Cytotoxic assessment of liver cancer cells (HepG2) with raw, functionalized multiwalled carbon nanotubes and their comparison with nanohydroxyapatite
⁎Corresponding author at: College of Science, Department of Zoology, P.O. Box 2455, King Saud University, Riyadh 114 51, Saudi Arabia. rwahab@ksu.edu.sa (Rizwan Wahab)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The carbon based nanostructured material exhibit unique and versatile properties and due this reason its largely applied in different fields. The present work was designed for to know the cytotoxic and genetic responses against liver cancer cells and was compared with the artificial bone implanted material hydroxyapatite (HA). The raw multiwalled carbon nanotubes (RMWCNTs) surfaces were turns to functionalized carbon nanotubes (FMWCNTs) via acid digestion process. The surface functional groups were identified through fourier transform infra red spectroscopy (FTIR) instruments, while structural detail of RMWCNTs, FMWCNTs and HA powder were observed via scanning electron microscopy (SEM) and transmission electron microscopy (TEM) consequently. The RMWCNTs, FMWCNTs and HAs powder were utilized against liver cancer (HepG2) cells to evaluate the rate of effectiveness against cells. The viability of grown cells were examined through MTT assay with the impact of doses of CNTs and HA powder (1 μg/mL, 2 μg/mL, 5 μg/mL,10 μg/mL, 25 μg/mL, 50 μg/mL, 100 μg/mL). The cells morphology was verified via inverted microscopy at different doses of CNTs and HA with control. Beside this, gene expression study was also performed for to know the apoptosis caused with CNTs and HA. The study was scrutinized with apoptotic and anti-apoptotic marker genes (p53, Bax, Caspase 3 and Bcl2) through quantitative polymerase chain reaction (qPCR). The qPCR data reveals that the up-regulation in gene expression and it shows that the CNTs, HAs are responsible for cells death with treatment at 50 μg/mL in 24 h incubation. The possible discussion related to cells death caused with CNTs and HAs were also described.
Keywords
RMWCNTs
FMWCNTs
Hydroxyapatite
FTIR spectroscopy
MTT
qPCR
1 Introduction
The polymeric nanostructured materials (PNMs) exhibit excellent properties in terms of their physicochemical characteristics (Agnello and Messina, 2020). Over a large number of materials, carbon based material exhibit a special place in material science, which is due to their unique electrical (Li et al., 2007) mechanical (Ding et al., 2020) and electrochemical properties (Mosqueda et al., 2014). Towards to this connection, benzene frame of carbon nanotubes (CNTs), exhibit multipurpose and immeasurable applications in various fields such as electronics (Meirinho et al., 2020), chemical sensors (Norizan et al., 2020), biological sensors (Xiong et al., 2020), catalyst (Hanif et al., 2020), energy storage (Geng et al., 2020) etc. CNTs are basically classified in two type’s single and multi-walled carbon nanotube (SW and MWCNTs) based on their sheets organizations in benzene ring (Liang et al., 2016). The size of SWCNT diameter ranges from ∼0.2–0.3 nm and length extents up to 1–2 µm, whereas the MWCNTs exhibit ∼10–15 nm and length exceeds up to 2–4 µm (Lee et al., 2020). The CNTs are largely utilized as an electrode material to detect the signals for various electrochemical and biological entities (Meirinho et al., 2020; Norizan et al., 2020). A long range of applications towards the electrochemical, storage and various other means are well documented (Agnello and Messina, 2020; Li et al., 2007; Ding et al., 2020). Including to this, it can also be utilized as a template material for the DNA interaction (Tardani et al., 2020) in drug delivery systems (Jha et al., 2020), retardation of bacterial proliferation (Frank et al., 2020), protein (Zhou et al., 2020), cell membranes via endocytosis or surface passivation processes (Liu and Boxer, 2020). In this direction, CNTs and HAs were employed for the cytological application with different cells and displayed comparative cytotoxicity studies with micro and nano range particles of graphite and MWCNTs respectively, with human lung epithelial cells (A549) (Azari and Mohammadian, 2020). In other work, rat alveolar macrophages (NR8383) were employed to examine the cytotoxicity of MWCNT for to evaluate inhalation toxicity of MWCNTs (Fujita et al., 2020). The CNTs were also used for the cytological study against human lung epithelial cells (A549) and murine macrophages (J774) (Kumarathasan et al., 2015). In another work, CNTs were utilized against human mesenchymal stem cells (MSCs) cultured in vitro and were compared in 3 and 60 days (Czarnecka et al., 2020). The CNTs were also employed to check the cytological behavior against the human breast cancer (MCF-7) cells (Garriga et al., 2020; Ozgen et al., 2020). The SW, MWCNT and graphene were used to examine the cytotoxic potential against human dermal fibroblasts (HDFs) and compared with fibro blast (L-929) cell line (Lee et al., 2012). In addition to these studies, HA powder were also utilized against cancer inhibition studies for different cells such as four different sizes of nano and micro sized crystals of hydroxyapatite for the cytotoxicity and endocytosis in renal epithelial cells (Sun et al., 2020). The HA powder was also utilized on rat aortic smooth muscle cell (A7 R5) injury and its phenotypic transformation were also investigated for the determination of cellular toxicity (Huang et al., 2019). A number of applications in various disciplines related to the CNTs and HAs were performed but very limited information is available related to cytological study with CNTs against liver cancer cells, which is a worldwide problem.
