Translate this page into:
Cytotoxic evaluation with aluminum oxide (Al2O3) nanoparticles in human cancer cells
* Corresponding author: E-mail address: javedahmad@ksu.edu.sa (J. Ahmad)
-
Received: ,
Accepted: ,
Abstract
Al2O3 (aluminum oxide) has a special quality that makes it easy to use in many industrial applications. There are several cytotoxic studies available in the literature, but not much is known about their biological function when Al2O3 is present at optimized concentrations. The aim of the study was to determine the cytotoxicity against lung (A-549) and colorectal (Caco-2) cancer cells at very minute concentrations (5, 10, 25, 50, 100, 250, and 500 µg/mL) of Al2O3NPs. Initially, several equipment were used to characterize the aluminum oxide (Al2O3). For this, tools like X-ray diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM), and Fourier transform infrared (FTIR) spectroscopy were employed. The results showed that the nanoparticles (NPs) had a diameter of approximately 10-15 nm. MTT was employed for cytotoxicity investigation, whereas the NRU test, ROS, and qPCR analysis were used for genotoxicity examination. MTT and Neutral Red Uptake (NRU) assay with aluminum oxides (Al2O3), which indicates that loss in cell viability, was used to analyze the cytotoxicity. The genes like p53, BAX, and CASPASE-3 were found to be up-regulated in qPCR data, whereas BCL-2 was found to be down-regulated. These findings suggest that ROS and genotoxicity pathways are responsible for apoptosis. Al2O3 exhibits a moderate and dose-dependent effect on cancer cells, according to the cytotoxicity tests. Al2O3 has the ability to cause cell death by genotoxicity and ROS formation, as evident from the analysis.
Keywords
Al2O3NPs
Cell lines
MTT
NRU
ROS
1. Introduction
Alumina oxide (Al2O3) and other metal oxides are commercially important nano particles (NPs). Aluminum (Al) in its micro- and nanoscale forms has great oxidizing power, light weight, and mechanical resilience. Because of their unique properties, Aluminum microparticles and related materials are largely used in culinary goods (Gehrke et al. 2015 and Vignal et al. 2016). The functionality of NPs and their impact on the environment and human health are closely linked to their physical characteristics, including physicochemical properties such as surface charge, size, shape, and porosity (Modena et al., 2019). Even when the raw components are the same, NPs have unique physicochemical properties based on the manufacturing techniques. Additionally, because of their increased surface area, high reactivity, smaller size, better capacity to pass biological barriers, and ability to change their characteristics in biological settings, NPs elicit different toxicities than naturally occurring particles (Li et al., 2008; Willhite et al., 2014). The toxic effect of Al-NPs on the organs is influenced by many experimental variables, including exposure time, route, and dose. Also, NPs have the ability to bind macrophages and stay in the liver and spleen for extended periods of time (Geraets et al., 2014). According to Park et al. (2015), animals absorb NPs through the skin, mouth, and respiratory systems. Once within the body, NPs can have harmful effects on an animal’s metabolism, protein expression, and structure (Canli et al., 2019). According to reports, Al2O3NPs cause cytokines release, DNA mutations, and ROS that are seriously harm to the immune system, liver, kidneys, and brain (Kim et al., 2018).
Furthermore, exposure to Al2O3NP can result in cytotoxicity, mitochondrial dysfunction, inflammatory reactions, genetic damage, and carcinogenicity (Yousef et al., 2019). After oral consumption, Al accumulates throughout the body and causes problems for humans. In large dosages, signs of renal failure, anemia, and neurobehavioral abnormalities have been reported (Krewski et al., 2007). Also, the kidneys, brain, and bones can collect Al2O3NPs. While these NPs can be inhaled and absorbed via skin, ingestion is common in the general population (Krause et al., 2020). The toxicokinetics and route of administration of Al2O3NPs indicate that they gather in the lungs before being directly injected into the systemic circulation. This circulatory blood then carries the particles to multiple organs, for instance, the parathyroid glands and thyroid, where they accumulate and cause physiological and histopathological disorders (Morsy et al., 2016).
The primary aim of this research is to examine the toxicity of γ-Al2O3NPs on A549 lung cancer cells and Caco-2 colorectal adenocarcinoma cells, as these are common cancers around the globe. The majority of packing materials contain γ-Al2O3NPs, which are also airborne. The aluminum NPs are largely used for the electrochemical, chemical, energy storage, solar cells, and related issues, but very limited studies are available for biological studies especially human cancer cells lines. The material was created using a solution procedure, and it underwent thorough investigation using the following techniques: X-ray diffraction (XRD), scanning electron microscope (SEM), Fourier transform infrared spectroscopy (FTIR), transmission electron microscope (TEM). The morphological structure of cells was investigated using microscopy, while MTT and Neutral red uptake (NRU) assays were used in cytotoxicity studies to determine the viability of the cells. Studies on the formation of reactive oxygen species (ROS) and RT-PCR were also carried out to determine the impact of cancer cells at various doses. Here is an explanation of a potential debate based on the findings and analysis.
