Translate this page into:
Curcumin palliative effects on sexual behavior, fertility and reproductive hormones disorders in mercuric chloride intoxicated mice offspring
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The present study was designed to investigate the impacts of mercuric chloride on offspring during pregnancy and lactation. Pregnant mice was selected and divided into various groups. To the experimental animal mercuric chloride and curcumin were incorporated. Only tap water was provided to the control group of animals. To the Group II and III animals, curcumin was administered at 150 and 300 ppm. Group IV animals received mercury chloride (10 ppm) and curcumin at two different doses (150 and 300 ppm). Experiment was started at the first day of pregnancy and treatment was continued up to PD 15 stage further, only water was provided. Male and female pups were separated and allowed for mating. Then, viability of sperm, sperm count and motility was tested. The hormonal level was analyzed from the serum sample. In the case of males, testosterone and FSH was analysed. In the case of female, progesterone and LH were detected from the serum sample. The present findings revealed decreased body weight, sexual behavior, viability of sperm and mating performance. Exposure of HgCl2 during lactation and gestation is highly dangerous. Exposure of HgCl2 affected fertility and social behavior during lactation and at the time of gestation. In this investigation, curcumin was used to minimize the toxic effect of HgCl2.
Keywords
Mercuric chloride
Infertility
Curcumin
Fertility
Mice offspring
1 Introduction
Mercury is one of the toxic heavy metals in the world and has been discovery very early (Parsons and Percival, 2005). The symbol “Hg” was derived from Latin. In earth, mercury occurs as Hg-bearing minerals and Hg, the most important form is cinnabar (Huang et al., 2007; Antonisamy et al., 2015). Mercury is used in various chemical industries. In chlorine production plants, it is widely used for the synthesis of chlorinated compounds and also used in the production of sodium hydroxide. Mercury has various aplications including to control weeds, fungi, bacteria and insects.
Curcumin (Cur) is a phytochemical present in the rhizome of Curcuma longa L. Cur is a phenol rich compound, diferuloylmethane (Balamurugan, 2015; Kannan and Agastian, 2015; Rathi et al., 2015). Curcuma longa L. comes under the family Zingiberaceae and widely distributed in Asian countries (Altintas et al., 2016). Cur has various benefits and is very much effective to treat various diseases and disorders including, coryza, anorexia, cough, sinusitis and hepatic diseases (Maheshwari et al., 2006). It is also used to control various diseases and also acts as antifungal, antiviral, anti-infectious, anti-inflammatory, anticarcinogenic, antimutagen, antiparasitic, and antioxidants (Ciftci et al., 2010; Valsalam et al., 2019a; Valsalam et al., 2019b). Curcumin has the potential to treat neurological disorders (Rajkumari et al., 2019; Gurusamy et al., 2019; Arokiyaraj et al., 2015). The neuroprotective effects of curcumin have been reported and this compound was found to be effective to treat various neurodegenerative diseases and disorders (Cole et al., 2007). The phytochemicals of curcumin showed various antioxidant properties (Ciftci et al., 2012). Abu-Taweel et al. (2013) analyzed the composition of commercial curcumin. Curcumin contains 77% curcumin, 17% de- methoxycurcumin and 3% bisdemethoxycurcumin. In this study, mercury chloride was treated to experimental animal and the protective role of curcumin on mercury chloride induced toxicity in mice was analyzed.
2 Materials and methods
2.1 Experimental animals
In this study, male and female Swiss–Webster mice with the age of about 12 weeks were selected. All animals are maintained in plastic cages (30 × 12 × 11 cm). The selected experimental animal was maintained at Saud University, Riyadh, Saudi Arabia. Ethical committee approval has been obtained.
