Cytotoxic activity and toxicity study of HF8, a poly-herbal formulation
⁎Corresponding author. nabutaha@ksu.edu.sa (Nael M. Abutaha)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Introduction
Breast cancer ranked the 2nd amongst all cancer in terms of mortality worldwide.
Objective
We evaluated the anticancer, apoptotic activity and toxicity of the polyherbal formulation, HF8, prescribed by the herbalist in Saudi Arabia.
Methods
HF8 was prepared by mixing different proportion of plants namely Rosmarinus officinalis. L, Vitis vinifera seeds, Cichorium intybus seeds, Trigonella foenum-graecum L seeds, Lavandula multifida L., Pistacia lentiscus resin, commiphora myrrha resin, and Viola odorata flower. The HF8 was evaluated for its cytotoxicity using human breast adenocarcinoma (MCF-7, and MDA-MB-231 cells) cell lines.
Results
The result revealed promising cytotoxicity on the MCF-7 (IC50: 107 µg/mL) and MDA-MB-231 (IC50: 89.5 µg/mL) cell lines. Further, HF8 was assessed for its apoptotic potential using acridine orange (AO)/ethidium bromide (EB) dual staining and Caspase-3/7. After incubation with MDA-MB-231, the HF8 inducted apoptosis through the activation of caspase-3/7. The toxicity of the HF8 was assessed using zebrafish and Swiss Albino mice. The zebrafish embryos did not show any noticeable toxicities or teratogenicity when treated with HF8 with a concentration from 0.001 to 300 µg/ml. In acute toxicity, no mortality detected after a single dose (5000 mg/kg) administration to Swiss Albino mice of both genders.
Conclusions
HF8 extract has the potential for development into a potent anticancer agent against breast cancer, albeit further studies are needed to evaluate the mechanism of action as well as validation of other drug development processes.
Keywords
Acute toxicity
Breast cancer
Poly-herbal formulation
Zebrafish
1 Introduction
Breast cancer the most often diagnosed cancer in women (Torre et al., 2015). Breast cancer is the 9th cause of death in females in the Kingdom of Saudi Arabia (KSA) in 2010 (Mokdad et al., 2014), and it is expected that the number will increase due to aging and the population’s growth in KSA (Ibrahim et al., 2008).
Unfortunately, the available therapeutic solutions for cancer are limited. The present therapies for the cancer include chemotherapy, surgery, hormonal therapy, and radiation therapy. The currently available therapy has very serious side-effects including appetite loss, anemia, constipation, thrombocytopenia, diarrhea, delirium, edema, nerve peripheral neuropathy, sexual health issues, alopecia, neutropenia, lymphedema, memory problems, and pain, (NCI, 2017).
In addition to these side effects drugs are costly and patent protected. There are many reports that confirm the resistance of tumor cells to drugs even the most recent discovered ones such as Abemaciclib (Guarducci et al., 2017), doxorubicin (AbuHammad and Zihlif, 2013), 5-fluorouracil (Takahashi et al., 2013), Gemcitabine (Yang et al., 2014), paclitaxel (Němcová-Fürstová et al., 2016), Taxane (Wang, 2014), and Vinblastine (Rosas-Ramírez et al., 2017). Therefore, there is a need for a new safe and improved chemotherapeutic agent for cancer treatment.
In this context, herbal formulations have recently received scientific attention as a promising candidate chemotherapeutic agent. The mixture of plants improves efficacy compared to the single because it targets many pathways in an additive or synergistic way. Herbalists use combination of different plants to achieve maximum therapeutic potential than the individual ones. These combinations are employed for the treatment of various disorders (Kang et al., 2015). These combinations are employed for the treatment of various disorders for example Hwang-Heuk-San used in treatment of cancer in traditional Korean medicine (Kang et al., 2015). Similarly, QYHJ, a seven-herb medicinal formula, is a Chinese formula used in treatment of cancer (Chen et al., 2014). DSHT, is known for anti-diabetic (Park et al., 2001), anti-hepatotoxic (Kim et al., 2004) and anti-obesity activities (Hussain et al., 2016)
Though most natural products are commonly recognized as safe, however, adverse effects may happen sometimes after consuming herbal products (Sharwan et al., 2015) such as dermatitis diarrhea, nausea, respiratory and cardiac failure, and death (Serrano, 2018). Current toxicology testing in preclinical studies includes in vitro and in vivo models. Usually these studies are expensive, time consuming and require a large number of animals. However, zebrafish (Danio rerio) offers several advantages for low cost, ease of maintenance, ease of breeding, high fertility, and transparent eggs that enable organogenesis much easier as compared to rodents (Gore et al., 2018).
