Cytotoxic secondary metabolites from mangrove-rhizosphere-associated fungus Emericella sp. strain SWR1718
⁎Corresponding authors at: Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Mutah University, 61710 Al-Karak, Jordan. rorfali@ksu.edu.sa (Raha Orfali), weaamnabil@mans.edu.eg (Weaam Ebrahim), ss_ebada@mutah.edu.jo (Sherif S. Ebada) sherif_elsayed@pharma.asu.edu.eg (Sherif S. Ebada)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Chemical exploration of mangrove-rhizosphere-associated fungus Emericella sp. strain SWR1718 (Aspergillaceae) was performed through various chromatographic workup procedures. The achieved results afforded one new natural compound, emericelactone E (1) in addition to known compounds (2–7). The planar structures of the purified compounds were unambiguously carried out using several spectroscopic methods. Both relative and absolute configurations of compound 1 were carefully determined based on NOESY experiments, coupling constants and comparing its optical rotation with related congeners. Moreover, a plausible biosynthetic pathway of 1 and its related derivatives is reported for the first time in our study. The cytotoxic potential of isolated metabolites was assessed toward three human tumor cell lines where some of them exhibited moderate activities compared to paclitaxel as a standard anticancer.
Keywords
Emericella
Mangrove
Emericelactone
Cytotoxic activity
1 Introduction
Fungi represent a ubiquitous group of eukaryotes that are currently consisting of seven principal phyla: Chytridiomycota, Ascomycota, Basidiomycota, Microsporidia, Glomeromycota, Neocallimastigomycota and Blastocladiomycota (Simões et al., 2013). There is no doubt that fungi are a very essential soil component which play the roles of both decomposers and plant symbionts (Liu et al., 2015). Fungi have been a burgeoning source for several new and bioactive natural products (Suryanarayanan et al., 2012). Particularly, fungal strains that can withstand environments having high salt content proved to biosynthesize unique biomolecules (Ebada and Ebrahim, 2020). Being one of the unique ecosystems which are dramatically threatened, mangroves are coastal biotopes which are considered as an important source of terrestrial organic matter. This is because they harbor a broad spectrum of microbes especially fungi (Simões et al., 2015). Although, several researchers have attempted to study these ecosystems from the microbiological point of view, yet few reports explored their fungal communities (Simões et al., 2015). In fact, a huge proportion of the mangrove ecosystem total biomass is consisting of microbes that encourage natural product researchers to investigate the mangrove-related microbial community especially the fungal content of these ecosystems (Alongi, 1988). Unfortunately, the reports on fungi in the soil zone that is located in the vicinity of the roots (mangrove rhizospheres) are very scanty and reports which are found are mostly based on culture-dependent investigations (Simões et al., 2015).
Emericella is one of the mangrove-associated genera which is known to produce several interesting metabolites featuring complex structural stereochemistry such as cyclopeptides (Oh et al., 2007; Malmstrøm, 1999), diketopiperazines (Xu et al., 2013), alkaloids (Zhang et al., 2011), polyketides (Pang et al., 2018a; Wei et al., 2005), merosesqui- and sesterterpenoids (Chen et al., 2019; Pang et al., 2018b; He et al., 2017). Either the extracts and/or pure metabolites isolated from the genus Emericella revealed a vast array of bioactivities such as antiviral (Zhang et al., 2011), antibacterial (He et al., 2017), antileishmanial (Alves et al., 2018) and insecticidal activities (Abraham et al., 2015).
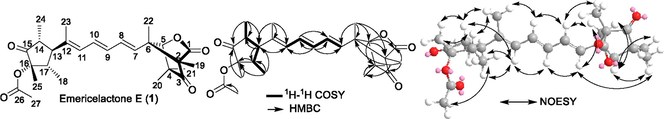
During our current research targeting the isolation and the identification of new and/or bioactive fungal secondary metabolites from mangrove-associated fungi (Ebrahim et al., 2012a, 2012b, 2013; Elissawy et al., 2019; Moussa et al., 2016; Umeokoli et al., 2019), we have isolated a mangrove-rhizosphere-associated fungus Emericella sp. strain SWR1718. This fungus was derived from the rhizosphere region of Avicennia marina (Forssk.) Vierh. (Acanthaceae) gathered at Jeddah coastline in Saudi Arabia. The phytobiological investigation for methanol extract prepared from liquid Wickerham medium of mangrove-rhizosphere-associated fungus Emericella sp. strain SWR1718 resulted in isolation of one unreported natural product, emericelactone E (1) (Fig. 1) along with six known metabolites belonging to various chemical classes namely; asperthecin (2) (Frisvad and Samson, 2004), nidulol (3) (Fujita et al., 1984), adenine (4) (Cao et al., 2019), adenosine (5), 3′-deoxyadenosine (6) and 3′-deoxy-5′-acetyladenosine (7) (Kawahara et al., 1992) (Fig. 1).