2. Material and methods
2.1 Synthesis of nanoparticles and their characterizations
γ-Al2O3NPs were synthesized by hydrothermally reacting aluminum nitrate nonahydrate (Al(NO3)3.9H2O) to produce γ-Al2O3NPs. Al(NO3)3.9H2O was treated with sodium hydroxide (NaOH) to reduce the amount of γ-Al2O3. The Al(NO3)3.9H2O and NaOH were supplied by Aldrich Chemical Corporation Co., U.S.A., and disposed of as purchased. Initially, 80 mL of distilled water in a 100 mL Teflon-lined beaker containing the Al (NO3)3.9H2O (12 mM, or 0.4501g) was dissolved while being constantly agitated. Drop by drop, very small amount of SDS (1×10-2 mM) and sodium hydroxide (NaOH, 0.3 M) were added to this solution until it entirely reacted. This solution’s pH was measured (Cole-Parmer, USA), and the result was 12.73. After being securely enclosed inside a steel jacket, the Teflon beaker was baked at 120°C for six hrs. The steel jacket was taken to a cool place and kept there overnight at 25°C after the response time had expired. Following many cleanings with solvent (acetone, alcohol, etc.) to eliminate any leftover impurities from the reaction, the recovered product was allowed to dry at room temperature for a full day in order to prepare it for further chemical and morphological analysis. The material was well characterized with XRD, SEM, TEM, and FTIR.
2.2 Reagents and plastic ware used for the biological study
Purchased from Sigma Chemical Company Pvt. Ltd. in St. Louis, MO, USA, the MTT [3-(4, 5-dimethylthiazol-2-yl)-2, 5 diphenyltetrazolium bromide] was used without modification other than dilution. Additionally, Dulbecco’s Modified Eagle Medium (DMEM) and MEM culture medium, antibiotics—antimycotic and fetal bovine serum (FBS) were acquired from Invitrogen in United States. Nunc, Denmark provided the plastic wares and other consumable materials required in the cell culture.
2.3 MTT assay
The MTT test was used to assess the viability of cells in both control and treated samples in accordance with the predetermined methodology (Siddiqui et al., 2008). To put it briefly, the cells were first cultivated on 96-well plates at a rate of 1 × 104/well, and they were nurtured for 24 h at 37°C in a humid atmosphere. For a full day, the cells were exposed to prepared sample γ-Al2O3NPs ranging in concentration from 5 to 500 µg/mL. Following a thorough mixing of the cells in the well plates, a stock solution of MTT (5 mg/mL in PBS) was added at a rate of 10 μL/well in 100 μL of cell suspension, and the mixture was incubated for an additional 4 hrs.
Following the exposure, the well plate was gently mixed with ∼200 µL of DMSO to aspirate the formazan product, and the wells were then rinsed with PBS. Thermo Scientific, Finland’s Multiskan Ex multiwall microplate reader was used to measure the solution’s optical analysis at 550 nm. The control cells were used to run under the same conditions and as a point of reference. The sample solution’s solvent of choice determines the maximum absorbance, and the following formulae were used to calculate the percentage of viable cells:
2.4 NRU assay
The NRU assay was utilized to evaluate the toxicity of both control and treated γ-Al2O3NPs cancer cells, in accordance with earlier reports (Borenfreund et al.,1985). The cancer cells were sown in 96-well plates at a density of 10,000/well. After 24 h, when the cells were fully grown, expose them to the substance at the necessary concentration (5-500 µg/mL) and keep them in an incubator for another 24 hrs. Following the exposure, the cells were cultured for an additional three hrs in NR media (50 µg/mL). Following this, the cells were cleaned and the dye was extracted using a solution of 1% acetic acid and 50% ethanol. At 540 nm, the developed color was examined.
2.5 Reactive oxygen species (ROS) generation
Consuming 2, 7-dichloro dihydrofluoresce in diacetate (DCFH-DA; Sigma Aldrich, USA) dye as a fluorescent agent, the production of ROS was investigated. This was examined using the methodology that was previously outlined (Zhao et al., 2014). After being exposed to the processed material for 24 hrs, the cells were thoroughly cleaned with PBS and incubated in DCFH-DA (20 μM) in a dark medium at 37°C for 30 minutes. After DCFH-DA’s reaction with both control and treated cells was finished, intracellular fluorescence was examined under a fluorescence microscope.
2.6 Isolation of total RNA and real-time PCR (RT-PCR)
A set of primers was used for the quantification of apoptotic and anti-apoptotic genes with the Qiagen RNeasy mini Kit. RNA was extracted from 3 × 105 cells/well of caco-2 and A549 cells that were left untreated and treated for 24 hrs with 200 μg/mL of γ-Al2O3NPs. The RNA was electrophoresed on a 2% agarose gel using the documentation system (Universal Hood II, Bio Rad, USA), and the total RNA purity was confirmed using a Nanodrop 8000 spectrophotometer (Thermo Scientific, USA). Following the kit instructions, MLV reverse transcriptase (GE Health Care, UK) was used to synthesis cDNA using 100 ng of oligo (dT) 12–18 primer and 2 μg of RNA. Using the Roche® LightCycler®480 (96-well block), the set of primers for the apoptotic and anti-apoptotic genes were quantified while adhering to the suggested cycling regimen. For the 20 µL reaction combination, use two times SYBR Green I from Roche Diagnostics with 100 ng of cDNA and 7.5 μM of each primer. The RT-PCR cycling settings are as follows: 40 cycles of 95°C for 15s of denaturation, 60°C for 20s of annealing, and 72°C for 20 s of elongation. The denaturation stage lasts 10 minutes. GAPDH served as an internal housekeeping control gene to normalize the data on expressed genes.