The preparation, HF8, is one of a polyherbal formulation prescribed by the herbalists in Saudi Arabia. The herbalists claim it to be an effective treatment of cancer. The anticancer activity of HF8 polyherbal formula and the toxicity haven't been studied. Therefore, we planned this study to investigate anticancer potential and its toxicity in mice and zebrafish embryos.
2 Materials and methods
2.1 Plant collections
Eight plants or plant products namely Rosmarinus officinalis L, Vitis vinifera seeds, Cichorium intybus seeds, Trigonella foenum-graecum L seeds, Lavandula multifida L., Pistacia lentiscus resin, commiphora myrrha resin, and Viola odorata flower were purchased as a dried herb from herbal drug store from Reef al yamen Co., Al Morooj, Riyadh, kingdom of Saudi Arabia and were authenticated based upon their microscopic and macroscopic characteristics in the Department of Botany and Microbiology (DBM), College of Science, King Saud University. The voucher samples were placed at the herbarium of DBM.
2.2 Plant extraction
R. officinalis (15 g), V. vinifera seeds (13.20 g), C. intybus seeds (12.5 g), T. foenum-graecum seeds (27 g), L. multifida (7.60 g), P. lentiscus resin (15.4 g), C. myrrha resin (18.5 g), and V. libanotica flower (14 g) were collected in a beaker and mixed until homogenized, to which 2.5 L of boiling distilled water (1:10, sample:water, w/v) was added. The mix was soaked for 1 h, filtered using cotton, and centrifuged (4000 rpm) for 3 min. The collected supernatant was evaporated by rotary evaporation under vacuum at 50 °C (Heidolph, Schwabach, Germany). The extract was given the name HF8.
2.3 Cytotoxicity assay
Cytotoxicity of HF8 was carried out by MTT (Life Technologies, USA) assay on MCF7 and MDA-MB-231 cells as reported previously (Abutaha, 2020). In brief, cells (5 × 105 cells/ well) were seeded into 24-well plates in 1000 μL medium. After attachment, cells were incubated with various gradient concentrations ranged between 25 and 250 µg/mL and further incubated for another 48 h to assess cytotoxicity. 200 μL MTT (5 mg/mL) was pipetted into each well and the plates were further incubated (37 °C) for extra 2 h. The media were aspirated and then 1000 μL 0.01% HCl-MeOH was transferred to each well to solubilize formazan crystals. The plates were read at 490 nm on a multi-well microplate reader (Thermo Fisher Scientific Inc., USA). Methanol was used as vehicle controls.
2.4 LDH assay
Cells were plated in 24-well plate until 70% confluency. The LDH release post HF8 treatment was assessed with a commercial kit based on the manufacturers’ instruction (Sigma). Absorbance at 490 nm was measured on a multi-well microplate reader.
2.5 AO and EB dual staining
The cells (1 × 105 cell/well) were incubated with HF8 extract (100 µg/mL) for 48 h and exposed a mixture of 10 μL ethidium bromide (1 mg/ml) and 1 μL of acridine orange (10 mg/ml) was added to the plate and after 5 min of dark incubation at 25 °C, multiple images of the stained cells were taken under inverted fluorescence microscope (EVOS, USA).
3 Caspase 3/7 detection
MDA-MB-231 cells were seeded in 24-well plates as mentioned above for the detection of caspase-3/7 induction using the caspase-3/7 green detection kit (2 μM) (Invitrogen, USA) as previously reported (Abutaha et al., 2020). Finally, images from each well were taken using a fluorescent microscope (EVOS, USA).
3.1 Experimental animals
Male and female Swiss Albino mice (26–30 g) were obtained from the Animal House facility, King Saud University. The mice were acclimatized for 5 days before the experiments. The mice were housed in plastic cages in regulated environment (23–25 °C, with a 12 h light/dark cycle) with a standard pellet diet and tap water ad libitum. All measures were carried out in accordance with the Animal Ethics Committee, Princess Nourah bint Abdulrahman University.
3.2 Acute oral toxicity study
An acute toxicity test was carried out according to Organization for Economic Cooperation and Development (OECD) guideline 420 for testing of chemicals (OECD, 2001). Mice of both sexes were used. HF8 extract was dissolved in water and given orally at a single dose of 5000 mg/kg (n = 6; 3 males and 3 females mice). The control group administered only distilled water. After administration of HF8 extract, mice were monitored for 24 h. The mice were observed once daily during the period for mortality, changes in appearance, pain, signs of illness, injury, and behavioral including salivation, fur, and lethargy.