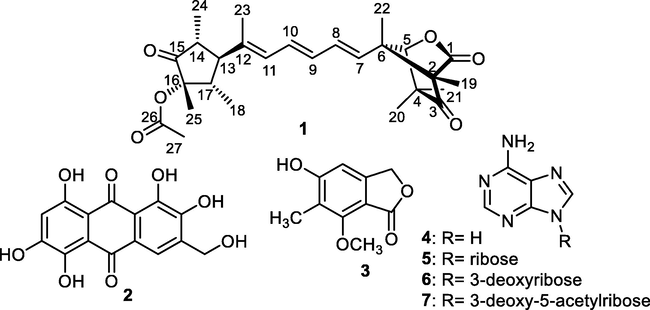
- Chemical structures of compounds (1–7).
In this study, we report isolation and structure elucidation of 1 including its relative and absolute configurations along with the results of assessing antiproliferative activity of isolated compounds against three different human tumor cell lines. HTB-176 cells are human lymphoblasts isolated from lymphoma. SW-620 cells are human colon cancer cells isolated from colon adenocarcinoma. HT-29 cells are human colorectal cancer cells isolated from colorectal adenocarcinoma.
2 Experimental
2.1 General experimental procedures
For column chromatography, Sephadex LH-20 (E. Merck, Darmstadt, Germany) was used as stationary phases. Aluminium ready-made TLC plates coated with silica gel F254 or RP-C18 (Merck, Darmstadt, Germany) were applied for Thin Layer Chromatography (TLC). Visualization was done at 254 nm and by spraying with ceric sulphate reagent. For HPLC applications, prominence Shimadzu LC Solution, (Shimadzu, Kyoto, Japan) was used that is equipped with a CBM-20A communication bus module, two LC-10AD pumps, a CTO-10A(C) column oven or Shim-pack VP-ODS (150 mm × 4.6 mm, 5.0 μm) analytical column and an SPD 10A(V) diode array detector (DAD). The used mobile phase is consisting of water supplemented with 0.1% TFA (A) and CH3OH (B) at a flow rate of 0.4 mL/min. Moreover, DAD detection channel was set at 254 nm wavelength. JASCO P-2000 Series polarimeter (JASCO Corporation, 2967-5, Tokyo, Japan) was used for determining optical rotations. Bruker AMX-700 spectrometer (Bruker, Faellanden, Switzerland) was implemented for 1H, 13C NMR and 2D NMR spectra using tetramethylsilane (TMS) as an internal standard. Agilent Triple Quadrupole 6410 QQQ LC/MS mass spectrometer (Agilent Technologies, Inc., USA) (for ESI negative and positive scan modes; nebulizer pressure at 60 psi with a gas flow rate of 12 L/min at 350 °C) and direct infusion method using CH3OH\H2O (1:1 v/v) was used for ionization (flow rate of 0.2 mL/min).
2.2 Plant and fungal strain materials
Emericella sp. strain SWR1718 was purified from rhizosphere soil of Avicennia marina sampled in Jeddah on the Saudi Arabian coastline in 2017 (Umeokoli et al., 2019). This fungal strain was deposited in one of authors’ laboratories (R.S.O.). The fungus was identified as Emericella sp. strain SWR1718 (GenBank accession number MN966856) according to the microbiological method previously described (Elnaggar et al., 2017).