2.2 Mercury and curcumin administration and experimental design
In this study, pregnant mice were divided into six goups. To the control group curcumin was not provided. The experimental group II and III was supplemented with Curcumin (150 and 300 ppm) and group IV was supplemented with only mercury chloride. In group V, curcumin was administered at two different doses (150 and 300 ppm). Experimental and control animal were fed with healthy diet and water. All experimental animals were further divided on the day of pregnancy. Analysis such as, sperm motility, sperm count and viability was analyzed. Male hormones were analyzed from the serum of male rats and the female hormone was tested in the female rats.
2.3 Total body weight analysis
Body weight is an important indicator to analyze metabolism. During experiments, the pups were frequently weighed up to PD40. The relative weight of liver, brain, prostate gland, seminal vesicle, vas deferens and epididymidis was analyzed.
2.4 Sexual behavior
The sexual activity tests carried out by introducing the experimental male mice in the home cage of the age matched estrous virgin females. The sexual activities of each male mouse had observed directly for three hours. The parameters recorded for sexual behavior were:
-
Latency of copulation (mounting): the time taken by the male mice to make the first attempt of mounting the female mice and making copulation.
-
The frequency of copulation: the number of copulation trials that will occur within a unit time scale will be frequency of copulation.
-
Duration of copulation: the total time spent by the males to copulate within the stipulated three hours observation will give the duration of copulation.
-
Approach and following: The time spent by the male near the female or follow her.
-
Threat and bite: The time spent by male in the process of the threat or biting the female.
-
The number of times the male sniffs the nose or vagina of the female.
-
Number of rears and wall rears: The number of times the male stands on its hind legs and hold its hands in the air or on the wall.
-
Number of the squat: The number of male lies on the floor of the cage away from the female.
-
Number of wash and digging: The number of times the animal cleans its body and scatters in the cage floor.
2.5 Fertility parameters
2.5.1 Sperm count, motility, and viability
In this study, sperm was counted from the epididymal region.
2.5.2 Mating test
Mating experiment was conducted using male and female mice treated at 2:1 ratio based on the instructions provided in WHO protocol (1983).
2.5.3 Analysis of hormones
The level of serum FSH and LG was analyzed as suggested by Peegel et al. (2005) and Pidoux et al. (2007) by double antibody radioimmunoassay method. The level of testosterone was analyzed by the method of radioimmunoassay according to the manufacture instructions.
2.5.4 Data analysis
The results were analyzed statistically using one-way analysis of variance (ANOVA) between control and experimental groups. Also Student-Newman-Keuls multiple comparison test was performed as suggested by Yamane (1973). The significance of sexual activity of the animal was analyzed using Mann-Whitney U test as described by Sokal and Rohlfe (1981).
3 Results
3.1 Body weight analysis
In the present study, body weight of the animal has decreased significantly due to the exposure of mercuric chloride. Table 1 and Fig. 1(A–H) showed marked depletion of absolute and relative body weight of the experimental animal. Also the organ such as, liver, brain, epididymidis, seminal vesicle, testis, vas deferens, and prostate gland weight was decreased considerably in the experimental animals treated with mercuric chloride than curcumin groups. Absolute and relative body weight of curcumin administered animal was increased than mercuric chloride experimental group. Body weight and other organs weight considerably reduced in mice offspring treated with mercuric chloride (Fig. 2A–D) (Table 1). The attenuating effect of curcumin on female animals were described and the variation was statistically significant (Fig. 2A–D). *,** and *** show statistically significant at P < 0.05, P < 0.01 and P < 0.001 respectively from the control and Cur groups.#,## and ### show statistically significant at P < 0.05, P < 0.01 and P < 0.001 respectively from Hg group by ANOVA and student's t-test.
Gender
Parameters
Doses (ppm)
Control
Cur (1 5 0)
Cur (3 0 0)
Hg (10)
Hg + Cur(1 5 0)
Hg + Cur(3 0 0)
Male
Body weight
39.3 ± 0.5
34.6 ± 0.8
36.1 ± 0.4
20.5*** ± 0.3
28.3# ± 0.5
32.4## ± 0.3
Brain A.W.