2.7 Statistical analysis
The obtained data is displayed as a mean ± SD and the statistical analysis was performed at significant level when P <0.05.
3. Results and discussion
3.1 X-ray diffraction pattern (XRD)
The XRD (Fig. 1) was used to identify the phases, full width half maxima (FWHM), and size of the generated powder. Several peaks including ˂102˃ 20.36, ˂113˃ 27.87, ˂221˃ 32.84, ˂311˃ 36.53, ˂115˃ 40.44, ˂040˃ 45.59, ˂242˃ 53.20, ˂511˃ 59.62, and ˂046˃ 67.01 were found and categorized based on the XRD pattern. The phases of the powder material are consistent with γ-Al2O3, as confirmed by JCPDS database card no. 46-1215, which has lattice constants of a = 7.934 Å, b = 7.956 Å, and c = 11.71 Å. The significant assigned peaks, such as <040> (45.59) and ˂046˃ (67.01), in addition to other peaks in the spectrum, clearly show the dimension of γ-Al2O3. The size was calculated using the well-known Scherer equation (Pakrashi et al., 2013; Sifontes et al., 2014). According to the crystallite size calculation, the average size is between 10-15 nm, with good crystalline properties and authenticate that the powder contains minuscule γ-Al2O3 NPs.
- X-ray diffraction pattern of synthesized γ-Al2O3NPs.
3.2 Morphological analysis of prepared nanostructured material
The structure was assessed by SEM, and the resulting image have been demonstrated in Fig. 2a. There are microscopic particles scattered across the surfaces, some of which are gathered together to resemble clusters, according to the SEM images (Fig. 2). Upon closer inspection at a higher magnification, the structural detail shows that each particle has smooth, clean surfaces and a spherical shape with a very small diameter. After a thorough analysis of the particle dimensions, the findings (Fig. 2a) show that each particle is spherical in shape, well-organized, and has a diameter of about ∼10-15 nm.
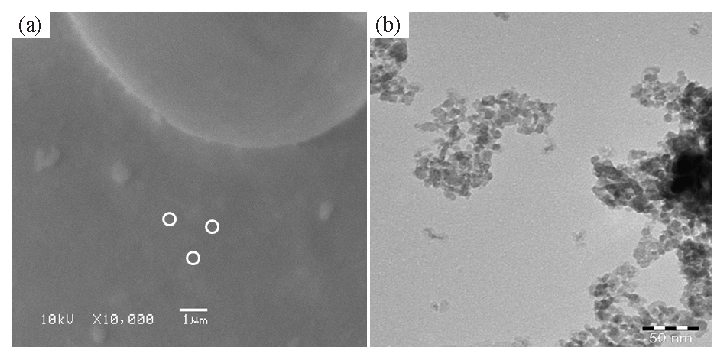
- (a) Display the SEM image of synthesized γ-Al2O3NPs received at low magnification. White circles show the individual fine particles, whereas (b) illustrates the TEM Image of γ-Al2O3 QDs. QDs: Quantum dots.
Fig. 2b displays the findings that were obtained after further examination of the structural observation using TEM. TEM indicates that whole surface in the picture (Fig. 2b) appears to be covered in a large number of small particles. The morphology and size of each particle were well-checked, based on TEM images. The findings demonstrate that every particle is distributed throughout the surfaces and has a spherical form, with an average size of about 10 nm (Fig. 2). The picture shows that entire particles are spherical, but few are grouped into formations that have distinct, oval-like morphologies. The finding indicates that when all the particles are gathered together, they form spheres. Each particle is scattered over the whole surface and is very small (Fig. 2). These justifications are in good agreement with results from other studies, such as XRD and SEM (Pakrashi et al., 2013; Noguchi et al., 2008; Chen et al., 2022).