2.3 Fermentation, extraction and isolation
Emericella sp. strain SWR1718 was cultured on sterile liquid Wickerham medium (malt 3.0 g, yeast 3.0 g, glucose 10.0 g, and peptone 5.0 g in 1000 mL distilled water) in 5 Erlenmeyer flasks (1 L each) statically at room temperature. The crude methanol extract of mycelial cells of liquid Wickerham medium weighed 280.1 mg after being evaporated under vacuum. This is followed by liquid–liquid fractionation between 90% aqueous MeOH and n-hexane. The aqueous MeOH extract (120 mg) was then purified by Sephadex LH-20 column (100 × 2.5 cm) using 100% methanol as an eluting solvent to yield eight subfractions (EM-1 to EM-8). Fraction EM-2 (39 mg) was then subjected to semi-preparative HPLC (H2O:MeOH, gradient elution) to yield 2 (0.4 mg) (purity: 99%), 4 (0.9 mg) (purity: 98%) and 7 (1.3 mg) (purity: 99%). Moreover, fraction EM-5 (28 mg) was further separated via semi-preparative HPLC (H2O:MeOH, gradient elution) to afford compound 1 (1.5 mg) (purity: 99%), 3 (0.3 mg) (purity: 97%) , 5 (1.2 mg) (purity: 98%) and 6 (0.7 mg) (purity: 98%).
Emericelactone E (1): colourless oil;
| pos. | δH (J inHz) a | δC, typea,b,c | δH (J inHz) d | δC, type b,c,d |
|---|---|---|---|---|
| 1 | 174.5, C | 172.6, C | ||
| 2 | 70.0, C | 68.4, C | ||
| 3 | 212.4, C | 211.2, C | ||
| 4 | 49.1, C | 46.6, C | ||
| 5 | 4.69, s | 90.4, CH | 4.89, s | 88.2, CH |
| 6 | 59.9, C | 58.5, C | ||
| 7 | 5.55, d (15.8) | 132.1, CH | 5.59, d (15.7) | 131.0, CH |
| 8 | 6.30, dd (15.8, 10.5) | 136.1, CH | 6.27, dd (15.7, 10.5) | 133.2, CH |
| 9 | 6.17, dd (14.7, 10.5) | 132.2, CH | 6.19, dd (14.7, 10.5) | 131.2, CH |
| 10 | 6.58, dd (14.7, 11.1) | 131.5, CH | 6.61, dd (14.7, 11.1) | 130.4, CH |
| 11 | 6.05, dd (11.1, 1.5) | 130.2, CH | 6.00, dd (11.1) | 128.3, CH |
| 12 | 138.5, C | 137.6, C | ||
| 13 | 1.94, t (11.8) | 58.9, CH | 1.97, t (11.9) | 56.2, CH |
| 14 | 2.67, dq (11.8, 7.0) | 45.4, CH | 2.56, dq (11.9, 6.7) | 43.5, CH |
| 15 | 217.7, C | 214.7, C | ||
| 16 | 85.7, C | 83.9, C | ||
| 17 | 2.73, dq (11.8, 7.0) | 39.6, CH | 2.61, dq (11.9, 6.8) | 37.8, CH |
| 18 | 0.92, d (7.0, 3H) | 11.7, CH3 | 0.82, d (6.8, 3H) | 11.4, CH3 |
| 19 | 1.19, s (3H) | 6.1, CH3 | 1.13, s (3H) | 5.8, CH3 |
| 20 | 1.26, s (3H) | 23.6, CH3 | 1.18, s (3H) | 22.9, CH3 |
| 21 | 1.11, s (3H) | 25.0, CH3 | 1.04, s (3H) | 24.4, CH3 |
| 22 | 1.37, s (3H) | 18.9, CH3 | 1.29, s (3H) | 18.4, CH3 |
| 23 | 1.77, d (1.5, 3H) | 11.8, CH3 | 1.70, s (3H) | 11.7, CH3 |
| 24 | 0.96, d (7.0, 3H) | 12.1, CH3 | 0.86, d (6.7, 3H) | 12.6, CH3 |
| 25 | 1.16, s (3H) | 17.2, CH3 | 1.09, s (3H) | 16.8, CH3 |
| 26 | 171.1, C | 169.6, C | ||
| 27 | 2.01, s (3H) | 20.8, CH3 | 1.99, s (3H) | 20.7, CH3 |
2.4 Cytotoxicity assay
Full details of cytotoxicity assay are present in supplementary materials.