3.3 Zebrafish embryo treatment
Wild type zebrafish (AB/Tuebingen tab-14) were obtained from zebrafish international resource center (ZIRC University of Oregon, USA) and kept under standard conditions as recommended (Westerfield, 2000). The embryos were obtained from natural pair wise breeding. The eggs were harvested soon after spawning and were washed once with egg water (distilled water supplemented with commercial sea salt 60 µg/ml and subsequently raised in embryos medium (KCl 0.17 mM, NaCl 5.03 mM, MgSO4·7H2O 0.33 mM, CaCl2·2H2O 0.33 mM, and Methylene blue 0.1% (w/v) in distilled water. The unfertilized and dead embryos were removed and round 7–10 embryos were transferred to each of 35 mm sterile glass culture dishes having embryo medium. The poly herbal formula was dissolved in distilled water to obtain a stock concentration of 10 mg/ml, the working concentrations (0.001, 0.01, 1, 10, 100 and 300 µg/ml were calculated and desired volume of the herbal formula was pipetted to the medium. The untreated embryos served as control. The desired criteria for in vivo toxicities (mortality, otoliths, eyes, circulation, and heart beat) were recorded after every 24 h post fertilization (hpf). The assay was repeated in triplicate using different embryos batches from different parents. The phenotype (toxicity) was only recorded as real if it has been observed in more than 60% of treated embryos and reproducible in all three biological trials.
4 Result
4.1 In vitro cytotoxicity assay
The HF8 revealed a broadly dose dependent cytotoxic activity on the MCF-7, and MDA-MB-231 (Fig. 1). The IC50 values of the HF8 against to MCF-7, and MDA-MB-231 were 107 and 87.9 µg/mL respectively (Fig. 1A). The most promising anticancer potential of the HF8 extract was against MDA-MB-231 cells.
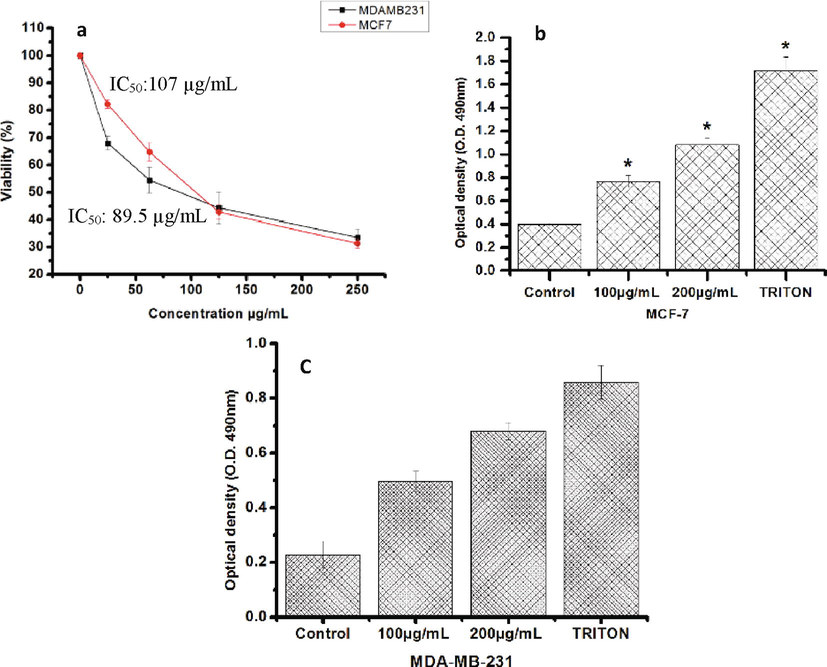
- Effect of HF8 on viability of MDA-MB- 231 and MCF-7. (a) Concentration-dependent inhibitory effect of HF8 on cellular growth of MDA-MB- 231 and MCF-7 cells. The cell viability was measured by MTT assay. (b and c) Lactate dehydrogenase assay showing its release from the cells after the addition of the 100 and 200 µg/ml of HF8. Values are the means ± SD (n = 3) *P < 0.05.
4.2 LDH cytotoxicity test
The cytotoxic effect of the HF8 was also evaluated by LDH release on treated cells at the concentration of 100 and 200 μg/mL after 48 h of treatment. HF8 caused a significant increase of LDH release in treated cells compared with methanol control cells (Fig. 1B and 1C).
4.3 Morphological assessment by AO/EB
The PH8 treated cells revealed nuclear morphological changes. MDA-MB-231 cells were stained with AO/EB after 48 h of treatment. No apoptosis was detected in the methanol control group (Fig. 2). In the experimental group, apoptotic cells marked by granular green nuclear fluorescence located in one side of cells (Fig. 1C). Necrotic cells marked by uneven orange-red staining at their periphery and increased in cells volume.