To keep this view the present work objective was to examine the cytotoxic evaluation of RMWCNTs, FMWCNTs and compared with HA powder against liver cancer (HepG2) cells correspondingly at very low doses of the material (1 to 100 μg/mL). The HepG2 cells were employed for the cytotoxicity study due to liver cancer is a worldwide problem. The RMWCNTs were cleansed and functionalized with acid digestion process and were well characterized. The surface bonding studies for the carbon nanotubes (CNTs) were examined via FTIR spectroscopy. The viability of cells was studies via MTT assay, whereas the gene expressions were examined with apoptotic gene with qPCR in presence of CNTs and HAs. The morphological changes due to the interaction of RMWCNTs, FMWCNTs, HAs were examined via microscopy including control. The possible discussion was also explained for the role of CNTs and HAs against liver cancer cells.
2 Material and methods
2.1 Experimental
2.1.1 (a) Functionalization of raw multiwalled CNTs (RMWCNTs and FMWCNTs)
The raw multiwalled carbon nanotubes were procured from Aldrich Chemical Co., Ltd U.S.A and purified through double deionized water (DDW) to remove the unwanted carbon particles and thereafter used for the acid treatment process. The RMWCNTs surfaces were functionalized via acid treatment process as per the previously reported protocol (Norizan et al., 2020; Liang et al., 2016). The recovered CNTs were examined in terms of their chemical, structural and biological observations (Liang et al., 2016).
2.1.2 (b) Synthesis of hydroxyapatite (HA) an artificial bone implant material
The HA powder is an artificial bone implant material, was prepared with the use of calcium chloride and disodium hydrogen phosphate. In a typical experiment about 5 × 10-2 M of calcium chloride (CaCl2) was dissolved in 100 mL of DDW. To this mixed with disodium hydrogen phosphate (0.5 M) was added drop by drop under constant stirring and form a white colour aqueous solution. The formed HA powder was filtered and washed many times with DDW and alcohol to eradicate the ionic impurities and desiccated the final product.
2.1.3 (c) Characterizations of RMWCNTs, FMWCNTs and HA
The raw and purified/functional CNTs were initially analyzed via FTIR (FTIR; Perkin Elmer-FTIR Spectrum-100, U.S.A) ranges from 400 to 4000 cm−1 with using KBr pellets. The structural assessment of RMWCNTs, FMWCNTs and HA powder were analyzed via SEM (Jeol, JED-2200 series, Japan), and TEM (JEOL, JEM 2010 at 200 kV, Japan). To analyse the structural analysis (SEM), RMWCNTs, FMWCNTs and HA powder were uniformly sprinkled on carbon tape and fix it on to the sample holder and analyzed. For more clear observation related to the morphology of CNTs and HA powder, were further analyzed via TEM. For TEM analysis, samples were sonicated in an ethanol for ∼10–15 min and to this solution, a copper grid (carbon-coated, 400 mesh, Sigma, Aldrich chem. Co. U.SA.) was dipped for 2–3 s and dried at room temperature and analyzed.
2.2 Cell culture (HepG2 cells) and treatment with CNTs and HAs
The HepG2 cells were obtained from ATCC (American Type Culture collection, Manassas, U.S.A) and thawed it for 2–3 min at 37 °C. The cells were transferred to the culture flasks with cultured medium (DMEM) including 12% fetal bovine serum (FBS), 0.2% sodium bicarbonate (NaHCO3), and antibiotic–antimycotic solution (100X, 1 mL/100 mL of medium) with 5% CO2 environment at 37 °C. The culture medium was refilled every other day and cells were subcultured once it reached to their maximum confluence (90%). Initially, the CNTs and HAs were used at high concentration and then after, diluted it at desired concentrations for the exposure of cells. The cells were plated in 6 and 96 well plates as per the condition and requirement of experiment.
