Translate this page into:
Cytotoxic and molecular assessment against breast (MCF-7) cancer cells with cobalt oxide nanoballs
⁎Corresponding author. rwahab@ksu.edu.sa (Rizwan Wahab)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
Among various types of cancer, the breast cancer is a very common and widely affected to the human beings especially female candidate. To keep this view the present work was designed to access the cytotoxic and apoptotic responses of breast (MCF-7) cancer cells with cobalt oxide nanoparticles (Co3O4NPs). The Co3O4NPs was prepared via solution process and well characterized. The materials crystallinity, size and phase were determined via XRD, whereas the morphology of Co3O4NPs was analyzed via SEM and TEM respectively. The Co3O4NPs were spherical in shape with an average diameter of (∼33±1). The cytotoxicity rate of breast (MCF-7) cells was much influenced and dose dependent (1, 2, 5, 10, 25, 50 and 100 μL/mL) with Co3O4NPs confirmed via the MTT and NRU assays. The rate of ROS was increased gradually from 105, 124,132 and 148 with different concentrations of Co3O4NPs (10, 25, 50 and 100 μL/mL) with control. The mRNA level of apoptotic markers with Co3O4NPs was up-regulated and it shows the apoptosis in cells. The study describes the cytotoxicity with Co3O4NPs in breast (MCF-7) cancer cell were apoptotic gene expression with possible discussion.
Keywords
Cobalt oxide NPs
Cytotoxicity
MCF-7 cells
Reactive oxygen species
RT-PCR
1 Introduction
Cancer is a divesting disease, which is not only affect the particular organs but disturb to the whole body system (Wahab et al., 2013). It’s happens due to uncontrolled progression of normal cells. Several factors influenced such as initiation, promotion and growth etc; are responsible for the transformation of a normal cell to a cancerous one (Bray et al., 2012). Over a number of cancer types, the breast cancer is very common to the entire world and normally diagnosed in female candidates (Nardin et al., 2020). In an estimated value the breast cancer grasp more than one million women’s world wide. The periodical statistics data to the incidence of this disease varied widely such as in 2008, about 421,000 cases were recovered for breast cancer, where as in 2009–2010, more than 49,500 women were diagnosed with breast cancer in Europe. From the breast cancer about 11,600 women’s and 75 men were died in 2010. The estimated data caused from this cancer is more than 458,000 women in 2008 worldwide. In 2008, the new cases 184,450 were appeared in persistent stages and this number varies to 230,480 in 2011 in USA. This estimated value is increases day by day and new cases (∼268,600) for breast cancer was identified in women, it also detected in men (∼2,670) in 2019 (Nardin et al., 2020). An average 42,170 women in the U.S. predictable to die in 2020 (Facts & Figures 2019-2020). A number of therapies have been proposed to cure this devastating disease such as chemotherapy, radiotherapy, proton beam therapy, hormone therapy, targeted drug therapy, clinical trials, cryoablation, immunotherapy etc, that causes so much suffering (Chen et al., 2020, Cammarata et al., 2020, Boing et al., 2020, Li et al., 2020). Including this, surgery is an another option from which the cancer cells were removed from the particular organ of the body such as curative, preventive diagnostic, staging, palliative, supportive, cryosurgery, laser, electro surgery and various others (He et al., 2020, Wahab et al., 2014).
In recent, the nanotechnology, which is a multi-disciplinary area of science, connects with various branches of basic and applied sciences, is a best alternative for to reduce the cancer cells at a very low cost, much effective against cancer cells and not harm to body system. The nanotechnogy has privilege to provide a range of fascinating nano and microstructures, which are widely useful to optoelectronic & biological applications (Siddiqui et al., 2015). Among a large number of metal and metal oxides nanostructures, the cobalt oxide nanostructures (Co3O4NSs) exhibit a unique properties in electronics, batteries, solar cells, energy storage, imaging, chemical and biological sensing, magnetic, catalysis (Li et al., 2005, Dong et al., 2014, Karuppiah et al., 2014, Sivachidambaram et al., 2017) etc. Various shapes and sizes endorse and exhibit a surface anisotropy behavior, which influences their magnetic response (Farhadi et al., 2013). The Co3O4NSs also have different properties such as hydrodesulfurization, photo catalyst, oxidation, electrochemical (Askarinejad et al., 2010, Packiaraj et al., 2019) studies. The cobalt oxide nanostructure is a p-type semiconductor, enhance band gap (1.29 to 3.34 eV) energy with enormous photosensitivity including enormous quantum efficiency (Vennela et al., 2019). Over a long range of physicochemical properties and their utilization in various areas, limited studies are available for the biological application of cobalt oxide to diminish the proliferation rate of cancer cells (Jarestan et al., 2020). Towards this direction work related to retardation of cancer cells such as chattopadhyay et al. describes the role of surface modification of cobalt oxide NPs for the biological activity. For this, different cell lines such as the human lymphocytes, T-cell lymphoma (Jurkat) and oral epithelial cancer (KB) were exposed with surface modified cobalt oxide NPs and achieved their anticancer activity (Chattopadhyay et al., 2012, Mauro et al., 2015, Arsalan et al., 2020, Rauwel et al., 2020). Due to the magnetic and high stability, the cobalt oxide NPs also utilized in hyperthermic treatment. This involves heating of tumor tissues in a temperature below 50 °C (Jose et al., 2020). In addition to this, the magnetic NPs can be easily conjugate with DNA, peptides, antibodies, enzymes and other biological molecules to form the nano-bio hybrids (Bossi et al., 2016). The cellular uptake of cobalt oxide NPs was examined via TEM to explore the new insights of mechanisms to entry in cells, distribution and their cellular environment (Papis et al., 2009). The Co3O4NPs were also utilized for the light-induced photodynamic therapy (PDT) and was employed to assess their photo and cytotoxic effects of materials against HepG2 cells (Iqbal et al., 2020).
