. Chem. Ecotoxicol.. 2021;3:85-90.
- [Google Scholar]
- Green synthesis of stable antioxidant, anticancer and photocatalytic activity of zinc oxide nanorods from Leea asiatica leaf. J. Biotechnol.. 2021;329:65-79.
- [Google Scholar]
- ZnO nanorod-induced apoptosis in human alveolar adenocarcinoma cells via p53, survivin and bax/bcl-2 pathways: role of oxidative stress. Nanomed. Nanotechnol. Biol. Med.. 2011;7(6):904-913.
- [Google Scholar]
- Route of administration induced in vivo effects and toxicity responses of Zinc Oxide nanorods at molecular and genetic levels. Int. J. Nano Dimens.. 2018;9(2):158-169.
- [Google Scholar]
- Zinc oxide nanorod clusters deposited seaweed cellulose sheet for antimicrobial activity. Int. J. Biol. Macromol.. 2018;112:1264-1271.
- [Google Scholar]
- Identification of target organs of copper nanoparticles with ICP-MS technique. J. Radioanal. Nucl. Chem.. 2007;272(3):599-603.
- [Google Scholar]
- Zinc oxide spiky nanoparticles: A promising nanomaterial for killing tumor cells. Mater. Sci. Eng., C. 2021;124:112071.
- [CrossRef] [Google Scholar]
- Reactive oxygen species mediated bacterial biofilm inhibition via zinc oxide nano particles and their statistical determination. PLoS ONE. 2014;9(11):e111289.
- [CrossRef] [Google Scholar]
- Anti-candidal activity and in vitro cytotoxicity assessment of graphene nanoplatelets decorated with zinc oxide nanorods. Nanomaterials.. 2018;8(10):752.
- [CrossRef] [Google Scholar]
- Epitaxial growth of the zinc oxide nanorods, their characterization and in vitro biocompatibility studies. J. Mater. Sci.: Mater. Med.. 2011;22(10):2301-2309.
- [Google Scholar]
- Synthesis, characterization and biocompatibility studies of zinc oxide (ZnO) nanorods for biomedical application. Nano-Micro Lett.. 2010;2(1):31-36.
- [Google Scholar]
- Induction of inflammation in vascular endothelial cells by metal oxide nanoparticles effect of particle composition. Environ. Health Perspect.. 2006;115(3):403-409.
- [Google Scholar]
- Mass balance of metolachlor in a grassed phytoremediation system. Environ. Sci. Technol.. 2007;41(11):4084-4089.
- [Google Scholar]
- Toxicity of different zinc oxide nanomaterials and dose-dependent onset and development of Parkinson’s disease-like symptoms induced by zinc oxide nanorods. Environ. Int.. 2021;146:106-179.
- [Google Scholar]
- Antimicrobial properties of ZnO nanomaterials: a review. Ceram. Int.. 2017;43(5):3940-3961.
- [Google Scholar]
- The control of cell adhesion and viability by zinc oxide nanorods. Biomaterials. 2008;29(27):3743-3749.
- [Google Scholar]
- Porous ZnO nanorod for targeted delivery of doxorubicin: in vitro and in vivo response for therapeutic applications. J. Mater. Chem.. 2012;22(45):24145.
- [CrossRef] [Google Scholar]
- Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods.. 1983;65(1-2):55-63.
- [Google Scholar]
- Detection of superoxide anion and hydrogen peroxide production by cellular NADPH oxidases. Biochim. Biophys. Acta Gen. Subj.. 2014;1840(2):757-767.
- [Google Scholar]
- Ethnoveterinary plants of Uttaranchal a review. Indian J. Tradit. Know.. 2007;6:444-458.
- [Google Scholar]
- Analysis of dihydroethidium fluorescence for the detection of intracellular and extracellular superoxide produced by NADPH oxidase. Free Radic. Res.. 2007;41(6):699-712.
- [Google Scholar]
- Synthesis, characterisation and dispersion of zinc oxide nanorods for biomedical applications. Micro & Nano Lett.. 2010;5(5):355-360.
- [Google Scholar]
- Intracellular heterogeneity in mitochondrial membrane potentials revealed by a J-aggregate-forming lipophilic cation JC-1. Proc. Natl. Acad. Sci.. 1991;88(9):3671-3675.
- [Google Scholar]
- In vitro cytotoxicity of gold nanorods in A549 cells. Environ. Toxicol. Pharmacol.. 2015;39(2):871-878.
- [Google Scholar]
- Zinc oxide nanorod based immunosensing platform for the determination of human leukemic cells. Talanta. 2019;200:378-386.
- [Google Scholar]
- Laurus nobilis leaf extract mediated green synthesis of ZnO nanoparticles: characterization and biomedical applications. Biomed. Pharmacother.. 2016;84:1213-1222.
- [Google Scholar]
- Low temperature solution synthesis and characterization of ZnO nano-flowers. Mater. Res. Bull.. 2007;42(9):1640-1648.
- [Google Scholar]
- Room temperature synthesis of needle-shaped ZnO nanorods via sonochemical method. Appl. Surf. Sci.. 2007;253(18):7622-7762.
- [Google Scholar]
- Photocatalytic activity of zinc oxide micro-flowers synthesized via solution method. Chem. Engg. J.. 2011;168(1):359-366.
- [Google Scholar]
- A non-aqueous synthesis, characterization of zinc oxide nanoparticles and their interaction with DNA. Synth. Metals.. 2009;159(23-24):2443-2452.
- [Google Scholar]
- ZnO nanoparticles induced oxidative stress and apoptosis in HepG2and MCF-7 cancer cells and their antibacterial activity. Colloids Surf., B. 2014;117:267-276.
- [Google Scholar]
- Biomedical Applications of Zinc Oxide Nanomaterials. Curr Mol Med.. 2013;13(10):1633-1645.
- [Google Scholar]
- In vitro toxicity studies of zinc oxide nano- and microrods on mammalian cells: A comparative analysis”. Mater. Lett.. 2016;179:90-94.
- [Google Scholar]