2.3 Reagents and consumables for the biological study
The chemical such as MTT [3-(4, 5-dimethylthiazol-2-yl)-2, 5 diphenyltetrazolium bro mide], was procured from Sigma Chem. Co. USA and used without any further modification except dilution. On the other hand, the cells culture medium Dulbecco's Modified Eagle Medium (DMEM), antibiotics-antimycotic and fetal bovine serum (FBS) were acquired from Invitrogen, Waltham, Massachusetts, USA. The plastic wares such as well plates (6 and 96), pipets, bottles and other consumables products for the cells culture were used from Nunc, Roskilde, Denmark.
2.4 MTT assay
The cells viability of control and treated samples were accessed via MTT assay as per the followed protocol previously (Siddiqui et al., 2008; Mosmann, 1983). Briefly, the cells were cultured in a specialized 96 well plates (1 × 104/well) with permissible to follow for 24 h at 37 °C with humidified environment. The cells were exposed with RMWCNTs, FMWCNTs and HAs from 1 to 100 μg/mL for 24 h. Once the cells were completely mixed in well plates, stock solution of MTT (5 mg/mL in PBS) was incorporated with the rate of 10–20 μL/well in 100 μL of cell suspension and further incubated for 4 h. In this well plates, MTT salt was reduces and to form a purple formazan salt in mitochondria of living cells. When the incubation period was completed, the well plate’s solutions was with draw from the pipette and in these wells ∼200 µL/well of DMSO was added for to aspirate the formazan product and mixed gently, a purple color visible at this stage and deepen over pipeting. The optical density of the solution was examined at 550 nm through multiwall micro-plate reader (Multiskan Ex, Thermo Scientific, Finland). The untreated or control cells were also employed as reference and to run with the same conditions. The value of maximum absorbance depends upon the employed solvent in sample solution and the percentage (%) viability of cells was calculated as per the mentioned below equation:
2.5 RNA isolation
The RNA was mined from RNeasy mini Kit (Qiagen) as per the manufacturer’s protocol. Initially, the RNA was extracted from the cancer cells ( HepG2) in a 6-well plates with control and treated samples (RMWCNTs, FMWCNTs and HA) at a concentration of 50 µg/ml for 24 h. The cDNA was formed from treated and untreated cells taking 1 µg of RNA by Reverse Transcriptase kit using MLV reverse transcriptase (GE Health Care, UK) as per the manufactures’ protocol. The qPCR was performed on Roche®Light Cycler®480 (96-well block) (USA) following the cycling program recommended. 2 μL (40 ng) of cDNA template added to the volume of 20 µL of reaction mixture. Relative ratios were calculated based on the 2–△△CT method. qPCR was monitored using the CFX96TM Real-Time qPCR Detection Systems (Bio-Rad) (Humes et al., 2017).
2.6 Statistical analysis
The recovered data is displayed as a mean ± SD. Statistical analysis was performed by student T-tests. Results were considered significant when P < 0.05.
3 Results and discussion
3.1 FTIR spectroscopy
The FTIR spectroscopy was used to analyse the chemical functional groups of RMWCNTs, FMWCNTs and HAs for chemical characteristics and illustrated as Fig. 1. Initially RMWCNTs were analyzed, where few peaks were observed at 3200–3600 cm−1 and 1631 cm−1, which indicates water molecule (H-O-H) and C⚌C peak in CNTs consequently (Fig. 1 (a)). The acid digested or functional CNTs shows the different signals at 3459 cm−1, depicts the –OH stretching vibration mode whereas signal at 1639 cm−1 denotes the C⚌C group of CNTs. Another signal was noticed at 1215 cm−1, which assign to C–H stretching band (Fig. 1 (b). The signal at 1049 cm−1 denotes to the carboxylic acid (CO OH–) functional group whereas the signal at 875 cm−1 signifies C–C band (Wahab et al., 2009) (Fig. 1(b)). The recovered bands and their positions designates that the CNTs are functionalized with carboxylic (–COOH) group. Fig. 1(c) shows the bands at 3400 cm−1, 1805 cm−1, 1072 cm−1, 856 cm−1 and 536 cm−1 are related to the hydroxyl (–OH), CO32−ions, Ca(OH)2, HPO42− and PO43− groups respectively, in the prepared HA powder.