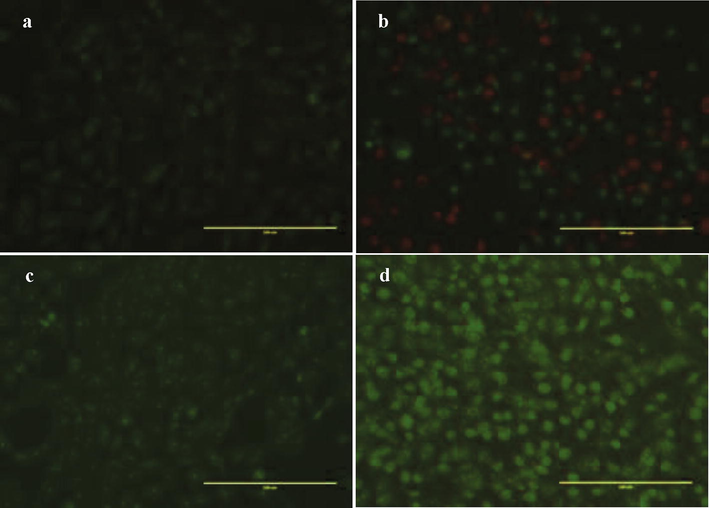
- Cellular staining using acridine orange/ethidium bromide of MDA-MB-231 and MCF-7 treated with HF8. (a) Negative control group MDA-MB-231 and (b) MCF-7: the nucleus located in the cell center. Experimental group (apoptotic cells) (c) MDA-MB-231 and (d) MCF-7 nucleus fluoresce green or orange and located in one side of cells. Necrotic cells appeared irregular red fluorescence (Magnification 200x).
4.4 Activation of caspase-3/7
We assessed the effect of the HF8 extract on caspase-3/7 activation. Treatment of MDA-MB-231 cells with HF8 induced caspase-3/7 activity, as detected by a bright fluorescence upon cleavage of DEVD (a four amino acid peptide), a fluorescent labeled peptide as compared with the control cells (Fig. 3).Fig. 4.
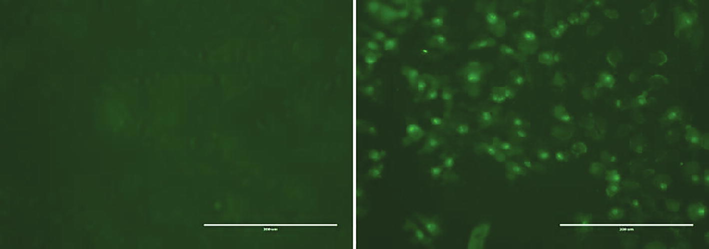
- HF8 induced apoptosis in MDA-MB-231; Apoptosis was assayed using caspase 3/7 assay. Cells fluoresce bright green due apoptosis. Images were taken using a fluorescence microscope at 20× magnification at 100 µg/mL. (A) Untreated controlled cells. (B) Treated cells.
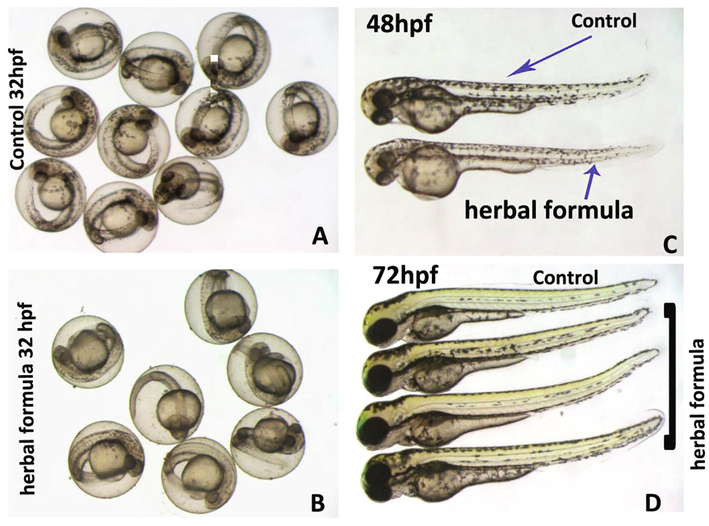
- The poly-herbal formulation did not induce toxicity in zebrafish embryo. Representative photomicrograph of zebrafish embryos either control or treated with poly-herbal formulation at concentration of 300 µg/ml are shown at various developmental stages. In order to avoid biases, the control and treated embryos shown here are at the same time intervals. A) The group of control embryos at 32 hpf. B) The group of zebrafish embryos which were treated overnight (∼16 h) with poly-herbal formulation are shown at 32 hpf developmental stage. The treated embryos did not show any obvious malformation or significant developmental delay. The treated embryos were at same developmental stage as compared to control embryos (A). The poly-herbal formulation proved to be nontoxic to zebrafish embryos after prolong exposure of 48 hpf (C) and 72hpf (D). The abbreviation used “hpf”; hours post fertilization.