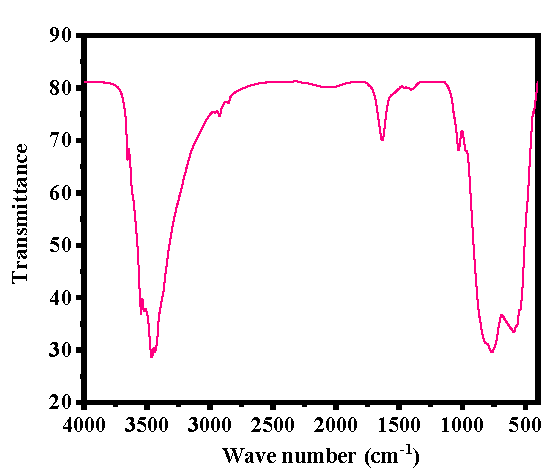
3.3 Chemical functional group analysis
FTIR spectroscopy was used to examine the functional groups of the generated powder in order to understand the material’s chemical footprint. Fig. 3 displays the spectrum data that was obtained. Several peaks in the 400–4000 cm-1 FTIR spectrum are associated with metal oxides and functional groups such as H-O-H, O–H, NO32– and γ-Al2O3. A broad and curved band observed between 3200-3600cm-1 is associated with a water molecule (3467 cm-1) (Pakrashi et al., 2013; Sifontes et al., 2014), whereas asymmetric stretching of hydroxyl (O-H) molecules were localized at 1647 cm-1. Additionally, the FTIR spectrum shows the nitrate (NO32-) molecule’s stretching and bending modes are linked to the bands centered at 1396 cm-1 and 1031 cm-1 respectively, while the shallow and pointed bands ranges ∼780 to 581 cm-1 denotes the presence of γ-Al2O3 (Pakrashi et al., 2013; Noguchi et al., 2008).

- FTIR spectrum of γ-Al2O3NPs.
3.4 Microscopic study of Caco-2 and A-549 cells treated with Al2O3
Following the procedures outlined in the materials and method section, the cancer (Caco-2 and A-549) cells were cultured, and after 24 hrs of incubation, their morphology was assessed using microscopy at a variety of chosen concentrations of γ-Al2O3NPs (5, 10, 25, 50, 100, 250, and 500 µg/mL) in contrast with the control (Fig. 4). The acquired image clearly shows that, at initial range of concentration (50 µg/mL) of γ-Al2O3NPs, no noticeable alteration was detected; nevertheless, when this concentration was enhanced to 500 µg/mL, the cells’ development was influenced. The recovered photos demonstrate how the treated material had a significant impact on cell development, which was dose-dependent. At the maximum 500 µg/mL concentration of γ-Al2O3NPs, the morphology and proliferation of cells were completely destroyed (Fig. 4). The processed γ-Al2O3NPs extensively destroyed the cells, as seen by the microscopic photographs. A-549 cells treated with the same quantities of γ-Al2O3NPs exhibit a very similar pattern (Fig. 5).
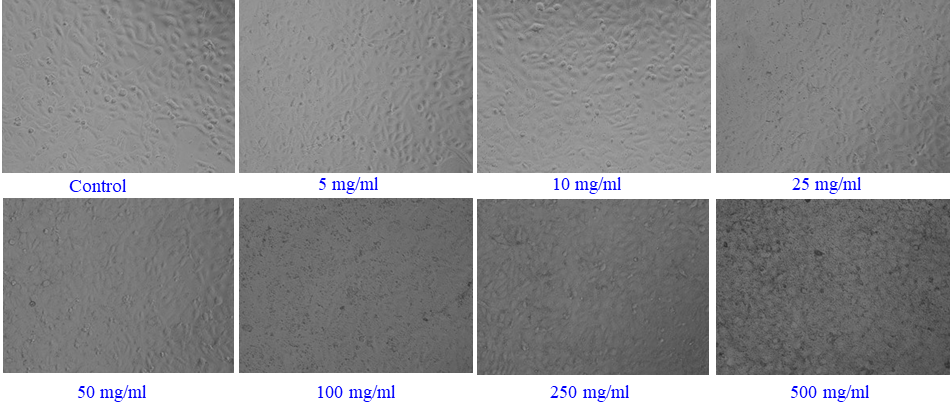
- γ-Al2O3NPs - induced morphological changes in Caco-2 cells. Cells were exposed to Al2O3-NPs at various concentrations for 24 hrs. Images were taken using a phase contrast inverted microscope at 20x magnification.

- γ-Al2O3NPs -induced morphological changes in A-549 cells. Cells were exposed to Al2O3-NPs at various concentrations for 24 hrs. Images were taken using phase contrast inverted microscope at 20x magnifications.
3.5 The induced cytotoxicity (MTT Assay) with Al2O3
The cytotoxicity of the cancer cells (A-549 and Caco-2 cells) was assessed by MTT assay using material concentrations ranging from 5, 10, 25, 50, 100, 250, and 500 µg/mL for a 24-hour incubation period. The collected data indicate that the synthesized γ-Al2O3NPs reduced the viability of cancer cells, and that the result was dependent on both concentration and dose. Using the MTT assay, the Caco-2 cells’ viability decreased at 24 hrs to 100.2%, 100.3%, 96%, 91%, 81%, 73%, and 68% (Fig. 6a) for concentrations of 5, 10, 25, 50, 100, 250, and 500 µg/mL. (p<0.05 for each). MTT assay reduces at 24 hrs 101%, 102%, 100%, 96%, 88%, 81%, and 71% for the concentrations of (5, 10, 25, 50, 100, 250, and 500 µg/mL), correspondingly (p<0.05 for each), in the case of A-549 cells viability (Fig. 6b). The results obtained indicates that the cancer cells was significantly altered at low concentrations but this was changed when the concentration increased.