3 Results and discussion
Emericelactone E (1) was recovered as a colourless oil that revealed pseudomolecular ion peaks in LRESIMS at m/z 457.6 [M+H]+ and at m/z 455.5 [M−H]−. Its molecular formula was ascertained to be C27H36O6 following its HRESIMS measurement that unravelled a pseudomolecular peak at m/z 457.2522 [M+H]+ (calcd for C27H37O6, 457.2590). The determined molecular formula undoubtedly indicated the existence of ten degrees of unsaturation. Its 13C NMR data (Table 1) disclosed the presence of 27 different resonances distinguished into nine quaternary carbon including two ketocarbonyl carbons (δC 217.7 and δC 212.4), two carboxyl carbons (δC 174.5 and δC 171.1), one olefinic carbon (δC 138.5) and four aliphatic carbons (δC 85.7, δC 70.0, δC 59.9 and δC 49.1). In addition, 13C NMR spectrum of 1 revealed five olefinic carbons at δC 136.1, δC 132.2, δC 132.1, δC 131.5 and δC 130.2 together with one oxygenated methine carbons at δC 90.4 and three aliphatic methine carbons (δC 58.9, δC 45.4 and δC 39.6). The remaining nine carbon resonances were all designated for methyl groups (δC 25.0, δC 23.6, δC 20.8, δC 18.9, δC 17.2, δC 12.1, δC 11.8, δC 11.7 and δC 6.1). Compound 1 exhibited a maximal absorption (λmax) at 288.6 nm in its UV spectrum. By exploring the reported literature and by comparison to the obtained data, compound 1 was suggested to be a related derivative to emericelactones A-D, polyketide fungal metabolites featuring a characteristic tetramethyloxabicyclo[2.2.1]heptanes-1,3-dione moiety (Pang et al., 2018a). The 1H NMR data of 1 (Table 1) further supported its proposed chemical class of fungal metabolites via revealing five olefinic protons at δH 6.58 (dd, J = 14.7, 11.1 Hz), δH 6.30 (dd, J = 15.8, 10.5 Hz), δH 6.17 (dd, J = 14.7, 10.5 Hz), δH 6.05 (dd, J = 11.1, 1.5 Hz) and δH 5.55 (d, J = 15.8 Hz), three aliphatic methines at δH 2.73 (dq, J = 11.8, 7.0 Hz), δH 2.67 (dq, J = 11.8, 7.0 Hz) and δH 1.94 (t, J = 11.8 Hz) along with an oxygenated singlet methine proton (δH 4.69). The 1H NMR data of 1 also disclosed the presence of six singlet methyl resonances at δH 2.01, δH 1.37, δH 1.26, δH 1.19, δH 1.16 and δH 1.11 together with three doublet methyl protons at δH 1.77 (d, J = 1.5 Hz), δH 0.96 (d, J = 7.0 Hz) and δH 0.92 (d, J = 7.0 Hz). Further confirmation of the unambiguous structure elucidation of 1 was achieved through the results obtained from extensive 2D NMR spectroscopic analyses (Fig. 2, Table 2) including 1H–1H COSY, HMBC, HMQC and NOESY experiments. The 1H–1H COSY spectrum (Fig. 2) displayed the existence of a triene moiety conjugating over C-7 to C-12 with an additional methyl group at C-12. The presence of this substructure was assured by the COSY spin system from H-7 to H-11 in addition to key HMBC correlations (Fig. 2, Table 2) from Me-23 at δH 1.77 (d, J = 1.5 Hz) to three carbons at δC 138.5 (C-12), δC 130.2 (C-11) and δC 58.9 (C-13). Further COSY correlations (Fig. 2, Table 2) were recognized featuring another spin system extending over Me-18/H-17/H-13/H-14/Me-24 together with key HMBC correlations from H-13 at δH 1.94 (t, J = 11.8 Hz) to C-15 (δC 217.7) and C-16 (δC 85.7), from H-14 at δH 2.67 (dq, J = 11.8, 7.0 Hz) to C-16 and C-17 (δC 39.6), from H-17 at δH 2.73 (dq, J = 11.8, 7.0 Hz) to C-12, C-14 (δC 45.4) and C-15, from Me-18 at δH 0.92 (d, J = 7.0 Hz) to C-13 and C-16, from Me-24 at δH 0.96 (d, J = 7.0 Hz) to C-13 and C-15 and from Me-25 at δH 1.16 (s) to C-15 and C-17 that altogether indicated the probable existence of a