Over the various applications of Co3O4NPs in different ways, very limited studies are available for to control the cancer cells growth, cellular cytotoxicity, gene expressions are via Co3O4NPs. To keep this view, the core objective of the current work is to elucidate the role of Co3O4NPs against breast cancer cells. The Co3O4NPs were synthesized via solution method with the use of cobalt acetate tetrahydrate (Co(CH3COO)₂.4H₂O) and sodium hydroxide (NaOH) under a very short refluxing temperature. The processed Co3O4NPs crystallinity, particle size and phases were estimated via X-ray diffraction (XRD) pattern, whereas the structural examinations were confirmed via scanning electron (SE) and transmission electron microscopy (TEM) respectively. The chemical functional positions were identified via Fourier transform infra-red (FTIR) spectroscopy. The cytotoxicity study was conducted against breast cancer cells (MCF-7) with using Co3O4NPs. The MCF-7 cells were widely selected because the breast cancer has a major health problem worldwide. The cells viability of cancer cells (MCF-7) were examined with Co3O4NPs with MTT assay. The apoptosis caused with Co3O4NPs was investigated with the accessible real-time polymerase chain reaction (RT-PCR) study also explained why the Co3O4NPs are useful to diminish the growth rate of cancer cells.
2 Experimental
2.1 Material and methods
2.1.1 Synthesis of cobalt oxide nanoparticles (Co3O4NPs)
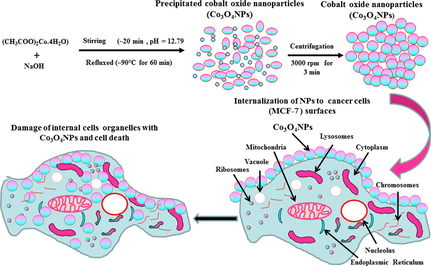
The Co3O4NPs were formed through cobalt acetate tetra hydrate (CH3COO)2Co·4H2O) and sodium hydroxide (NaOH). The chemicals for the formation of NPs were acquired from Sigma Aldrich chemical Co., USA and used as received. Acetate salt of cobalt and alkali NaOH were assorted in 100 mL of deionized double distilled water (DDDW) with a final concentration of 15 mM (0.18681 g) and 0.2 M (0.2 g), respectively, with the constant stirring conditions for ∼ 20–30 min. The solution pH was measured (corning PH meter 430 red, cole-parmer, U.S.A) and it reaches to 12.79. Once cobalt salt was completely dissoluted, the solution was refluxed at ∼ 90 °C for 60 min in a refluxing pot. The solution color turns from red to black with increase of temperature of refluxing pot. When the reaction was completed, the formed precipitated product was kept for cooling at room temperature for about ∼ 24 h. The obtained product was transferred in a centrifuge tubes (∼50 mL capacity) and centrifuge (Eppendorf, 5430R, Centrifuge, Germany). The solution was centrifuged at 3000 rpm for 3 min and dried at room temperature in a glass petri dishes. The powder was analyzed in terms of their morphological and chemical characteristics.
2.1.2 Characterizations of synthesized material
The prepared powder crystallinity, phases, crystallite size were identified via XRD (PAN alytical XPert Pro, U.S.A.) with CuKα source (λ = 1.54178 Å) from 20 to 70° with 6° angle rotation/min speed. The morphology of synthesized product was analyzed via SEM (JSM −6380 LA, Japan). For the analysis of SEM, synthesized product was homogeneously sprinkled on a black colored carbon tape and forms a layer. The cobalt oxide coated sample carbon tape was transferred to the specialized glass chamber for sputtering with thin conducting layer of platinum (pt) for 3 s to enhance the conductance of the material. Further the morphological evaluation was also confirmed via TEM (JEOL, JEM JSM 2010, Japan) and the procured morphological images were collected at 100 kV current. The chemical functional identification of synthesized Co3O4NPs was analyzed via FTIR (Perkin Elmer-FTIR Spectrum-100, U.S.A.) spectroscopy ranges from 400 to 4000 cm−1. For this, very small amount of the powder was used for FTIR analysis, mixed with KBr to form the pellet under high-pressure (∼4 tons). The pellet was fixed to the sample holder and analyzed the FTIR at room temperature.
2.2 Cell culture (MCF-7 cells) and treatment of cobalt oxide NPs
The breast cancer cells (MCF-7) was grown in a medium (DMEM/MEM) including 12% fetal bovine serum (FBS), 0.2% sodium bicarbonate, and antibiotic–antimycotic solution (100 X, 1 mL/100 mL) with moist environment (5% CO2 & 95% O2) at 37 °C. Earlier for the experiments, the cells viability were evaluated by trypan blue dye as per the protocol (Siddiqui et al., 2008) and shows the viability more than 95% were only used in the study. The cells were employed between 10 and 12 passages to treat the cells with nanostructures. The Co3O4NPs were used at high concentration and then subsequently diluted at desired different concentrations for the exposure of cells. The cells were plated in 6-well or 96-well plates as per the experimental requirement.