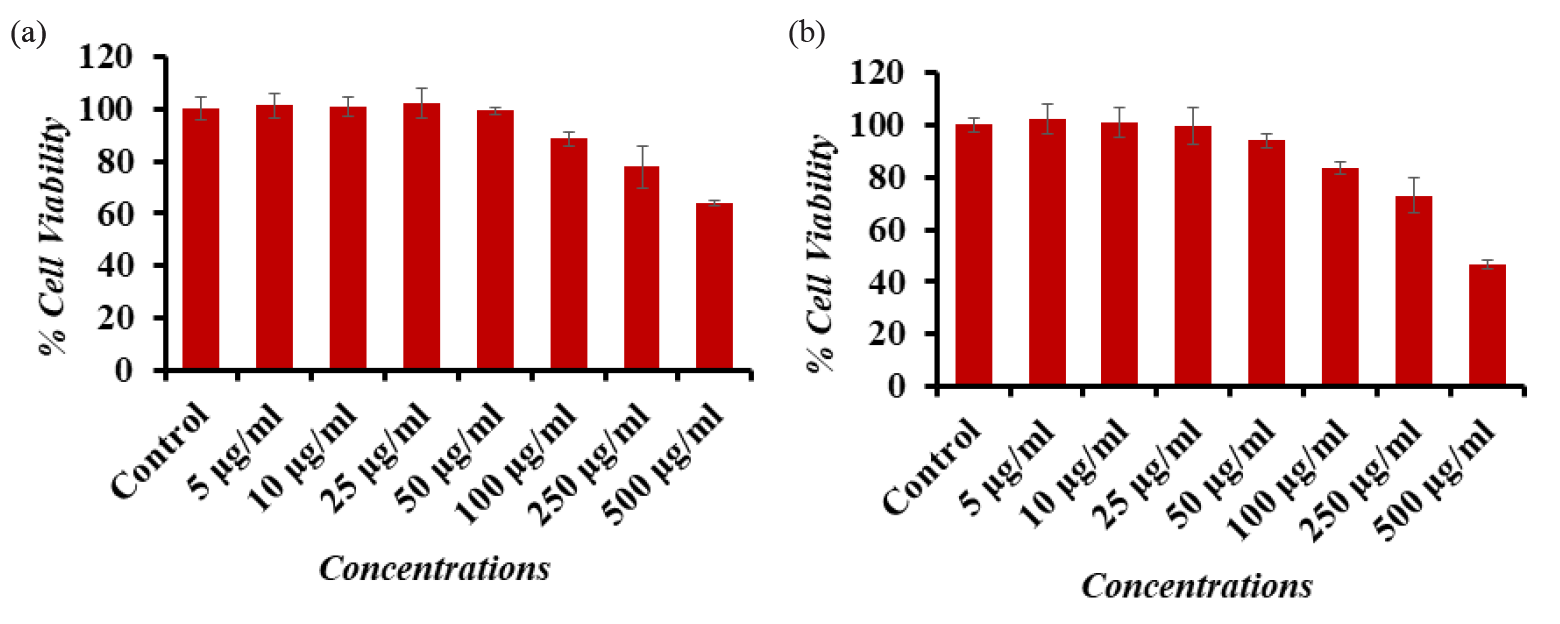
- Cytotoxic assessment of Al2O3-NPs in (a) Caco-2 cells and (b) A-549 cells by MTT assay. Cells were exposed to various concentrations of Al2O3-NPs for 24 hrs. The values are mean ± SE of three independent experiments (p<0.05). SE: Standard error.
3.6 NRU assay assessment of cancer cells with γ-Al2O3NPs
Similar findings were also observed in the NRU assay, which has been covered in the section on materials and methodology. The created materials had no effect on the two cancer cells that were used at first, but as the concentration or dose of NPs increased, the cells decreased, and the effect was dose-dependent. When comparing the doses of 5, 10, 25, 50, 100, 250, and 500 µg/mL to the Caco-2 cells, the NRU test showed decreases at 24 hrs of 99%, 97%, 96%, 90%, 80%, 72%, and 64% (Fig. 7a). Each drop was significant at p <0.05. For the amounts of (5, 10, 25, 50, 100, 250, and 500 µg/mL), respectively, the NRU assay showed declines at 24 hrs of 99%, 105%, 100%, 105%, 90%, 83%, and 73% (Fig. 7b). Each of these decreases was significant (p <0.05).