trimethylcyclopentan-1-one moiety with one oxygenated aliphatic quaternary carbon at C-16. By comparing spectroscopic data of 1 with the reported literature (Pang et al., 2018a), it turned out to be a close derivative to emericelactone B but with a higher molecular weight by 42 amu. Moreover, the methyl singlet at δH 2.01/δC 20.8 that was correlated via HMBC spectrum (Fig. 2) with a carboxyl carbon at δC 171.1 indicates the presence of an additional acetyl group in 1 explaining its higher molecular weight compared to emericelactone B. Based on NOESY correlations (Fig. 2, Table 2) along with the coupling constant values, all the three double bonds in (1) were distinguished all to be in E-configurations resembling their comparable positions in emericelactone B. The relative configuration of the tetramethyloxabicyclo[2.2.1]-heptanes-1,3-dione unit was recognized to be (2S*, 5R*, 6S*) similar to those reported for ukulactones A-C (Kaifuchi et al., 2015; Mori et al., 2011) together with emericelactones A-D (Pang et al., 2018a) as well by comparing their NMR data of the respective moiety. In addition, NOESY spectrum of 1 supported the suggested relative configuration by cross correlations of H-5 with H-8 and Me-22; and Me-19 with H-7. The relative configuration of trimethylcyclopentan-1-one moiety was determined based on NOE cross correlations of H-14 and H-17 with Me-25, H-13 with Me-18 and Me-24 indicating that H-14, H-17 and Me-25 similarly oriented, while H-13, Me-18 and Me-24 oriented toward the opposite side of the structure.
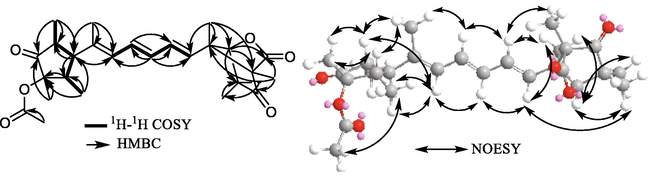
- Key 1H–1H COSY, HMBC and NOESY correlations of compound 1.
| pos. | 1H–1H COSY | 1H–13C gHMBC | NOESY |
|---|---|---|---|
| 5 | – | C-1; C-2; C-3; C-4; C-19; C-22 | H-8, Me-20, Me-21, Me-22 |
| 7 | H-8 | C-2; C-5; C-6; C-9; C-22 | Me-19, Me-20, Me-22 |
| 8 | H-7; H-9 | C-6; C-7; C-9; C-10 | H-5, Me-20, Me-22 |
| 9 | H-8; H-10 | C-7; C-8; C-10; C-11 | H-7, H-11 |
| 10 | H-9; H-11 | C-8; C-9; C-11; C-12 | H-8, Me-23 |
| 11 | H-10; Me-23 | C-9; C-10; C-12; C-13; C-23 | H-9, H-13, Me-18, Me-24 |
| 13 | H-14; H-17 | C-11; C-12; C-14; C-17; C-23; C-24 | H-11, Me-18, Me-24, Me-27 |
| 14 | H-13; Me-24 | C-12; C-13; C-15; C-24 | H-17, Me-23, Me-25 |
| 17 | H-13; Me-18 | C-12; C-13; C-16; C-18; C-25 | H-14, Me-23, Me-25 |
| 18 | H-17 | C-13; C-16; C-17 | H-13, Me-24, Me-27 |
| 19 | – | C-1; C-2; C-3; C-6 | H-7 |
| 20 | – | C-3; C-4; C-5; C-21 | H-5, H-7, H-8 |
| 21 | – | C-3; C-4; C-5; C-20 | H-5 |
| 22 | – | C-2; C-5; C-6; C-7 | H-5, H-7, H-8 |
| 23 | H-11 | C-11; C-12; C-13 | H-10 |
| 24 | H-14 | C-13; C-14; C-15 | H-11, H-13, H-18 |
| 25 | – | C-15; C-16; C-17 | H-14, H-17 |
| 27 | – | C-26 | H-13, Me-18 |
Interestingly, the presence of a single hydroxylated carbon at C-16 suggested that it is the only possible position to be acetylated supported by its more downfield carbon resonance (δC 85.7) compared to its corresponding carbon in the non-acetylated congener, emericelactone B (δC 75.9) (Pang et al., 2018a). Another evidence strengthened the placement of acetyl group at C-16 is the NOE interaction figured out between Me-27 with H-13 and Me-18. By comparing 1D/2D NMR data of 1 and emericelactone B which obviously revealed close values along with their biosynthetic similarities and their close optical rotation values, the absolute configuration of 1 was deduced to be as 2S,5R,6S,13S,14R,16S,17S. As a conclusion, compound 1 was determined to be 16-O-acetylemericelactone B which was given a trivial name emericelactone E.