2.3 Reagents and consumables for the biological study
The MTT [3-(4, 5-dimethylthiazol-2-yl)-2, 5 diphenyltetrazolium bromide], was obtained from Sigma Chem.Co.USA and castoff without any further modification except dilution, besides this the Dulbecco's Modified Eagle Medium (DMEM) and MEM culture medium, antibiotics-antimycotic and FBS were bought from Invitrogen, USA. The plastic wares and other consumables products for cells culture were used from Nunc, Denmark.
2.4 MTT assay
Cancer cells viability with (treated) and without Co3O4NPs (control) were accessed via MTT assay as per the previous followed protocol (Siddiqui et al., 2008, Mosmann. 1983). Initially, cells were cultured in a specialized 96 well plates (rate of 1 × 104/well) with permissible to follow for 24 h at 37 °C with humidified environment. The cells were intermingled with Co3O4NPs from 1 to 100 µg/mL for 24 h. When the cells were completely inter mixed in well plates, stock solution of MTT (5 mg/mL in PBS) was amalgamated with rate of 10 μL/well in 100 μL of cell suspension and further incubated for 4 h. After the completion of incubation period, the well plate’s solution was with draw from the pipette and in these wells ∼ 200 µL of DMSO was added for to aspirate the formazan product and mixed gently. The optical characteristic of the solution was measured at 550 nm with micro-plate reader (Multiskan Ex, Thermo Scientific, Finland). The control cells were also employed as a reference and to run with the same conditions. The value of maximum absorbance depends upon the employed solvent in sample solution and the level of viability of cells % was calculated as per the equations mentioned below:
2.5 NRU assay
The cytotoxic assessment was also confirmed with and without Co3O4NPs via neutral red uptake (NRU) assay as per the previously described procedure (Borenfreund and Puerner , (1985, Siddiqui et al., 2010). The MCF-7 cells (1 × 104/well) cells were sowed in a specified 96 well plates. Once the cells were completely grown (after 24 h) were exposed to desired conc (1–100 μg/mL) of Co3O4NPs and kept for 24 h in an incubator. When the exposure was completed, cells were further incubated in NR medium (50 µg/mL) for 3 h. Thereafter, cells were washed and dye was extracted in 1% acetic acid and 50% ethanol solution. The develop color was read at 540 nm.
2.6 Generation of reactive oxygen species (ROS)
The ROS was measured with 2, 7-dichloro dihydrofluoresce in diacetate (DCFH-DA; Sigma Aldrich, USA) dye as a fluorescence agent as per the previously described method (Zhao and Riediker, 2014). Once the cells were exposed with the material for 24 h and then after, cells were rinsed well with PBS and further nurtured for 30 min in DCFH-DA (20 μM) in dark place at 37 °C. When the reaction of DCFH-DA with cells control and treated (with Co3O4NPs) cells completed, cells were examined with using fluorescence microscope.
2.7 mRNA expressions with cancer cells (MCF-7)
The RNA isolation was performed with cultured cells of MCF-7 cells in a 6-well plates control and treated sample of Co3O4NPs at concentration of 50 µg/mL for 24 h. The RNA was mined from RNeasy mini Kit (Qiagen) as according to manufacturer’s protocol. The cDNA was synthesized from treated and untreated cells taking 1 µg of RNA by Reverse Transcriptase kit using MLV reverse transcriptase (GE Health Care, UK) as per the manufactures’ protocol. The RT-PCR was performed on Roche® Light Cycler®480 (96-well block) (USA) following the cycling program recommended. 2 μL (40 ng) of cDNA template included the 20 µL volume to this reaction mixture (Huang et al., 2021, Bejarbaneh et al., 2020).
3 Result and discussion
3.1 X-ray diffraction pattern (XRD)
Fig. 1 shows the XRD of processed powder via solution process. The XRD pattern defines the crystallinity, crystallite size and phases of the powder material. The well assigned peaks were identified in XRD spectrum and is related to the face centered cubic (FCC) structures and well matched with the JCPDS card No. 76–1802 with Co3O4. The peaks and their positions such as 31.26 〈2 2 0〉, 36.84<, 38.59<, 44.83<, 55.68<, 59.38<, 65.25 < illustrates the lattice plane of 220 and 311 for Co3O4 crystal (Fig. 1) and well justified with the previously published literature of pure Co3O4 (Divyapriya et al., 2019, El-Dossoki et al., 2020). The intense peak indicates that powder exhibit good crystallinity, small particles size etc (Cullity 1978). The particles size was found to be ∼ 33 nm, calculated with using sherrer formula. The obtained X-ray spectrum shows only the Co3O4 peaks without any other impurities, which indicates the purity of the sample.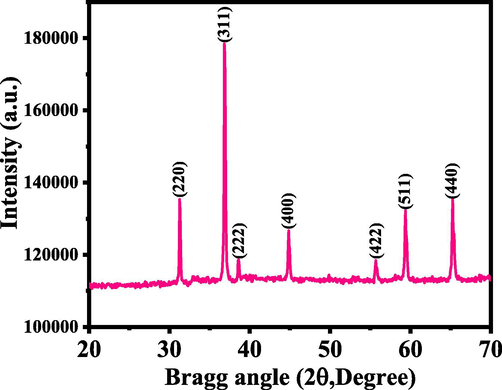
X-ray diffraction (XRD) pattern of the prepared cobalt oxide nanoparticles (Co3O4NPs).