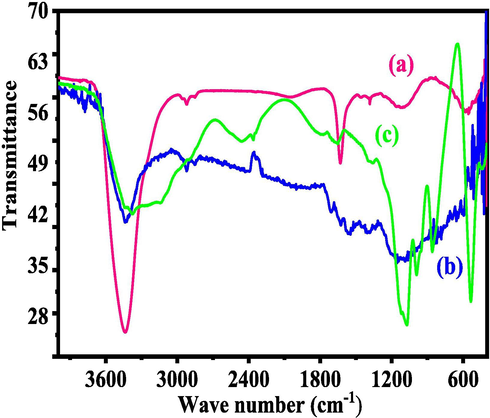
- Fourier transform infrared spectroscopy (FTIR) shows functional detail of RMWCNTs (a), FMWCNTs (b) and HA (c).
3.2 Morphology of CNTs and HA
The structural scrutiny was performed via SEM and TEM for RMWCNTs, FMWCNTs and HA powder and presented as Fig. 2. The acquired image display several carbon particle structures with (Fig. 2(a)) an aggregation, whereas other image (Fig. 2(b)) shows FMWCNTs, which are separated and seems to be several ropes likes structures. From the observation, the estimated size of an individual coiled rope like structure is ∼2–4 μm length and ∼10–15 nm diameters respectively. The HA powder seems lumpy, aggregated and arranged as a network like structures (Fig. 2(c)). Further for more clarification, the RMWCNTs, FMWCNTs and HA were again analyzed with TEM and presented as Fig. 2 (d-f). The recovered information from the SEM are in consistent and shows that the RMWCNTs (Fig. 2d) exhibit smooth surfaces, whereas the acid digested CNTs surfaces were rough and granulated on the surfaces of NTs (Fig. 2e) due to acid treatment (Fig. 2e). From TEM image, it’s very clear that an individual CNT exhibit 2–4 μm long whereas the diameter reaches to ∼10–15 nm (Fig. 2e). The HA powder molecules are interlinked with other molecule and form a leaf/network shaped structure and analogous SEM observation (Fig. 2f).
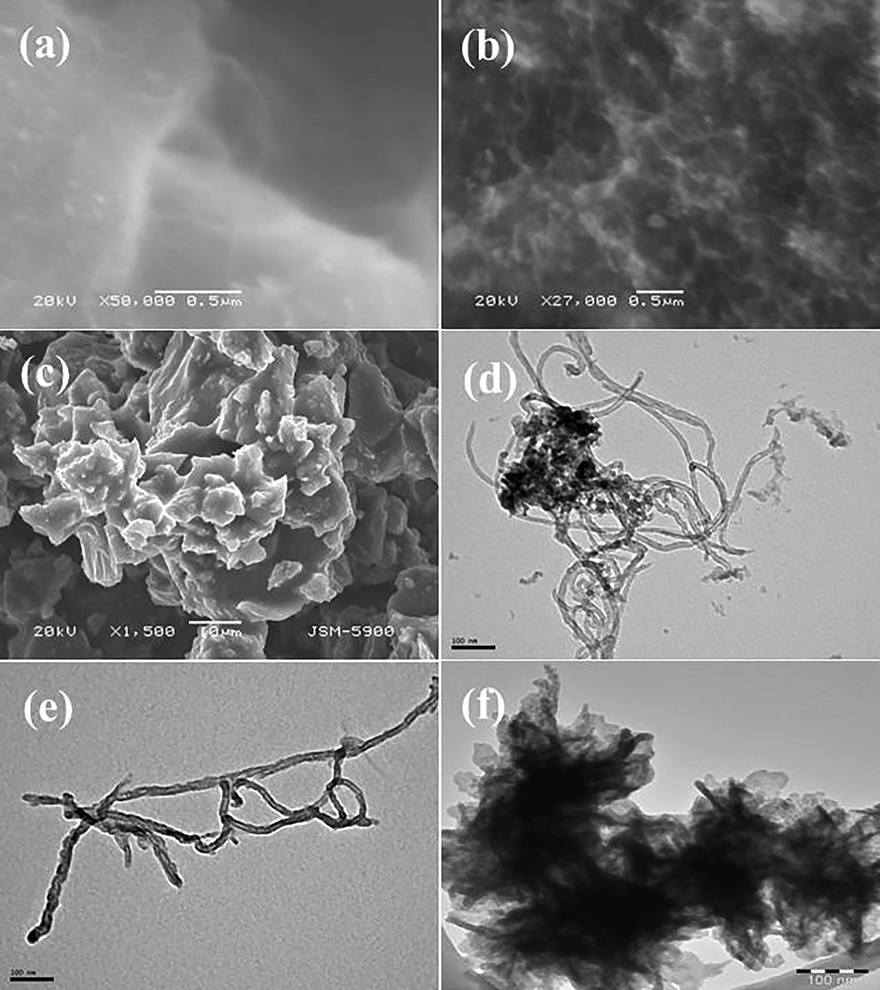
- The scanning electron microscopic (SEM) images of RMWCNTs (a), FMWCNTs (b) and HA (c) powder images consequently. Also transmission electron microscopic (TEM) image display the RMWCNTs (d) and FMWCNTs (e) including HA powder (f) respectively.