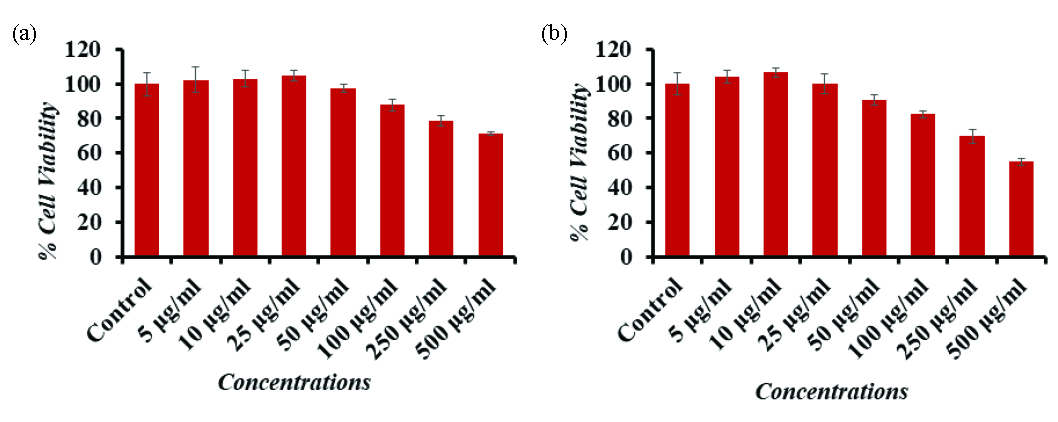
- Cytotoxic assessment of Al2O3-NPs in (a) Caco-2 cells and (b) A-549 cells by NRU assay. Cells were exposed to various concentrations of Al2O3-NPs for 24 hrs. The values are mean ± SE of three independent experiments (p<0.05). SE: Standard error.
3.7 ROS generation in cells with γ-Al2O3NPs
Following exposure of Caco-2 and A-549 cells to γ-Al2O3NPs at 100, 250 and 500 μg/mL for 24 hrs, a chronological order of ROS formation was analyzed (Fig. 8) with control. It was seen that in comparison to control cells, ROS increased when Al2O3 levels rose. In comparison to the control (100%), the ROS increased at 100 μg/mL, 250 μg/mL, and 500 μg/mL, measuring 126, 165, and 184% in Caco-2 cells, whereas it increased in A-549 cells to 135, 173, and 195%, respectively.
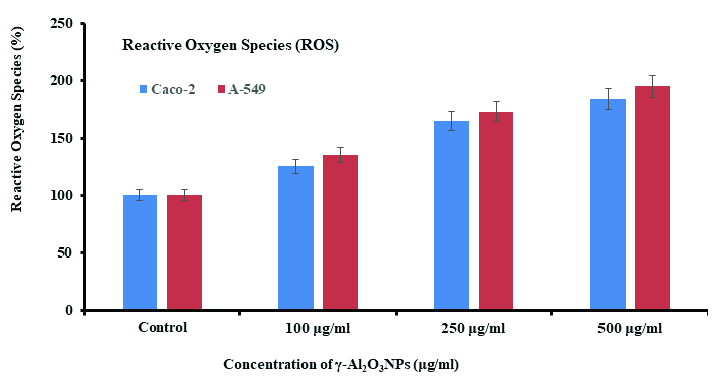
- ROS generation induced by ZnFe2O4NPs in Caco-2 and A-549 cells exposure to γ-Al2O3NPs at 100, 250, and 500 μg/mL with a control for 24 hrs. The values are mean ± SE of three independent experiments (p<0.05). SE: Standard error.
3.8 Real time PCR induced by γ-Al2O3NPs
Caco-2 and A549 cells were exposed to γ-Al2O3NPs for 24 hrs at a concentration of 250 μg/mL to investigate the mRNA level using RT-PCR. The mRNA levels of genes (p53, BAX, CASP3, and BCLl-2) were assessed using RT-PCR. Changed the Caco-2 and A549 cells’ apoptotic gene regulation significantly (p<0.05 for each gene). The pro-apoptotic gene Bax and the tumor suppressor gene p53 have elevated mRNA levels. Additionally, we noticed that cells treated with γ-Al2O3NPs had increased expression of the Caspase-3 gene. In cells exposed to γ-Al2O3NPs, the expression of the anti-apoptotic gene Bcl-2 was downregulated (Fig. 9).

- Gene expression study of Caco-2 and A-549 cells with γ-Al2O3NPs (250 µg/mL) induced mRNA fold change, apoptotic genes (p53, BAX, and CASP3), and anti-apoptotic gene (BCL-2) were calculated for 24 hrs. For the internal control, GAPDH was used. The values are mean ± SE of three independent experiments (p<0.05). SE: Standard error.