4.5 Acute oral toxicity study
The HF8 at 5000 mg/kg showed no detrimental effect on the behavior of the mice for 14 days of monitoring. Physical observations showed no tremors, salivation, diarrhea, skin changes, fur, eyes, and behavior patterns of the mice. There was no mortality nor weight loss in the mice tested (data not shown). The LD50 of this herbal formula was therefore expected to be more than 5000 mg/kg.
4.6 Zebrafish
The zebrafish embryos did not show any noticeable toxicities or teratogenicity when treated with poly-herbal formulation with a concentration from 0.001 to 300 µg/ml. The cumulative toxicities are reported in Table 1 and it is quite evident that the HF8 was very much safe to zebrafish embryos and the numbers of mortality were very much similar to control embryos. Similarly, the defects in otoliths, eyes and circulation in treated embryos were insignificant as compared to control. Zebrafish embryos hatched normally around 48 hpf, and the hatching of the treated embryos with herbal formulation was recorded around 72hpf to have synchronized developmental stage.
| Concentration | Embryo toxicity | Otoliths | Eyes | Somite | Tail detachment | Heart beat | Blood circulation | Hatching | Active swimming | |
|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± S.D | Mean ± S.D | Mean ± S.D | Mean ± S.D | Mean ± S.D | Mean ± S.D | Mean ± S.D | Mean ± S.D | Mean ± S.D | ||
| 24hpf | Control | 00 ± 00 | 0. ± 00 | 0. ± 00 | 0. ± 00 | 0. ± 00 | 0. ± 00 | 0. ± 00 | 0. ± 00 | 0. ± 00 |
| 0.001 | 1 ± 1 | 0. ± 00 | 0. ± 00 | 0. ± 00 | 0. ± 00 | 0. ± 00 | 0. ± 00 | 0. ± 00 | 0. ± 00 | |
| 0.01 | 0.66 ± 0.57 | 0. ± 00 | 0. ± 00 | 0. ± 00 | 0. ± 00 | 0. ± 00 | 0. ± 00 | 0. ± 00 | 0. ± 00 | |
| 1 | 1 ± 1 | 0. ± 00 | 0. ± 00 | 0. ± 00 | 0. ± 00 | 0. ± 00 | 0. ± 00 | 0. ± 00 | 0. ± 00 | |
| 10 | 2.33 ± 0.57 | 0. ± 00 | 0. ± 00 | 0. ± 00 | 0. ± 00 | 0. ± 00 | 0. ± 00 | 0. ± 00 | 0. ± 00 | |
| 100 | 3.33 ± 0.57 | 0. ± 00 | 0. ± 00 | 0. ± 00 | 0. ± 00 | 0. ± 00 | 0. ± 00 | 0. ± 00 | 0. ± 00 | |
| 300 | 4.33 ± 0.57 | 0. ± 00 | 0. ± 00 | 0. ± 00 | 0. ± 00 | 0. ± 00 | 0. ± 00 | 0. ± 00 | 0. ± 00 | |
| 48hpf | 0. ± 00 | 0. ± 00 | 0. ± 00 | 0. ± 00 | 0. ± 00 | 0. ± 00 | 0. ± 00 | 0. ± 00 | 0. ± 00 | |
| Control | 0. ± 00 | 0. ± 00 | 0. ± 00 | 0. ± 00 | 0. ± 00 | 0.33 ± 0.75 | 0.33 ± 0.57 | 0. ± 00 | 0. ± 00 | |
| 0.001 | 0. ± 00 | 0. ± 00 | 0. ± 00 | 0. ± 00 | 0. ± 00 | 0 | 0. ± 00 | 0. ± 00 | 0. ± 00 | |
| 0.01 | 0. ± 00 | 0. ± 00 | 0. ± 00 | 0. ± 00 | 0. ± 00 | 0.33 ± 0.75 | 0. ± 00 | 0. ± 00 | 0. ± 00 | |
| 1 | 0. ± 00 | 0. ± 00 | 0. ± 00 | 0. ± 00 | 0. ± 00 | 0.33 ± 0.75 | 0. ± 00 | 0. ± 00 | 0. ± 00 | |
| 10 | 0. ± 00 | 0. ± 00 | 0. ± 00 | 0. ± 00 | 0. ± 00 | 1.33 ± 0.75 | 0.66 ± 0.57 | 0. ± 00 | 0. ± 00 | |
| 100 | 1.330. ± 0.57 | 0. ± 00 | 0. ± 00 | 0. ± 00 | 0. ± 00 | 2.666667 | 1 ± 0.0 | 0. ± 00 | 0. ± 00 | |
| 300 | 2.660. ± 1.15 | 0. ± 00 | 0. ± 00 | 0. ± 00 | 0. ± 00 | 5. ± 00 | 1 ± 00 | 0. ± 00 | 0. ± 00 | |
100% of embryos which were treated with poly-herbal formulation with ≥300 µg/ml did not hatch up to 72hpf, while the all the control embryos hatched successfully at this developmental stage. The hatching of embryos depends on variable factors and it could also be an indirect effect of chemical or extracts which have been used to treat the embryos. The failure to hatch of zebrafish embryos which were treated with HF8 need to be further explored, but we think that this could be a secondary effect as we have not seen any teratological signs and also the treated embryos did not have any noticeable signs of developmental delay.