3.3 Morphology of HepG2 cells and their dose dependent treatment effect
The cells were cultured in a specified 75 mm2 flask and grow at maximum confluence, it was further harvested with trypsin and treated with RMWCNTs, FMWCNTs and HAs at different concentration from 1 μg/mL, 2 μg/mL, 5 μg/mL, 10 μg/ mL, 25 μg/ mL, 50 μg/ mL and 100 μg/mL with control to check the effect of CNTs, HA and incubated further at 24 h incubation time (Fig. 3). As per the images, it seems that cells were initially mono nucleated and once the inculcation time was reached at 24 h, start to further proliferate. Further inculcation completely damages the cells. In this experiment, the small amount of CNTs covered the surface of selected positions, whereas once the CNTs doses were increases from 50 μg/mL and 100 μg/mL and it roofed the entire surface of the cells (Fig. 3). This is hypothesized that the thin ropes of CNTs (RMWCNTs and FMWCNTs) structures exhibit the capability to enter easily to towards cell membranes, cytoplasm, cells organelles and creates the possibility of damage of cells. A similar trend was also found in case of HA, which shows the dose dependent toxicity with cancer cells. From the microscopic images, it’s realized that the as the conc of CNTs and HA increases the viability of cells were damaged with interference of NTs (Fig. 3) (Aoki and Saito, 2020).

- Microscopic images of HepG2 cells with RMWCNTs, FMWCNTs and HAs shown at different concentrations (1 to 100 μg/mL) at 24 h incubation period.
3.4 MTT assay or cytotoxicity assessment
The recovered data from MTT assay reveals that the viability of cells is in concentration/dose dependent (Fig. 4). The viability of cells with the RMWCNTs doses at 1 μg/ mL, 2 μg/mL, 5 μg/mL, 10 μg/mL, 25 μg/mL, 50 μg/mL and 100 µg/mL recorded as 103%, 103%, 103%, 81%, 75%, 70% and 65% respectively by MTT assay at 24 h (Fig. 4). In case of FMWCNTs viability of cells are more effective as compared to the RMWCNTs at different doses are 1 μg/mL, 2 μg/mL, 5 μg/mL, 10 μg/ mL, 25 μg/mL, 50 μg/mL and 100 µg/mL recorded as 99%, 93%, 91%, 84%, 79%, 71% and 63% respectively by MTT assay at 24 h (Fig. 4). The viability of cells with polymeric HAs shown a similar inclination for the cancer cells at different doses are 1 μg/mL, 2 μg/mL, 5 μg/mL, 10 μg/ mL, 25 μg/mL, 50 μg/mL and 100 µg/mL recorded as 95%, 93%, 93%, 90%, 83%, 71% and 62% respectively by MTT assay at 24 h (Fig. 4). The current work also corroborated with previously published literature (Aoki and Saito, 2020) and indicates that polymeric material express the efficacy to control the proliferation of cancer cells at dose dependent manner.
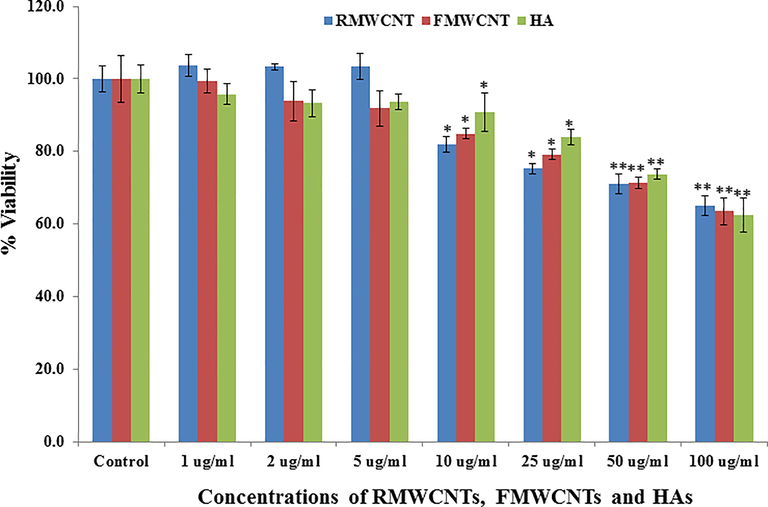
- The MTT assay of RMWCNTs, FMWCNTs and HAs at different concentration (1–100 μg/mL) at 24 h incubation period with control solution. Experiments were performed in triplicate manner. *p < 0.05, **p < 0.01 Vs Control.