It is noteworthy mentioning that emericelactone E (1) was carefully detected in the original HPLC chromatogram of total methanol extract of Emericella sp. strain SWR1718 in this study. Its identity was confirmed by means of its UV spectrum and LCMS to assure that it is a genuine natural product. In addition, its deacetylated ancestor, emericelactone B, cannot be traced in the same extract.
After collecting NMR data and performing biological assays on the freshly isolated compound 1, a trial was done in order to elucidate the absolute configuration by ECD. An ECD spectrum was obtained (please see supplementary material, Fig. S12) indicating that compound 1 was decomposed either upon storage or by the effect of deuterated DMSO used for NMR measurement.
Based on careful search in the reported literature, a plausible biosynthetic pathway for emericelactone (1) (Fig. 3) was deduced. It was proposed to be emerging from a polyketide biosynthetic cascade resembling those reported for structurally related fungal metabolites such as ukulactones (Kaifuchi et al., 2015; Mori et al., 2011), prugosenes (Lang et al., 2007) and wortmannilactones (Liu et al., 2018).
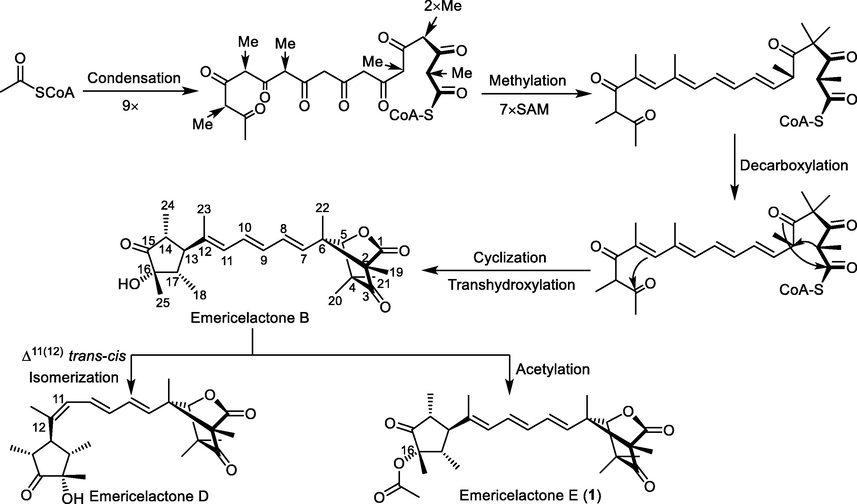
- A plausible biosynthetic pathway of emericelactone E (1).
The cytotoxic activities of compounds (1–7) were tested for against three human cancer cell lines using the MTT assay. The obtained results (Table 3) disclosed that most of these compounds possess moderate antiproliferative activities against the tested cell lines. Amongst the tested compounds, nidulol (3) revealed moderate cytotoxic activity against the three tested cell lines HTB-176 (lymphoma), SW-620 (colon) and HT-29 (colorectal adenocarcinoma) with IC50 values of 18.6, 15.7 and 36.9 µM, respectively, compared to paclitaxel as a standard cytotoxic agent (IC50 of 18.7–23.3 µM). Interestingly, the isolated compounds have not exhibited any remarkable toxicity against CCD-18 (normal colon) cell line.