3.2 Morphological results of Co3O4NPs
3.2.1 (a) SEM results
The morphology was examined for the processed powder with SEM images and presented as Fig. 2. From the low magnified image (Fig. 2a-b) of Co3O4NPs, it seems that small nanostructures were arranged with the collection of several tiny particles and it seems that these NPs are linked composed and from a spheres shaped structure. Further for more clarification of an individual particle, shape and size of NP was confirmed at high magnified image (Fig. 2c). From recovered image designates that estimated diameter of each individual particle is in range of ∼ 33 ± 1 nm (Fig. 2d). The obtained SEM analysis is well justified and in consistent with the XRD (Fig. 1).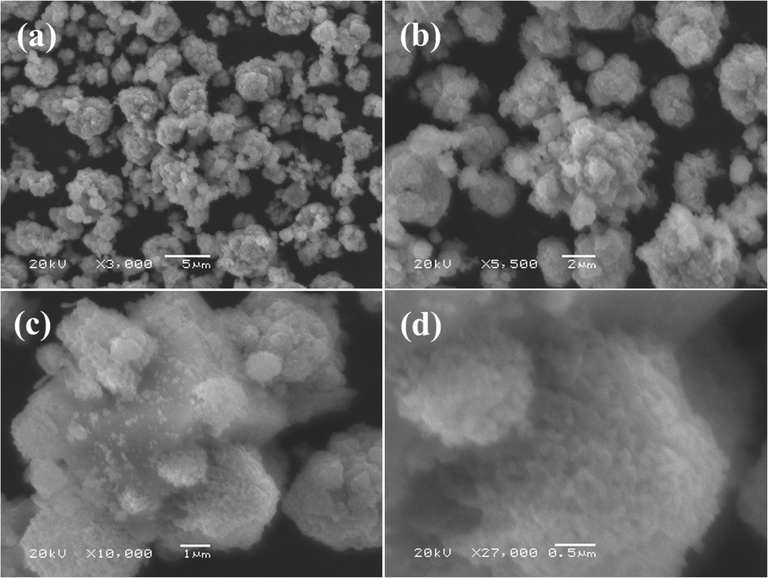
Scanning electron microscopy (SEM) images of cobalt oxide nanoparticles (Co3O4NPs): (a-b) illustrates low magnified images whereas (c-d) shows the high magnified image, which depicts the surface morphology of the cobalt oxide nanoparticles (Co3O4NPs), the average size of each NP is ∼ 33 ± 1 nm.
3.2.2 (b) TEM results
For more authentic observation, the grown powder was also analyzed with TEM at an above described procedure in section 2.2 and the obtained data is presented as Fig. 3. TEM image is also in consistent with the SEM images. Number of several nanometer scale particles (NPs) is seen and size of an individual NP ranges to ∼ 33 ± 1 nm. The obtained image confirms the spherical shaped NPs surfaces are smooth, clear and consisted with the SEM (Fig. 2) images.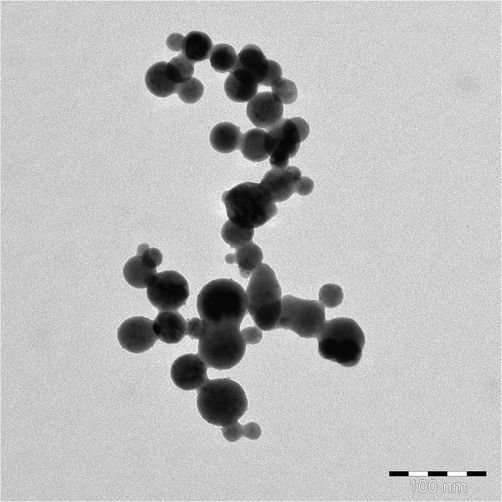
Transmission electron microscopy (TEM) displayed the low magnification image of the cobalt oxide nanoparticles (Co3O4NPs, size ∼ 33 nm) synthesized at ∼ 90 °C.
3.2.3 (c) FTIR results
To know the foot prints of utilized chemicals with functional groups, FTIR spectroscopy was utilized in the range of 400–4000 cm−1. A wide and shallow peak ranges between 3200 and 3600 cm−1 illustrates the O–H stretching mode whereas an intense peak was observed at 1633 cm−1 for H-O–H molecule (Fig. 4)). The absorption peak at ∼ 665 cm−1 is correlated to O-C-O absorption and band at 574 cm−1 depict the Co-O vibration metal oxygen group. From the observed FTIR data it’s confirm that no any other band was observed related to other functional group in the spectrum, which shows the purity of the material (Divyapriya et al., 2019, El-Dossoki et al., 2020).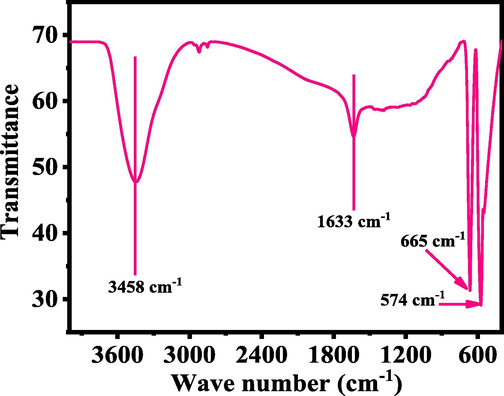
Typical Fourier transform infra-red (FTIR) spectroscopy of grown cobalt oxide nanoparticles (Co3O4NPs).