4. Discussion
After being synthesized using a solution procedure, the γ-Al2O3NPs were analyzed via XRD, which revealed details on the phases, FWHM, and crystallite size of the material. Using TEM and FTIR spectroscopy, the structural details and chemical bonding of a single particle were evaluated. The MTT assay was used to determine the reason behind the proliferation of cancer cells and their impact at different doses of γ-Al2O3NPs. In addition to ROS and gene analysis, very modest quantities (5, 10, 25, 50, 100, 250, and 500 µg/mL) of γ-Al2O3NPs were chosen and submitted for the MTT test study. The successful completion of the cytotoxic examination was attributed to the demonstration of γ-Al2O3NPs ability to inhibit cancer cell proliferation in a dose-dependent fashion. The MTT results show that at initial doses of 5, 10, and 25 µg/mL, γ-Al2O3NPs have no effect on the surface of cancer cells. However, a dose-dependent cell inhibition was seen as NPs concentrations increased to 50, 100, 250, and 500 µg/mL. The present study reports that NPs have been demonstrated to significantly inhibit cancer cells (Subramaniam et al., 2020). We may believe that essential factors are responsible for diminishing the rate of cancer cells, such as chemical and physical properties of the material (shape of the nanostructures, pH, concentrations, culture media, concentrations, particle interaction, cell conglomerates, etc. (Wahab et al., 2014). In this case, the cytoplasmic elements of the cancer cells were harmed by the absorption of γ-Al2O3NPs. When these particles penetrate the cells, the deformed structures cause the interior organelle structures to become chaotic (Nikolova et al, 2023; Malik et al. 2022). Furthermore, the other factors, such as free radicals in cell structures created due to the interaction of NPs, generate ROS and induce apoptosis in cancer cells (Djouina et al., 2016; Kapoor et al., 2024). In the current study, the fully cultivated cells were exposed to γ-Al2O3NPs, which caused them to start creating an envelope that covered the whole surface of the cancer cells. Because of their incredibly low density, γ-Al2O3NPs attached themselves to the cancer cells and started to cover the whole surface of the cells. Due to the absorption of NPs, the cell surfaces deteriorate with increasing concentration with incubation period. These γ-Al2O3NPs cause damage to the inner cell organelles, which finally becomes complete (Kapoor et al., 2024).
5. Conclusion
As per the current work’s overview, Aluminum oxide NPs (γ-Al2O3NPs) were synthesized, characterized, and utilized to evaluate the cytotoxic potential against Caco-2 and A549 cells’ cancer cells. The γ-Al2O3NPs were prepared via a solution process and characterized via X-ray diffraction pattern, which revealed that a single crystallite’s size is estimatedly ∼10-15 nm, whereas the developed structure’s morphology was determined by TEM. The analysis reveals that there are a number of particles (∼10-15 nm) scattered at the surfaces, which are spherical in shape and in agreement with the XRD observation. The FTIR analysis of the synthesized materials shows the functional features of NPs. The microscopic photos of the interactions between Caco-2 and A549 cancer cells’ reveal that the γ-Al2O3NPs with a concentrations (1, 2, 5, 10, 25, 50, and 100, 250 and 500 µg/mL with control) crystallites invaded the inner membrane of the cancer cells, damaging the cells’ organelles and ultimately causing the cells to die. A series of dose-dependent results were obtained for incubation times of 24 hrs against cancer cells. At low concentration, the interaction was not affected, while the NPs concentration increase, the cells’ death was greatly affected. The ROS levels increased due to the formation of free radicals in the cells, which inhibits the growth of cancer cells. The gene expression studies are also in good agreement with the MTT, NRU, and ROS and are fully dose-dependent. Therefore, the concentration of NPs is a crucial factor for designing a growth inhibitor for cancer cells with established standard quality.
Acknowledgment
The authors are grateful to the Deanship of Scientific Research, King Saud University, for funding through Vice Deanship of Scientific Research Chairs; Chair for DNA Research.
CRediT authorship contribution statement
Dr. Javed Ahmad: Conceptualization, Methodology, Software, Validation, Formal Analysis, Investigation, Resources, Data Curation, Writing – Original Draft, Writing – Review & Editing, Supervision, Project Administration, Funding Acquisition. Dr. Rizwan Wahab: Methodology, Software, Validation, Formal Analysis, Resources, Data Curation, Writing – Original Draft, Writing – Review & Editing. Dr. Maqsood A. Siddiqui: Methodology, Validation, Data Curation. Dr. Nida Nayyar Farshori: Methodology, Validation. Dr. Quaiser Saquib: Methodology, Software, Validation, Formal Analysis, Resources, Data Curation, Writing – Review & Editing, Project Administration, Funding Acquisition. Dr. Abdulaziz A. Al-Khedhairy: Resources, Writing – Review & Editing, Supervision, Project Administration, Funding Acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Declaration of Generative AI and AI-assisted technologies in the writing process
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
References
- Toxicity determined in vitro by morphological alterations and neutral red absorption. Toxicol Lett. 1985;24:119-124. https://doi.org/10.1016/0378-4274(85)90046-3
- [Google Scholar]
- Response of the antioxidant enzymes of rats following oral administration of metal-oxide nanoparticles (Al2O3, CuO, TiO2) Environ Sci Pollut Res Int. 2019;26:938-945. https://doi.org/10.1007/s11356-018-3592-8
- [Google Scholar]
- The nephrotoxic potential of polystyrene microplastics at realistic environmental concentrations. J Hazard Mater. 2022;427:127871. https://doi.org/10.1016/j.jhazmat.2021.127871
- [Google Scholar]
- Toxicological consequences of experimental exposure to aluminum in human intestinal epithelial cells. Food Chem Toxicol. 2016;91:108-116. https://doi.org/10.1016/j.fct.2016.03.008
- [Google Scholar]
- H. Tissue distribution and elimination after oral and intravenousadministration of different titanium dioxide nanoparticles in rats. Part. Fibre Toxicol. 2014;11:30.