490.8 ± 0. 9
450.1 ± 0.4
470.4 ± 0.5
380.1*** ±0.2
410.5# ± 0.6
430.4## ± 0.8
Brain R.W.
20.7 ± 1.8
15.9 ± 1.5
18.1 ± 1.1
9.2*** ± 1.6
12.3 ± 1.2
14.2# ± 0.9
Liver A.W.
390.2 ± 0.12
350.3 ± 0.11
370.1 ± 0.11
190.6** ± 0.13
250.3## ± 0.16
300.3### ± 0.18
Liver R.W.
10.12 ± 0.14
8.21 ± 0.11
9.13 ± 0.13
5.23*** ± 0.13
6.11 ± 0.11
7.14# ± 0.15
Testis A.W.
220.1 ± 0.13
190.2 ± 0.17
202.12 ± 0.12
130.14*** ± 0.12
160.21# ± 0.14
190.16### ± 0.18
Testis R.W.
80.10 ± 0.16
70.21 ± 0.19
77.14 ± 0.17
41.18*** ± 0.16
55.17# ± 0.19
65.27## ± 0.14
Epididymidis A.W.
46.1 ± 2.1
40.4 ± 1.5
43.2 ± 1.1
19.3*** ± 1.2
30.4# ± 1.3
39.8## ± 1.5
Epididymidis R.W.
0.28 ± 1.3
0.21 ± 0.9
0.25 ± 1.1
0.13*** ± 0.11
18.1### ± 0.17
20.5### ± 0.10
Vas deferens A.W.
12.4 ± 0.8
10.40.5
11.9 ± 0.6
4.2*** ± 0.3
7.1## ± 0.1
10.2### ± 0.7
Vas deferens R.W.
0.08 ± 1.1
0.05 ± 0.9
0.06 ± 1.0
0.02 ± 0.6
0.04 ± 0.4
0.05# ± 0.7
Seminal V. A.W.
107.5 ± 1.4
76.1 ± 2.3
95.2 ± 2.1
57.9*** ± 1.4
61.4# ± 1.5
72.1## ± 1.2
Seminal V. R.W.
0.49 ± 1.0
0.41 ± 1.9
0.45 ± 1.3
0.18*** ± 2.2
0.30## ± 2.1
0.35### ± 1.9
Prostate A.W.
39.0 ± 2.1
28.3 ± 1.4
33.4 ± 1.3
15.4*** ± 1.8
22.4# ± 1.1
26.7## ± 1.7
Prostate R.W.
0.18 ± 1.2
0.14 ± 1.1
0.16 ± 1.2
0.10 ± 0.6
0.11 ± 0.7
0.13 ± 0.6
Female
Body weight
37.6 ± 0.9
28.1 ± 0.7
33.4 ± 0.5
20.2** ± 0.5
24.5# ± 0.7
27.6## ± 0.9
Brain A.W.
430 ± 1. 9
375 ± 1.4
410.5 ± 1.6
250*** ± 1.2
300.1# ± 1.5
367.2### ± 1.9
Brain R.W.
20.1 ± 1.8
15.4 ± 1.4
18.1 ± 1.3
9.4** ± 1.7
13.4# ± 1.4
14.9## ± 1.5
Liver A.W.
370 ± 0.12
320 ± 0.13
348 ± 0.12
170*** ± 0.11
280###0.17
310### ± 0.17
Liver R.W.
14.9 ± 0.24
12.4 ± 0.24
13.9 ± 0.21
7.8** ± 0.23
9.1 ± 0.28
13.7# ± 0.24
Ovaries A.W.
9.3 ± 0.8
7.5 ± 0.4
8.1 ± 0.7
5.8** ± 0.6
7.1# ± 0.5
7.4# ± 0.3
Ovaries R.W.