3.3 Morphological change of cancer cells (MCF-7) with Co3O4NPs
The cancer cells (MCF-7) were cultured and their morphological evaluation was observed via microscopy at 24 h incubation periods with a number of concentrations (25, 50 and 100 µg/mL) ranges of Co3O4NPs (Fig. 5). The images were captured for MCF-7 cancer cells and used as control and for their interactions with different concentrations of Co3O4NPs respectively (Fig. 5). It’s evident that initially the cells were nucleated and once their confluences were reached to their optimum level (∼70–80%,) treated with the Co3O4NPs with different concentrations (25, 50 and 100 µg/mL) and analyzed. From the obtained images, it reveals that there is not much noteworthy alternation was observed at an initial range of concentration 25 µg/mL, but once this range of concentration of Co3O4NPs upsurges to 50 to 100 µg/mL, the growth of cells was much influenced with Co3O4NPs and it was dose dependent. At low concentration 25 µg/mL its doesn’t shows any change in cells morphology, whereas at 50 and 100 µg/mL, the cells was destroyed (Fig. 5). From the microscopic images it’s very clear that cells were damaged with the Co3O4NPs.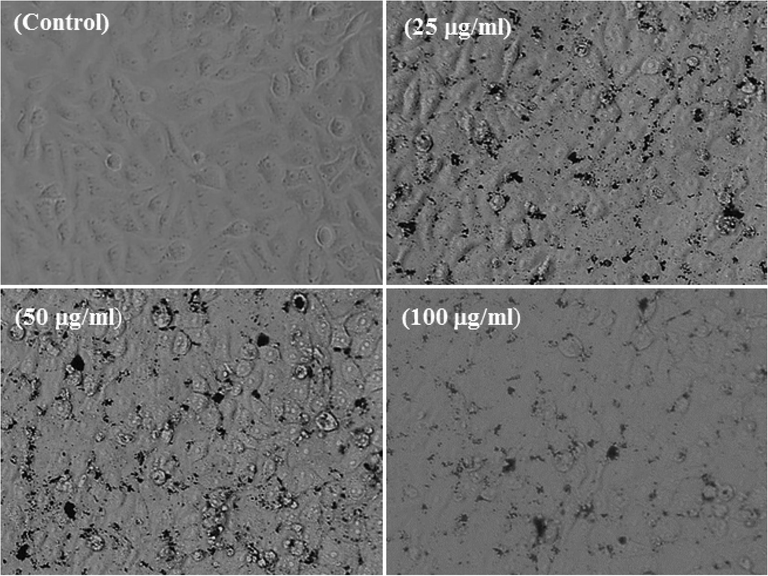
Structural change in MCF-7 cells with exposed to cobalt oxide nanoparticles (Co3O4NPs) for 24 h. Images were taken under the phase contrast inverted microscope.
3.4 The induced cytotoxicity (MTT assay) with processed Co3O4NPs
As mentioned in the material and methods that the control (MCF-7) and treated (MCF-7 cells with Co3O4NPs) sample of cells were exposed in a range of different concentrations (ranges from 1 to 100 µg/ml) for 24 h incubation. Cells cytotoxicity was examined via MTT assay. From the obtained data it shows that the viability of cancer cells was diminished with Co3O4NPs and data was concentration/dose-dependent. For the MCF-7 cells viability, MTT assay was decreases at 24 h 99%, 101%, 89%, 81%, 71%, 65% and 51% (Fig. 6) for the concentrations of 1, 2, 5, 10, 25, 50 and 100 μg/mL correspondingly (p < 0.05 for each). From the data its shows that at initial concentration, it was not much affected whereas when the concentration of processed material is increase the cytotoxicity was much influenced.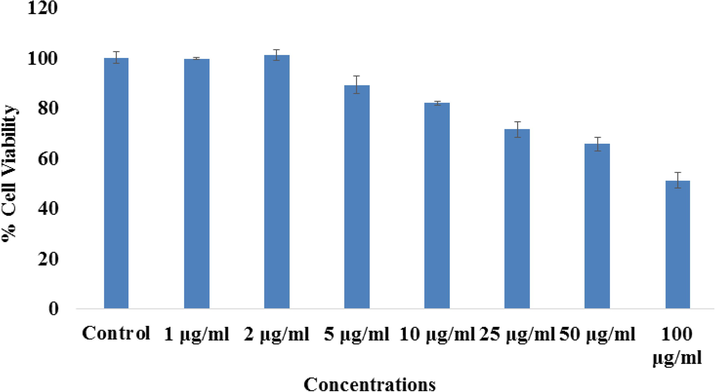
The study of cytotoxicity via MTT assay in MCF-7 cells succeeding the exposure of cobalt oxide nanoparticles (Co3O4NPs) for 24 h. The experiments were conducted in triplicate manner (Mean ± SD triplicate).