A functional heart with active circulation is visible around 24–30 hpf in zebrafish embryos. The active circulation and heart beat in treated embryos were recorded around 48hpf in order to allow maximum duration in which 100% embryos would have synchronized active circulation.
5 Discussion
Natural products have been studied as anticancer chemotherapeutic agents, in vivo and in vitro (Wu et al., 2004). However, there is limited information on the medicinal value of polyherbal formulation commonly used by traditional healer in general and in the Middle East in particular. Therefore, the aim of the present study was to evaluate the HF8 potency on cell proliferation inhibition, induction of apoptosis, toxicity on mice and zebrafish embryos which could help in proving the claim of their anticancer activity and safety.
Both the MTT and LDH assay were used to assess the cell viability, confirm the reliability and avoid misestimating of the toxicity of the HF8. In this study, HF8 showed a dose-dependent reduction in the cell viability. The present study also revealed that the LDH release from the cells increased significantly when incubated with HF8 as compared with the control. Therefore, LDH release may be resulted from the cytotoxic nature of the HF8 and thus, assure its antitumor activity (Sivalokanathan et al., 2006). LDH is reliable signal of cytotoxicity because its release is a marker of irreversible death due to the cell membrane damage (Xia et al., 2007), early necrosis and the late stage of apoptosis (Do et al., 2003).
In herbal drugs discovery, necrosis may take place together with apoptosis this has been found in many chemotherapeutic drugs such as 5′flurouracil, doxorubicin, cisplatin and cladribine which have both apoptotic and necrotic properties (Guchelaar et al., 1992); (Mailloux et al., 2001). Phyto-compounds that induce apoptosis are promising candidates for cancer therapy. The key mechanism of anticancer drugs is by induction of apoptosis (Liu et al., 2015). Natural products have been reported into induce apoptosis in vivo and in vitro.
One of the best approaches for apoptosis detection is microscopic observation of cell morphology (Abdel Wahab et al., 2009). MDA-MB-231 cells incubated with HF8 demonstrated anticancer activity through the apoptotic pathway. The changes in cell shape after 48 h were characterized by a rounding of cells, detachment, and the protrusion of apoptotic bodies. Apoptosis is marked by certain morphological changes such as cell shrinkage, rounding of cells, detachment, blebbing, apoptotic bodies and nuclear fragmentation that were observed. Further apoptosis induction was confirmed by using AO/EB fluorescent by AO/EB stain using florescent microscopy revealed that PH8 treated cells displayed nuclear morphological changes. AO/EB stain helps to distinguish between normal, early and late apoptotic cells, and necrotic cells (Biffl et al., 1996). Treatment with the HF8 showed brightly shining nuclei, a sign of cells undergoing and cells appeared irregular red fluorescence a hallmark of cells undergoing necrotic.
Caspases are proteins that regulate cell death to maintain homeostasis; they carry out these tasks through proteolytic cleavage at specific aspartate residue of their substrates (McIlwain et al., 2015). Apoptotic caspases are classified into two subgroups, effector caspases and initiator caspases (Shioiri et al., 2009).
After incubation of cell line with HF8 we studied caspase-3/7 activity. In this work, MDA-MB-231 cells treated with HF8 showed caspase-3/7 activation analyzed at 48 h of incubation (Fig. 3). Taken together, the results revealed that HF8 possesses dual-ability of cell death, could be due to the crude nature of the HF8, where all bioactive molecules are mixed together and may act independently or in synergism, thus contributing to this dual-ability of cell death properties.
The anticancer activity of HF8 and the knowledge about the toxicology of the HF8 are lacking. Hence, the current study aimed to assess the anticancer activity and the acute toxicity in vivo and in vitro. HF8 at 5000 mg/kg produce no deleterious effect on the tested mice. There are no changes in the mice body weights after dosing may show that the HF8 is not toxic. The body weight changes reflect the general health status of an animal (Yuet Ping et al., 2013). Therefore; this study revealed that the HF8 has an LD50 value higher than 5000 mg/kg. Technically, the limit test is not used for assessing the exact LD50 value, but it helps in classifying the herbal extract (Roopashree et al., 2009). Based on the chemical classification of acute toxicity suggested by OECD, the HF8 extract was assigned class 5 (LD50 >5000 mg/kg), which is considered the lowest toxicity class. Substances with LD50 values higher than 5000 mg/kg are considered to be safe (Kennedy Jr et al., 1986).