0.25 ± 0.14
0.21 ± 0.41
0.24 ± 0.13
0.14* ± 0.23
0.18 ± 0.26
0.20# ± 0.29
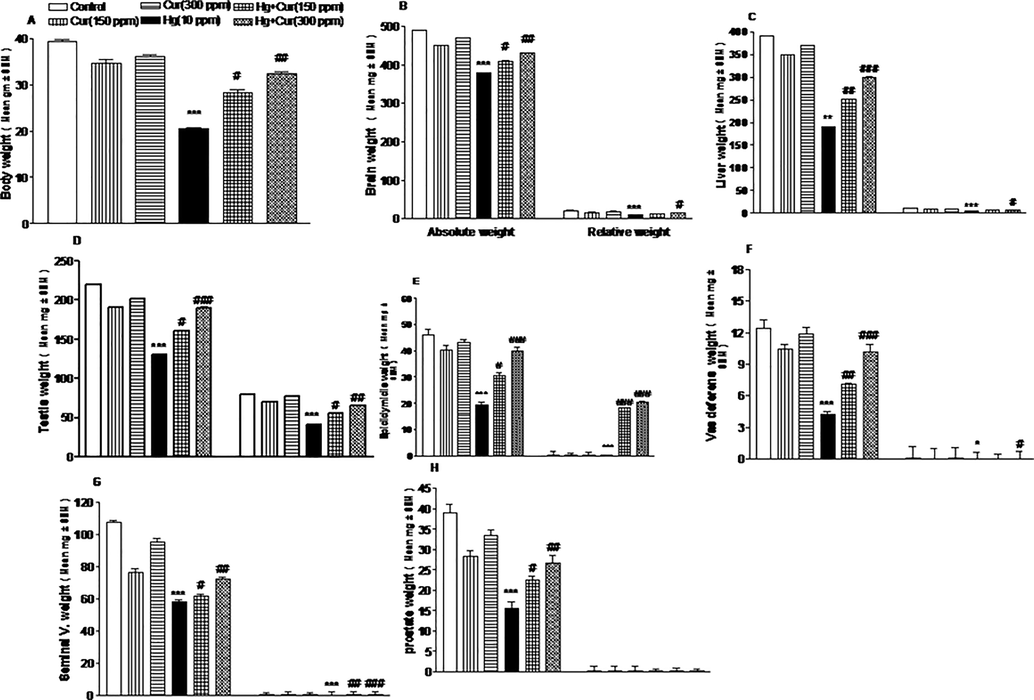
A–H Effect of perinatal exposure on the body, absolute and relative weight of male mice offspring. A, body weight; B, absolute and relative weight of brain; C, liver; D, testis; E, epididymidis; F, vas deferens; G, seminal vesicle and H, prostate. *,** and *** show statistically significant at P < 0.05, P < 0.01 and P < 0.001 respectively from the control and Cur groups. #,## and ### show statistically significant at P < 0.05, P < 0.01 and P < 0.001 respectively from Hg group by ANOVA and student's t-test.