3.5 Cytotoxicity study via NRU assay in MCF-7 cells with Co3O4NPs
Including MTT assay, the cytotoxic measurement was also confirmed with and without Co3O4NPs via NRU assay as detailed in the materials and method. A similar trend was also observed in NRU assay. From the obtained data it depict that the viability of cancer cells at initial doses of Co3O4NPs is not much affected, whereas once the concentration/doses increases, the cancer cells was diminished. For the MCF-7 cells, NRU assay was decreases at 24 h 102%, 98%, 90%, 80%, 67%, 60% and 56% (Fig. 7) for the concentrations of 1, 2, 5, 10, 25, 50 and 100 μg/mL correspondingly (p < 0.05 for each).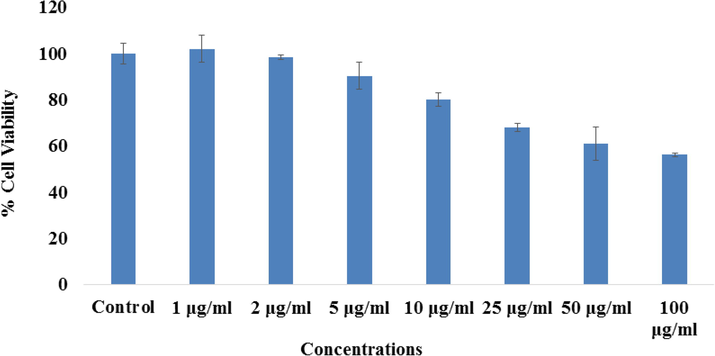
The study of cytotoxicity via NRU assay in MCF-7 cells succeeding the exposure of cobalt oxide nanoparticles (Co3O4NPs) for 24 h. The experiments were conducted in triplicate manner (Mean ± SD triplicate).
3.6 Induced ROS generation in MCF-7 with Co3O4NPs
A sequential trend for ROS generation was detected in MCF-7 cells after the exposure of Co3O4NPs at 100 μg/mL concentrations for 24 h (Fig. 8). The ROS is increases with the Co3O4NPs and it’s evident from the images (Fig. 8A) as compared to control cells. An increase of 148.00 ± 11.7% was observed in ROS generation at 100 μg/mL, as compared to control (Fig. 8B).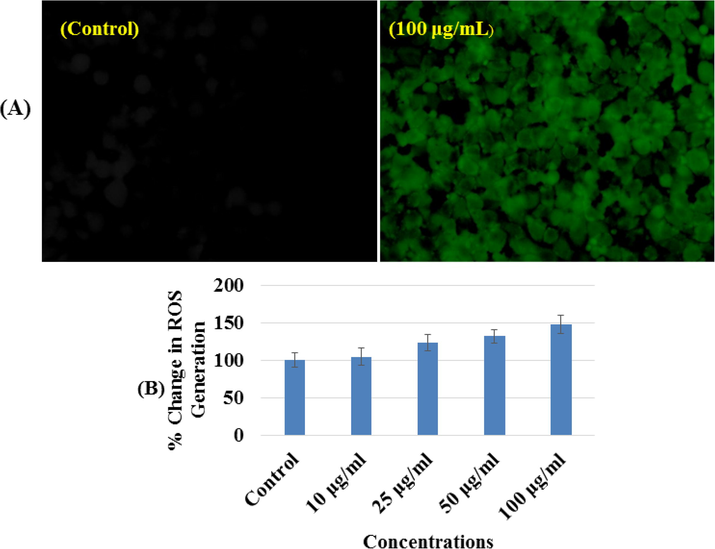
(A) Representative images of cobalt oxide nanoparticles (Co3O4NPs) induced reactive oxygen species (ROS) generation in MCF-7 cells exposed for 24 h. (B) Percent change in reactive oxygen species (ROS) generation with MCF-7 at different concentrations of cobalt oxide nanoparticles (Co3O4NPs) for 24 h.