Overall the HF8 found to be safe in zebrafish embryos as we have not noticed any severe toxicities in treated zebrafish embryos which were treated even with very high concentration (300 µg/ml), which mean that this herbal formulation even though having a encouraging anticancer potential in tested human cancer cell lines did not affect the normal tissues or at least normal embryonic development in zebrafish.
Plants reported in this study are rich in different classes of bioactive compounds including phenols, flavonoids terpenoids and many more (Gerlach et al., 2010; Zhou and Raffoul, 2012; Remila et al., 2015; Moore et al., 2016; Jancic et al., 2017; Yemmen et al., 2017; Al-Dabbagh et al., 2018; Mesquita et al., 2019; Aisa et al., 2020) that could have played an important role in the cytotoxic activity presented in the HF8 extract.
The present studies demonstrated that HF8 was an effective inhibitor against MCF-7, and MDA-MB-231 cell proliferation, and induced apoptosis. However, the use of any substance as a pharmacological drug depends on the balance between its therapeutic potential and toxicological effects. The results showed that the HF8 did not produce toxic effect towards mice and zebrafish larvae, suggesting that the anticancer activity of HF8 might be safe to use as pharmacological drug.
Acknowledgements
This work was funded by the Deanship of Scientific Research at Princess Nourah bint Abdulrahman University, through the Research Groups Program Grant no. (RGP-1442-0033).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- In vitro ultramorphological assessment of apoptosis induced by zerumbone on (HeLa) J. Biomed. Biotechnol. 2009
- [Google Scholar]
- Gene expression alterations in doxorubicin resistant MCF7 breast cancer cell line. Genomics. 2013;101(4):213-220.
- [Google Scholar]
- Anticancer, antioxidant, and acute toxicity studies of a Saudi polyherbal formulation, PHF5. Open Chem.. 2020;18(1):472-481.
- [Google Scholar]
- Chemical constituents and their pharmacological activities of plants from Cichorium genus. Chin. Herb. Med. 2020
- [Google Scholar]
- Antioxidant and anticancer activities of Trigonella foenum-graecum, Cassia acutifolia and Rhazya stricta. BMC Complement. Altern. Med.. 2018;18(1):1-12.
- [Google Scholar]
- Interleukin-6 delays neutrophil apoptosis via a mechanism involving platelet-activating factor. J. Trauma Acute Care Surg.. 1996;40(4):575-579.
- [Google Scholar]
- Chinese herbal medicine suppresses invasion-promoting capacity of cancer-associated fibroblasts in pancreatic cancer. PloS One. 2014;9(4):e96177
- [Google Scholar]
- Preferential induction of necrosis in human breast cancer cells by a p53 peptide derived from the MDM2 binding site. Oncogene. 2003;22(10):1431-1444.
- [Google Scholar]
- Anticancer and chemosensitizing abilities of cycloviolacin O2 from Viola odorata and psyle cyclotides from Psychotria leptothyrsa. Pept. Sci.. 2010;94(5):617-625.
- [Google Scholar]
- The zebrafish: A fintastic model for hematopoietic development and disease. Wiley Interdiscipl. Rev.: Develop. Biol.. 2018;7(3):e312
- [Google Scholar]
- Mechanisms of resistance to CDK4/6 inhibitors in breast cancer and potential biomarkers of response. Breast Care. 2017;12(5):304-308.
- [Google Scholar]
- Combination therapy with cisplatin: Modulation of activity and tumour sensitivity. Clin. Oncol.. 1992;4(6):388-393.
- [Google Scholar]
- Daesiho-Tang is an effective herbal formulation in attenuation of obesity in mice through alteration of gene expression and modulation of intestinal microbiota. PloS One. 2016;11(11):e0165483
- [Google Scholar]
- The present and the future of breast cancer burden in the Kingdom of Saudi Arabia. Med. Oncol.. 2008;25(4):387-393.
- [Google Scholar]
- Biologically active compounds and antioxidant capacity of Cichorium intybus L. leaves from Montenegro. Italian J. Food Sci.. 2017;29(4)
- [Google Scholar]
- Anti-inflammatory effects of Hwang-Heuk-San, a traditional Korean herbal formulation, on lipopolysaccharide-stimulated murine macrophages. BMC Complement. Altern. Med.. 2015;15(1):447.
- [Google Scholar]
- Estimation of acute oral toxicity in rates by determination of the approximate lethal dose rather than the LD50. J. Appl. Toxicol.. 1986;6(3):145-148.