3.5 mRNA gene expressions and activity of caspase with RMWCNTs, FMWCNTs and HAs
The qPCR was used to know the mRNA expression levels of apoptotic marker genes (e.g. p53, Bax, caspase-3 and Bcl2) in HepG2 cells exposed with RMWCNTs, FMWCNTs and HAs at 50 µg/mL for 24 h. From the recovered data’s, it can be easily seen that a noteworthy change was examined in mRNA level of apoptotic markers (p53, Bax, casp-3 and Bcl2) genes in HepG2 cells, when exposes with RMWCNTs, FMWCNTs and HAs (Fig. 5). The suppression gene of mRNA tumor supports the apoptosis induction by foreign material and observed the enzymatic activities of caspase-3 at the concentrations of 50 µg/ml. In case of RMWCNTs, the selected gene were express the up regulations whereas Bcl2 express down-regulation and fold changes for p53(2.0), Bax (4.0), Casp3 (1.7) and Bcl2 (0.62) correspondingly (Fig. 5), Similar treand of up and down (Bcl2) regulations were also found in case of FMWCNTs sample and the fold changes for p53 (2.1), Bax (4.0), Casp3 (1.8) and Bcl2 (0.67) congruently (Fig. 5). The HAs express the up-regulation in mRNA expression and the fold changes for p53 (2.2), Bax (4.2), Casp3 (1.9) exept the Bcl2 (0.70) respectively (Fig. 5). The obtained results displayed RMWCNTs, FMWCNTs and HAs induces the apoptosis in cells in presence of marker genes (Fig. 5) (Srivastava et al., 2011).
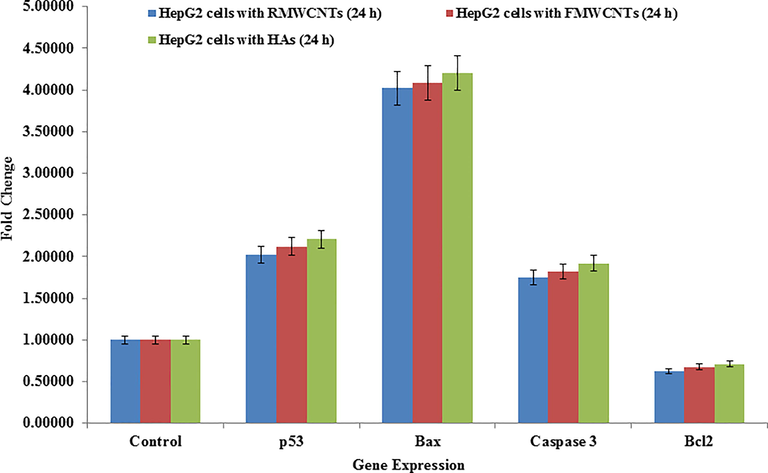
- mRNA expression of apoptosis marker genes by quantitative polymerase chain reaction (qPCR) analysis in HepG2 cells with RMWCNTs (a), FMWCNTs (b) and HA (c) at 50 mg/mL concentration for 24 h. qPCR was achieved with Roche LightCycler ®480 soft-ware (version 1.5). The GAPDH gene was used as a control to normalize data. The data is accessible as the mean ± SD of three identical experiments with three replicates manner. Significantly different compared with the control group (p < 0.05 for each).