| Compound | CCD-18a | IC50 (µM) | ||
|---|---|---|---|---|
| % Cell viabilityb | HTB-176a | SW-620a | HT-29a | |
| Emericelactone E (1) | 89 ± 2.89 | 28.3 ± 1.76 | 46.4 ± 1.78 | NAc |
| Asperthecin (2) | 95 ± 3.75 | 36.2 ± 1.34 | NAc | 82.8 ± 1.07 |
| Nidulol (3) | 72 ± 2.77 | 18.6 ± 2.23 | 15.7 ± 2.77 | 36.9 ± 1.75 |
| Adenine (4) | 111 ± 3.76 | NAc | NAc | NAc |
| Adenosine (5) | 92 ± 1.57 | 42.9 ± 1.06 | 85.3 ± 2.01 | 99.6 ± 1.96 |
| 3′-Deoxyadenosine (6) | 112 ± 2.14 | NAc | NAc | NAc |
| 3′-Deoxy-5′-acetyladenosine (7) | 95 ± 3.34 | 40.7 ± 2.06 | 77.8 ± 2.14 | 75.2 ± 1.58 |
| Paclitaxel | 20.4 ± 0.78 | 18.7 ± 1.57 | 23.3 ± 0.41 | |
4 Conclusion
Seven secondary metabolites including one new compound, emericelactone E (1), were identified from a crude methanol extract of mangrove-rhizosphere-associated fungus Emericella sp. strain SWR1718. Moreover, we demonstrate a plausible biosynthesis of a unique class of compounds to which emericelactone E belongs for the first time in our study. Interestingly, nidulol (3) revealed moderate cytotoxic activity against the three tested cell lines HTB-176 (lymphoma), SW-620 (colon) and HT-29 (colorectal adenocarcinoma) with IC50 values of 18.6, 15.7 and 36.9 µM, respectively, compared to paclitaxel as a standard cytotoxic agent (IC50 of 18.7–23.3 µM).
Acknowledgments
The authors acknowledge the Deanship of Scientific Research at King Saud University, Saudi Arabia for funding this study through the work project No. (RG-1440-032).
Disclosure statement
The authors declare that there is no conflict of interest.
References
- Insecticidal activity of ethyl acetate extracts from culture filtrates of mangrove fungal endophytes. Mycobiology. 2015;43:137-149.
- [Google Scholar]
- Bacterial productivity and microbial biomass in tropical mangrove sediments. Microb. Ecol.. 1988;15:59-79.
- [Google Scholar]
- Alves, D.R., de Morais, S.M., Tomiotto-Pellissier, F., Vasconcelos, F.R., Freire, F.d.C.O., da Silva, I.N.G., Cataneo, A.H.D., Miranda-Sapla, M.M., Pinto, G.A.S., Conchon-Costa, I., 2018. Leishmanicidal and fungicidal activity of lipases obtained from endophytic fungi extracts. PLoS One 13, e0196796
- Antimicrobial lavandulylated flavonoids from a sponge-derived Streptomyces sp. G248 in East Vietnam Sea. Mar. Drugs. 2019;17:e529.
- [CrossRef] [Google Scholar]
- Emerones A-C: three novel merosesquiterpenoids with unprecedented skeletons from Emericella sp. XL029. Org. Biomol. Chem.. 2019;17:8450-8455.
- [Google Scholar]
- Quinoisobutyride A, an acyclic antibacterial tetrapeptide incorporating an unprecedented heterocyclic amino acid residue from the hypersaline lake-derived fungus Penicillium simplicissimum strain WSH17. Phytochem. Lett.. 2020;36:95-98.
- [Google Scholar]
- Decalactone derivatives from Corynespora cassiicola, an endophytic fungus of the mangrove plant Laguncularia racemosa. Eur. J. Org. Chem.. 2012;2012:3476-3484.
- [Google Scholar]
- Pullularins E and F, two new peptides from the endophytic fungus Bionectria ochroleuca isolated from the mangrove plant Sonneratia caseolaris. Mar. Drugs. 2012;10:1081-1091.
- [Google Scholar]
- Unusual octalactones from Corynespora cassiicola, an endophyte of Laguncularia racemosa. Tetrahedron Lett.. 2013;54:6611-6614.
- [Google Scholar]
- New secondary metabolites from the mangrove-derived fungus Aspergillus sp. AV-2. Phytochem. Lett.. 2019;29:1-5.
- [Google Scholar]
- Two new triterpenoids and a new naphthoquinone derivative isolated from a hard coral-derived fungus Scopulariopsis sp. Fitoterapia. 2017;116:126-130.