3.7 Gene expressions study
The RT-PCR was utilized to understand the mRNA levels of apoptotic marker genes (e.g. p53, Bax, caspase-3 and BCl2) in MCF-7 cells interacted with Co3O4NPs oxide at 50 µg/ mL for 24 h. A significant change was observed in mRNA levels in apoptotic markers (p53, Bax, caspase-3 and BCl2) genes in MCF-7 cells, when exposure with Co3O4NPs (Fig. 9). The mRNA of tumor suppression gene supports the apoptosis induction by foreign material (NPs) and observed the enzymatic activities of caspase-3 at the concentrations of 50 µg/ml. In all the selected gene, the observations shows that the expression were in up-regulation except BCl2 and the fold changes for the p53 (2.2), Bax (4.1), Casp3 (1.9) and BCl2 (0.71) respectively (Fig. 9). The Results showed that NPs induced the apoptotic enzymes (caspase-3) in a dose-dependent manner (Fig. 9).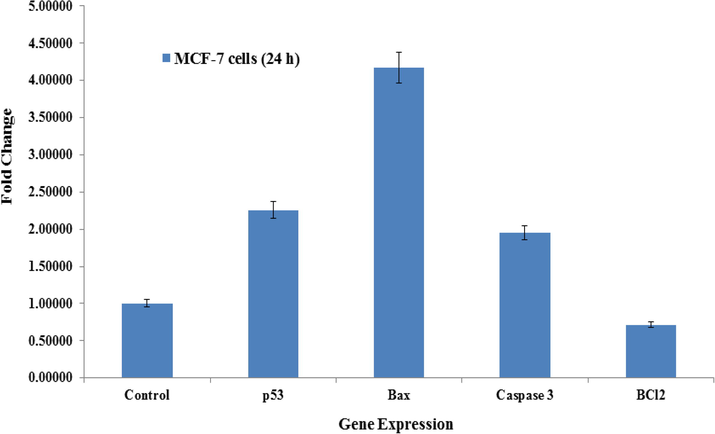
mRNA expression of apoptosis marker genes by real-time polymerase chain reaction (RT-PCR) analysis in MCF-7 cells with cobalt oxide nanoparticles (Co3O4NPs) at 50 mg/mL concentration for 24 h. RT-PCR data was achieved with Roche Light Cycler®480 soft-ware (version 1.5). The glyceraldehyde 3-phosphate dehydrogenase (GAPDH) gene was used as a control to normalize data. The data is accessible as the mean ± SD of three identical experiments with three replicates manner. *Significantly different compared with the control group (p < 0.05 for each).
4 Conclusions
The summary of the present work shows that the Co3O4NPs (size ∼ 33 ± 1 nm) were prepared via solution process and well characterized. The Co3O4NPs were utilized at very low concentration (1 to 100 μg/mL) against MCF-7 cancer cells, which signifies that the cytotoxicity of MCF-7 cells is dose-dependent and influence against cancer cells validated via MTT and NRU assays. The apoptosis or cells death cased with Co3O4NPs enhance at an optimized doses of nanostructure concentration. The current work implies the possibility to use processed nanostructures will be much effective against cancer cells and will be useful as an inorganic based nanodrug. To testify the prepared nanostructures, a detailed investigation as well as their sustainability under biological environments is required. The study epitomize that Co3O4NPs induces the cytotoxicity, apoptosis in MCF-7 cancer cells via p53, Bax, and caspase pathways. It also expected that Co3O4NPs to be intervened via ROS generation and oxidative stress. The overall recovered data suggest that the NPs are responsible to control the growth of cancer cells. Due to very small size it can be quickly and easily reach to the cells organelles as compared to other complex structures of drugs. The utilization of inorganic based nano structured materials for cancer studies can be possible to reduce the cost of the drugs also these nanostructured based studies reduces the anxiety of surgery for deprived patient.
Acknowledgement
The authors are grateful to the Deanship of Scientific Research, King Saud University for funding through Vice Deanship of Scientific Research Chairs.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- American Cancer Society. Breast Cancer Facts & Figures 2019-2020. Atlanta: American Cancer Society, Inc. 2019. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/breast-cancer-facts-and-figures/breast-cancer-facts-and-figures-2019-2020.pdf
- Exploring the Interaction of Cobalt Oxide Nanoparticles with Albumin, Leukemia Cancer Cells and Pathogenic Bacteria by Multispectroscopic, Docking, Cellular and Anti bacterial Approaches. Inter. J. Nanomed. 2020;15:4607-4623.
- [Google Scholar]
- Catalytic performance of Mn3O4 and Co3O4 nanocrystals prepared by sonochemical method in epoxidation of styrene and cyclooctene. Appl. Surf. Sci.. 2010;256(22):6678-6682.
- [Google Scholar]
- Synthesis of Cobalt Hydroxide Nano-flakes Functionalized with Glutamic Acid and Conjugated with Thiosemicarbazide for Anticancer Activities Against Human Breast Cancer Cells. Biol. Trace Elem. Res.. 2020;198(1):98-108.
- [Google Scholar]
- Cobalt oxide nanoparticles can enter inside the cells by crossing plasma membranes. Sci. Rep.. 2016;6:22254.
- [Google Scholar]
- Effects of exercise on physical outcomes of breast cancer survivors receiving hormone therapy–A systematic review and meta-analysis. Maturitas. 2020;141:71-81.
- [Google Scholar]
- Toxicity determined in vitro by morphological alterations and neutral red absorption. ToxicologyLett. 1985;24(2-3):119-124.
- [Google Scholar]
- Global cancer transitions according to the Human Development Index (2008–2030): a population-based study. Lancet Oncol.. 2012;13(8):790-801.
- [Google Scholar]
- Molecular Investigation on a Triple Negative Breast Cancer Xenograft Model Exposed to Proton Beams. Int. J. Mol. Sci.. 2020;21(17):6337.
- [CrossRef] [Google Scholar]
- Chen K., Wei1 J., Ge C., Xia W., Shi Y., Wang H., Jiang X., 2020. Application of autoplanning in radiotherapy for breast cancer after breast‑conserving surger, Scientific Reports 10:10927
- Surface-modified cobalt oxide nanoparticles: new opportunities for anti-cancer drug development. CancerNano. 2012;3(1-6):13-23.
- [Google Scholar]
- Elements of X-ray Diffraction. Reading, MA: Addison-Wesley; 1978.