- [Google Scholar]
- Innovations in nanotechnology for water treatment. Nanotechnol Sci Appl. 2015;8:1-17. https://doi.org/10.2147/NSA.S43773
- [Google Scholar]
- Metallic nanoparticles in cancer: Types, green synthesis, applications, tumor microenvironment and toxicity considerations. J Drug Deliv Sci Tech. 2024;92:105307. https://doi.org/10.1016/j.jddst.2023.105307
- [Google Scholar]
- Twenty-eight-day repeated inhalation toxicity study of aluminum oxide nanoparticles in male Sprague-Dawley rats. Toxicol Res. 2018;34:343-354. https://doi.org/10.5487/TR.2018.34.3.343
- [Google Scholar]
- Human health risk assessment for aluminium, aluminium oxide, and aluminium hydroxide. J Toxicol Environ Health B Crit Rev. 2007;10:1-269. https://doi.org/10.1080/10937400701597766
- [Google Scholar]
- Aluminum and aluminum oxide nanomaterials uptake after oral exposure - a comparative study. Sci Rep. 2020;10:2698. https://doi.org/10.1038/s41598-020-59710-z
- [Google Scholar]
- The role of oxidative stress in ambient particulate matter-induced lung diseases and its implications in the toxicity of engineered nanoparticles. Free Radic Biol Med. 2008;44:1689-1699. https://doi.org/10.1016/j.freeradbiomed.2008.01.028
- [Google Scholar]
- Metal nanoparticles: Biomedical applications and their molecular mechanisms of toxicity. Chem Pap. 2022;76:6073-6095. https://doi.org/10.1007/s11696-022-02351-5
- [Google Scholar]
- Studies on fate and toxicity of nanoalumina in male albino rats. Toxicol Ind Health. 2016;32:200-214. https://doi.org/10.1177/0748233713498462
- [Google Scholar]
- Nanoparticle characterization: What to measure? Adv Mater. 2019;31:e1901556. https://doi.org/10.1002/adma.201901556
- [Google Scholar]
- Updates on biogenic metallic and metal oxide nanoparticles: Therapy, drug delivery and cytotoxicity. Pharmaceutics. 2023;15:1650. https://doi.org/10.3390/pharmaceutics15061650
- [Google Scholar]
- Rapid synthesis of γ-Al2O3 nanoparticles in supercritical water by continuous hydrothermal flow reaction system. J Supercrit Fluids. 2008;46:129-136. https://doi.org/10.1016/j.supflu.2008.04.011
- [Google Scholar]
- Ceriodaphnia dubia as a potential bio-indicator for assessing acute aluminum oxide nanoparticle toxicity in fresh water environment. PLoS One. 2013;8:e74003. https://doi.org/10.1371/journal.pone.0074003
- [Google Scholar]
- A 13-week repeated-dose oral toxicity and bioaccumulation of aluminium oxide nanoparticles in mice. Arch Toxicol. 2015;89:371.
- [Google Scholar]
- Preparation of functionalized porous nano-γ-Al2O3 powders employing colophony extract. Biotechnol Rep (Amst). 2014;4:21-29. https://doi.org/10.1016/j.btre.2014.07.001
- [Google Scholar]
- Influence of cytotoxic doses of 4-hydroxynonenal on selected neurotransmitter receptors in PC-12 cells. Toxicol In Vitro. 2008;22:1681-1688. https://doi.org/10.1016/j.tiv.2008.07.001
- [Google Scholar]
- Assessment of the cytotoxicity of cerium, tin, aluminum, and zinc oxide nanoparticles on human cells. J Nanopart Res. 2020;22 https://doi.org/10.1007/s11051-020-05102-3
- [Google Scholar]
- Gut: An underestimated target organ for Aluminum. Morphologie. 2016;100:75-84. https://doi.org/10.1016/j.morpho.2016.01.003
- [Google Scholar]
- ZnO nanoparticles induced oxidative stress and apoptosis in HepG2 and MCF-7 cancer cells and their antibacterial activity. Colloids Surf B Biointerfaces. 2014;117:267-276. https://doi.org/10.1016/j.colsurfb.2014.02.038
- [Google Scholar]
- Systematic review of potential health risks posed by pharmaceutical, occupational and consumer exposures to metallic and nanoscale aluminum, aluminum oxides, aluminum hydroxide and its soluble salts. Crit Rev Toxicol. 2014;44:1-80. https://doi.org/10.3109/10408444.2014.934439
- [Google Scholar]
- Hepato-renal toxicity of oral sub-chronic exposure to aluminum oxide and/or zinc oxide nanoparticles in rats. Toxicol Rep. 2019;6:336-346. https://doi.org/10.1016/j.toxrep.2019.04.003
- [Google Scholar]
- Detecting the oxidative reactivity of nanoparticles: A new protocol for reducing artifacts. J Nanopart Res. 2014;16 https://doi.org/10.1007/s11051-014-2493-0
- [Google Scholar]