- [Google Scholar]
- The preventive effect of Daesiho-tang on liver damage induced by acetaminophen in the rats. Korean J. Orient. Med. Prescr.. 2004;12(2):139-154. 15
- [Google Scholar]
- Dual AO/EB staining to detect apoptosis in osteosarcoma cells compared with flow cytometry. Med. Sci. Monit. Basic Res.. 2015;21:15.
- [Google Scholar]
- Anticancer drugs induce necrosis of human endothelial cells involving both oncosis and apoptosis. Eur. J. Cell Biol.. 2001;80(6):442-449.
- [Google Scholar]
- Caspase functions in cell death and disease. Cold Spring Harbor Perspect. Biol.. 2015;7(4)
- [Google Scholar]
- Exploring the anticancer properties of essential oils from family Lamiaceae. Food Rev. Int.. 2019;35(2):105-131.
- [Google Scholar]
- The state of health in the Arab world, 1990–2010: an analysis of the burden of diseases, injuries, and risk factors. Lancet. 2014;383(9914):309-320.
- [Google Scholar]
- Anticancer effects of rosemary (Rosmarinus officinalis L.) extract and rosemary extract polyphenols. Nutrients. 2016;8(11):731.
- [Google Scholar]
- NCI, 2017. Side Effects of Cancer Treatment National Cancer Institute.
- Characterization of acquired paclitaxel resistance of breast cancer cells and involvement of ABC transporters. Toxicol. Appl. Pharmacol.. 2016;310:215-228.
- [Google Scholar]
- Effects of Daesiho-tang and its component groups on diabetes, free radical and antioxidative defense system in alloxan induced diabetic rats. Korean J. Orient. Med. Prescr.. 2001;9(1):289-317. 14
- [Google Scholar]
- Antioxidant, cytoprotective, anti-inflammatory and anticancer activities of Pistacia lentiscus (Anacardiaceae) leaf and fruit extracts. Eur. J. Integr. Med.. 2015;7(3):274-286.
- [Google Scholar]
- Roopashree, T.S., Dang, R., Rani, R.S., Narendra, C., 2009. Acute oral toxicity studies of antipsoriatic herbal mixture comprising of aqueous extracts of Calendula officinalis, Momordica charantia, Cassia tora and Azadirachta indica seed oil.
- Resistance-modifying activity in vinblastine-resistant human breast cancer cells by oligosaccharides obtained from mucilage of chia seeds (Salvia hispanica) Phytother. Res.. 2017;31(6):906-914.
- [Google Scholar]
- Toxic plants: knowledge, medicinal uses and potential human health risks. Environ. Ecol. Res.. 2018;6(5):487-492.
- [Google Scholar]
- Toxicity profile of traditional herbal medicine. J. Ayur. Herb. Med.. 2015;1(3):81-90.
- [Google Scholar]
- Caspase-3 is activated and rapidly released from human umbilical vein endothelial cells in response to lipopolysaccharide. Biochim. Biophys. Acta Mol. Basis Dis.. 2009;1792(10):1011-1018.
- [Google Scholar]
- Effects of Terminalia arjuna bark extract on apoptosis of human hepatoma cell line HepG2. World J. Gastroenterol.. 2006;12(7):1018.
- [Google Scholar]
- Establishment of a 5-fluorouracil-resistant triple-negative breast cancer cell line. Int. J. Oncol.. 2013;43(6):1985-1991.
- [Google Scholar]
- Westerfield, M., 2000. The zebrafish book: a guide for the laboratory use of zebrafish. http://zfin.org/zf_info/zfbook/zfbk.html.
- Antihepatoma activity of Physalis angulata and P. peruviana extracts and their effects on apoptosis in human Hep G2 cells. Life Sci.. 2004;74(16):2061-2073.
- [Google Scholar]
- Mechanism of 2-methoxyestradiol-induced apoptosis in myelodysplastic syndrome MUTZ-1 cell line. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2007;15(2):296-301.
- [Google Scholar]
- Gemcitabine resistance in breast cancer cells regulated by PI3K/AKT-mediated cellular proliferation exerts negative feedback via the MEK/MAPK and mTOR pathways. OncoTargets Ther.. 2014;7:1033.
- [Google Scholar]
- Antioxidant activities, anticancer activity and polyphenolics profile, of leaf, fruit and stem extracts of Pistacia lentiscus from Tunisia. Cell. Mol. Biol. (Noisy-le-Grand France). 2017;63(9):87-95.
- [Google Scholar]
- Acute and subchronic toxicity study of Euphorbia hirta L. methanol extract in rats. BioMed Res. Int. 2013
- [Google Scholar]