- Development of a novel graphene/ Co3O4 composite for hybrid capacitive deionization system. Desalination. 2019;451:102-110.
- [Google Scholar]
- Co3O4 Nanoparticles with Multi-Enzyme Activities and Their Application in Immunohistochemical Assay. ACS Appl. Mater. Interfaces. 2014;6(3):1959-1970.
- [Google Scholar]
- El-Dossoki F., Rady S and Hosny N., 2020. Synthesis of Co3O4 nanoparticles from amino acids mixed ligands and its adsorption properties. Alfa. J Basic & App Sci 1:1-12.
- Synthesis, characterization, and investigation of optical and magnetic properties of cobalt oxide (Co3O4) nano particles. J. Nanostructure in Chem. 2013;3:69.
- [Google Scholar]
- Resection of liver metastases from breast cancer: a multicentre analysis. Clin. Transl. Oncol.. 2020;22(4):512-521.
- [Google Scholar]
- Cobalt oxide nanoparticle-synergized protein degradation and phototherapy for enhanced anticancer therapeutics. Acta Biomaterialia. 2021;121:605-620.
- [Google Scholar]
- Photodynamic therapy, facile synthesis, and effect of sintering temperature on the structure, morphology, optical properties, and anticancer activity of Co3O4 nanocrystalline materials in the HepG2 cell line. J. Photochem and Photobiology A: Chem. 2020;386:112130.
- [CrossRef] [Google Scholar]
- Preparation, characterization, and anticancer efficacy of novel cobalt oxide nanoparticles conjugated with thiosemicarbazide. Biotech. 2020;10(5)
- [CrossRef] [Google Scholar]
- Jose J., Kumar R., Harilal S., Mathew GE., Parambi DGT., Prabhu A.,Uddin M.S., Aleya L., Kim H., Mathew B., 2020. Magnetic nanoparticles for hyperthermia in cancer treatment: an emerging tool, Environmental Sci.Poll.Res 27:19214-19225.
- A novel enzymatic glucose biosensor and sensitive non-enzymatic hydrogen peroxide sensor based on graphene and cobalt oxide nanoparticles composite modified glassy carbon electrode. Sens. Actuators, B. 2014;196:450-456.
- [Google Scholar]
- Facile strategy by hyaluronic acid functional carbon dot-doxorubicin nanoparticles for CD44 targeted drug delivery and enhanced breast cancer therapy. Inter. J. Pharmaceutics. 2020;578:119122.
- [CrossRef] [Google Scholar]
- Co3O4 Nanomaterials in Lithium-Ion Batteries and Gas Sensors. Adv. Func. Mater. 2005;15(5):851-857.
- [Google Scholar]
- Cobalt Oxide Nano particles: Behavior towards Intact and Impaired Human Skin and Keratinocytes Toxicity. Int. J. Environ. Res. Public Health. 2015;12(7):8263-8280.
- [Google Scholar]
- Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65(1-2):55-63.
- [Google Scholar]
- Breast Cancer Survivorship, Quality of Life, and Late Toxicities. Front Oncol.. 2020;10:864.
- [Google Scholar]
- Electrochemical Investigations of Magnetic Co3O4 Nano particles as an Active Electrode for Supercapacitor Applications. J. Super conductivity and Novel Magnetism. 2019;32(8):2427-2436.
- [Google Scholar]
- Engineered cobalt oxide nanoparticles readilyenter cells. ToxicologyLett. 2009;189(3):253-259.
- [Google Scholar]
- Assessing Cobalt Metal Nanoparticles Uptake by Cancer Cells Using Live Raman Spectroscopy. Inter. J. Nanomed. 2020;15:7051-7062.
- [Google Scholar]
- Molybdenum nanoparticles-induced cytotoxicity, oxidative stress, G2/M arrest, and DNA damage in mouse skin fibroblast cells (L929) Colloids Surf. BBiointerfaces. 2015;125:73-81.
- [Google Scholar]
- Influence of cytotoxic doses of 4-hydroxynonenal on selected neuro transmitter receptors in PC-12 cells. Toxicol In Vitro.. 2008;22(7):1681-1688.
- [Google Scholar]
- Protective potential of trans-resveratrol against 4-hydroxynonenal induced damage in PC12 cells. Toxicol. In Vitro. 2010;24(6):1592-1598.
- [Google Scholar]
- novel synthesis protocol for Co3O4 nanocatalysts and their catalytic applications. RSC Adv. 2017;7(62):38861-38870.
- [Google Scholar]
- Structural and Optical Properties of Co3O4 Nanoparticles Prepared by Sol- gel Technique for Photocatalytic Application. Int. J. Electrochem. Sci. 2019;14:3535-3552.
- [Google Scholar]
- ZnO Nanoparticles Induce Oxidative Stress in Cloudman S91 Melanoma Cancer Cells. J. Biomed Nanotech. 2013;9(3):441-449.
- [Google Scholar]
- ZnO nanoparticles induced oxidative stress and apoptosis in HepG2and MCF-7 cancer cells and their antibacterial activity. Colloids Surf. BBiointerfaces. 2014;117:267-276.
- [Google Scholar]
- Detecting the oxidative reactivity of nanoparticles: a new protocol for reducing artifacts. J Nanopart Res.. 2014;16(7):2493.
- [Google Scholar]