3.6 Discussion
The CNTs and HAs both are unique material with polymeric and physicochemical characteristics including high surface, electrical conductivity and including functional properties. Although, with unique physicochemical characteristic limited information is available towards the direction to control the proliferation of cancer cells. Based on the recovered results such as FTIR, SEM, TEM, microscopy, MTT and qPCR study, the cells toxicity influences with used RMWCNTs, FMWCNTs, and HAs and are depends upon their unique size, shape, chemical composition, surface functionalization’s, endocytic uptake of cells, DNA interactions and others various factors (Bergamaschi, 2008). Based on these observations, a possible mechanism and related cytotoxicity of cancer cells with RMWCNTs, FMWCNTs, and HAs are described. As it’s illustrious that the size of RMWCNTs and FMWCNTs are having very small (D = 10–15 nm, L = 2–4 µm), whereas the HAs are also exhibit a similar characteristics and comes in the nanorange with network like structure. The rope like structures, which have very small diameter and longer length are capable to penetrate the cell membrane of cancer cells. Once it enters in to the cancer cells, attack to the cells internal organelles surface of cells. Regarding this, several hypothesis related to the internalization of foreign material towards cancer cells were documented (Wahab et al., 2014). As per the previous study, cell membranes exhibit small pore like passages which facilitates to enter CNTs in to cells and damaged the upper layer of cell walls (Coccini et al., 2010). In other reports, the small nanostructures can be easily entered in to the cells cytoplasm and these small nanostructured adducts react with cell’s organelles and diminished the growth of cancer cells. In this case, CNTs were used from low to high concentration and shows the damage of cancer cells as dose dependent. Similar observations were also found in HAs, where very small particles are combined together and to form adduct and these adducts react to cells also their high density of materials in liquid medium strongly favors the interaction with cells and to enhanced the damage of cancer cells (Wahab et al., 2009; Bergamaschi, 2008; Coccini et al., 2010). The qPCR results also confirm that the apoptosis in cells caused with foreign material (RMWCNTs, FMWCNTs and HAs), which further confirms the cytotoxicity of cancer cells.
4 Conclusions
In current work summarizes and displayed the differential cytotoxicity of raw , functional carbon nanotubes and it’s compared with the artificial bone implanted hydroxyapatite. The characterizations for instance FTIR was utilized for RMWCNTs and FMWCNTs identification including the HAs whereas the structural assessments were analyzed via SEM and TEM, reveals that the individual size of nanotubes ranges from 10 to 15 nm in diameter and length reached to 2–4 μm and HAs size is ranges to ∼50 nm consequently. The analysis revealed that FMWCNTs surfaces were rough and granulated whereas RMWCNTs surface were smooth. The nanostructures were employed for the cytotoxicity study against HepG2 cancer cells at varied concentrations (1–100 μg/mL). Sequential changes were observed in all toxicity assessments and it clearly confirms from the MTT assay that it was concentrations/dose dependent manner. The apoptosis in cells were checked with qPCR study, illustrates that those treatments with nanostructures influence the growth of liver cancer cells.
For the eradication of cancer cells in human beings a number of physical approaches in terms of therapies were applied such as chemotherapy, radiotherapy, immunotherapy etc. These physical means are costly and doesn’t provide the full satisfaction because if any cells remain it grows again and form a tumor in the body. The high surgical cost restrict and very difficult to afford to normal and middle income families. Therefore an inclusive mechanism is required to form a fruitful therapy at a very low cost. The CNTs and HAs, which are nanostructured materials and exhibit high surface to volume ratio, which enables it to enter directly in to the cells and are the best alternative for to diminish the growth of cancer cell at a very low cost and to deliver a great input in cancer studies. The carbon and polymeric based nanostructured materials is the best alternative against cancer studies and it can possibly diminish the cost of available drugs also such studies curtail the nervousness against surgery for deprived patient.
Acknowledgements
The authors are grateful to the Deanship of Scientific Research, King Saud University for funding through Vice Deanship of Scientific Research Chairs.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Optical and electronic properties of carbon-based nano materials and composites. J. Carbon Res C. 2020;6(2):36.
- [Google Scholar]
- Biocompatibility and carcinogenicity of carbon nanotubes as biomaterials. Nanomaterials (Basel).. 2020;10(2):264.
- [Google Scholar]
- Comparing in vitro cytotoxicity of graphite, short multi-walled carbon nanotubes, and long multi-walled carbon nanotubes, Enviro nmental Sci and Poll. Res.. 2020;27:15401-15406.
- [Google Scholar]
- Non-functionalized multi-walled carbon nanotubes alter the para-cellular permeability of human airway epithelial cells. Toxicol. Lett.. 2008;178:95-102.
- [Google Scholar]
- Effects of water-soluble functionalized multi-walled carbon nanotubes examined by different cytotoxicity methods in human astrocyte D384 and lung A549 cells. Toxicology. 2010;269:41-53.
- [Google Scholar]
- Cytotoxic or Not? Disclosing the toxic nature of carbonaceous nanomaterials through nano-bio interactions. Materials. 2020;13:2060.
- [Google Scholar]
- Dispersing of functionalized CNTs in Si–O–C ceramics and electro magnetic wave absorbing and mechanical properties of CNTs/Si–O–C nano composites. Ceram. Int.. 2020;46(4):5407-5419.
- [Google Scholar]
- Influence of polymer type and carbon nanotube properties on carbon nanotube/polymer nanocomposite biodegradation. Sci. Total Environ.. 2020;742:140512
- [Google Scholar]
- Cytotoxicity profiles of multi-walled carbon nanotubes with different physico-chemical properties. Toxicol. Mech. Methods. 2020;30(7):477-489.