- [Google Scholar]
- Emericella venezuelensis, a new species with stellate ascospores producing sterigmatocystin and aflatoxin B1. System. Appl. Microbiol.. 2004;27:672-680.
- [Google Scholar]
- O-Methylation effect on the carbon-13 nuclear magnetic resonance signals of ortho-disubstituted phenols and its application to structure determination of new phthalides from Aspergillus silvaticus. Chem. Pharm. Bull.. 1984;32:2622-2627.
- [Google Scholar]
- Bioassay-guided isolation of antibacterial metabolites from Emericella sp. TJ29. J. Nat. Prod.. 2017;80:2399-2405.
- [Google Scholar]
- A nucleoside derivative from Emericella nidulans. Phytochemistry. 1992;31:1409-1410.
- [Google Scholar]
- Ukulactone C, a new NADH-fumarate reductase inhibitor produced by Talaromyces sp. FKI-6713. J. Gen. Appl. Microbiol.. 2015;61:57-62.
- [Google Scholar]
- New pentaenes from the sponge-derived marine fungus Penicillium rugulosum: structure determination and biosynthetic studies. Tetrahedron. 2007;63:11844-11849.
- [Google Scholar]
- Pyrosequencing reveals fungal communities in the rhizosphere of Xinjiang jujube. BioMed Res. Int.. 2015;2015
- [CrossRef] [Google Scholar]
- The activities of wortmannilactones against helminth electron transport chain enzymes, structure-activity relationships, and the effect on Trichinella spiralis infected mice. J. Antibiot.. 2018;71:731-740.
- [Google Scholar]
- Unguisins A and B: New cyclic peptides from the marine-derived fungus Emericella unguis. J. Nat. Prod.. 1999;62:787-789.
- [Google Scholar]
- Ukulactones A and B, new NADH-fumarate reductase inhibitors produced by Penicillium sp. FKI-3389. Tetrahedron. 2011;67:6582-6586.
- [Google Scholar]
- Tetrahydroanthraquinone derivatives from the mangrove-derived endophytic fungus Stemphylium globuliferum. Tetrahedron Lett.. 2016;57:4074-4078.
- [Google Scholar]
- Induced production of emericellamides A and B from the marine-derived fungus Emericella sp. in competing co-culture. J. Nat. Prod.. 2007;70:515-520.
- [Google Scholar]
- Emericelactones A-D: Four novel polyketides produced by Emericella sp. XL 029, a fungus associated the leaves of Panax notoginseng. Tetrahedron Lett.. 2018;59:4566-4570.
- [Google Scholar]
- Emericellins A and B: Two sesquiterpenoids with an unprecedented tricyclo [4, 4, 2, 1] hendecane scaffold from the liquid cultures of endophytic fungus Emericella sp. XL 029. Fitoterapia. 2018;131:55-58.
- [Google Scholar]
- Simões, M.F., Pereira, L., Santos, C., Lima, N., 2013. Polyphasic identification and preservation of fungal diversity: Concepts and applications. In: Management of Microbial Resources in the Environment. Springer. pp. 91–117.
- Soil and rhizosphere associated fungi in gray mangroves (Avicennia marina) from the Red Sea – a metagenomic approach. Genom. Proteom. Bioinf.. 2015;13:310-320.
- [Google Scholar]
- Fungal endophytes: an untapped source of biocatalysts. Fungal Divers.. 2012;54:19-30.
- [Google Scholar]
- A new depsidone derivative from mangrove sediment derived fungus Lasiodiplodia theobromae. Nat. Prod. Res.. 2019;33:2215-2222.
- [Google Scholar]
- Shimalactone A, a novel polyketide, from marine-derived fungus Emericella variecolor GF10. Tetrahedron. 2005;61:8054-8058.
- [Google Scholar]
- Secoemestrin D, a cytotoxic epitetrathiodioxopiperizine and emericellenes A-E, five sesterterpenoids from Emericella sp. AST0036, a fungal endophyte of Astragalus lentiginosus 1. J. Nat. Prod.. 2013;76:2330-2336.
- [Google Scholar]
- Antiviral isoindolone derivatives from an endophytic fungus Emericella sp. associated with Aegiceras corniculatum. Phytochemistry. 2011;72:1436-1442.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2020.05.008.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