- [Google Scholar]
- Toxicity of carbon nanomaterials and their potential application as drug delivery systems. In vitro studies in Caco-2 and MCF-7 Cell Lines. Nanomaterials. 2020;10(8):1617.
- [Google Scholar]
- Structure design and composition engineering of carbon-based nanomaterials for lithium energy storage. Adv. Energy Mater.. 2020;10(10):1903030.
- [Google Scholar]
- NiCo–N-doped carbon nanotubes based cathode catalyst for alkaline membrane fuel cell. Renewable Energy. 2020;154:508-516.
- [Google Scholar]
- Shape-dependent toxicity and mineraliz ation of hydroxyapatite nanoparticles in A7R5 aortic smooth muscle cells. Sci. Rep.. 2019;9:18979.
- [Google Scholar]
- overcoming qRT-PCR interference by select carbon nanotubes in assessments of gene expression. Bio Techn.. 2017;63(2):81-84.
- [Google Scholar]
- Smart carbon nanotubes for drug delivery system: A comprehensive study. J. Drug Deliv. Sci. Tech.. 2020;58(101):811.
- [Google Scholar]
- Cytotoxicity of carbon nanotube variants: A comparative in vitro exposure study with A549 epithelial and J774 macrophage cells. Nanotoxicology. 2015;9(2):148-161.
- [Google Scholar]
- Influence of powder and liquid multi-wall carbon nanotubes on hydration and dispersion of the cementitious composites. Appl. Sci. 2020;10:7948.
- [Google Scholar]
- Cytotoxicity evaluations of pristine graphene and carbon nano tubes in fibroblastic cells. J. Korean Phys. Soc.. 2012;61:873-877.
- [Google Scholar]
- Target membrane cholesterol modulates single influenza virus membrane fusion efficiency but not rate. Biophys. J.. 2020;118(10):2426-2433.
- [Google Scholar]
- Structure-dependent electrical properties of carbon nanotube fibers. Adv. Mater.. 2007;19(20):3358-3363.
- [Google Scholar]
- Multi-walled carbon nanotubes functionalized with a ultrahigh fraction of carboxyl and hydroxyl groups by ultrasound-assisted oxidation. J. Mater. Sci.. 2016;51:3513-3524.
- [Google Scholar]
- Electrochemical properties of oxygen- enriched carbon-based nanomaterials. J. Electroanalytical Chem. 2020;873:114420
- [Google Scholar]
- Electromechanical Properties of Carbon Nanotubes. J. Phys. Chem. C. 2014;118(25):13936-13944.
- [Google Scholar]
- Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65(1–2):55-63.
- [Google Scholar]
- Carbon nanotubes: functionalisation and their application in chemical sensors. RSC Adv.. 2020;10:43704-43732.
- [Google Scholar]
- Glycopolymer decorated multiwalled carbon nanotubes for dual targeted breast cancer therapy. J. Mater. Chem. B. 2020;8:3123-3137.
- [Google Scholar]
- Influence of cytotoxic doses of 4-hydroxynonenal on selected neuro transmitter receptors in PC-12 cells. Toxicol. In Vitro. 2008;22:1681-1688.
- [Google Scholar]
- Multi-walled carbon nanotubes induce oxidative stress and apoptosis in human lung cancer cell line-A549. Nanotoxicology. 2011;5(2):195-207.
- [Google Scholar]
- Size-dependent cytotoxicity of hydroxyapatite crystals on renal epithelial cells. Inter. J. Nanomed.. 2020;15:5043-5060.
- [Google Scholar]
- Experimental evidence of single-stranded DNA adsorption on multiwalled carbon nanotubes. J. Phys. Chem. B. 2020;124(12):2514-2525.
- [Google Scholar]
- Immobilization of DNA on nano-hydroxyapatite and their interaction with carbon nanotubes. Synth. Metals. 2009;159:238-245.
- [Google Scholar]
- ZnO nanoparticles induced oxidative stress and apoptosis in HepG2and MCF-7 cancer cells and their antibacterial activity. Colloids Surf., B. 2014;117:267-276.
- [Google Scholar]
- DNAzyme-mediated genetically encoded sensors for ratiometric imaging of metal ions in living cells. Angew. Chem.. 2020;132(5):1907-1912.
- [Google Scholar]
- Design, preparation and measurement of protein/CNTs hybrids: A concise review. J. Mater. Sci. Tech.. 2020;46:74-87.
- [Google Scholar]