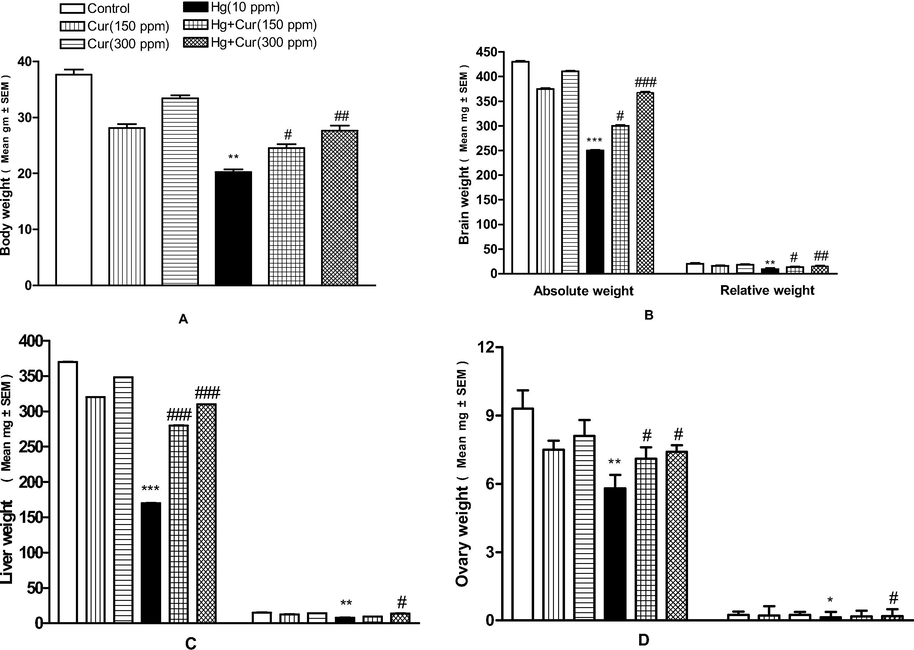
A–D Effect of perinatal exposure on the body, absolute and relative weight of female mice offspring. A, body weight; B,absolute and relative weight of brain; C, liver and D, ovary. *,**and*** show statistically significant at P < 0.05, P < 0.01 and P < 0.001 respectively from the control anf Cur groups. #,## and ### show statistically significant at P < 0.05, P < 0.01and P < 0.001 respectively from Hg group by ANOVA and student's t-test.
3.2 Sexual behavior
Mercuric chloride significantly affected sexual behavior in experimental animal and the behavior analysis was statistically significant. Naso-Nasal and Naso-Genital contacts, pelvic thrust and bite decreased, however the bite was increased and was statistically significant (Table 2A). The behavior such as, rears, wall rears, digging, wash were decreased considerably and was statistically significant (Table 2B). However, squat increased in experimental animal than curcumin treated animals. The protective role of curcumin on Hg toxicity in experimental animal is tabulated (Table 2B). *, ** and *** significantly different at (p < 0.05, p < 0.01 and p < 0.001) respectively from the control and Cur groups. #, ## and ### significantly different at (p < 0.05, p < 0.01 and p < 0.001) respectively from Hg group by ANOVA and Mann-Whitney U test. ** and *** show significantly different at (p < 0.01 and p < 0.001) respectively from the control and Cur groups. # and ### show significantly different at (p < 0.05, p < 0.01 and p < 0.001) respectively from Hg group by ANOVA and Mann-Whitney U test.
Group
Median number (with ranges) of acts and postures
Approach
Following
Number of Naso-Nasal contact
Number of Naso-Genital contact
Mount
Pelvic thrust
Threat
Bite
Control
21.00 (18.00–30.00)
33.00 (32.00–42.00)
63.00 (60.00–72.00)
33.00 (30.00–60.00)
9.00 (3.00 –10 0.00)
6.00 (6.00–9.00)
9.00 (5.00–12.00)
6.00 (3.00–9.00)
Cur (150 ppm)
15.00 (10.00–25.00)
23.00 (18.00–28.00)
45.00 (28.00–43.00)
26.00 (18.00–30.00)
6.00 (4.00–6.00)
4.00 (3.00–5.00)
6.00 (3.00–8.00)
6.00 (2.00–8.00)
Cur (300 ppm)
18.00 (12.00–20.00)
30.00 (18.00–31.00)
51.00 (30.00–57.00)
30.00 (28.00–39.00)
7.00 (3.00–8.00)
5.00 (4.00–7.00)
7.00 (3.00–8.00)
5.00 (3.00–7.00)
Hg(10 ppm)
6.00 * (0.00–9.00)
10.00 *** (0.00–9.00)
28.00 ** (25.00–33.00)
11.00 ** (6.00–13.00)
1.00** (0.00–2.00)
0.00 *** (0.00–2.00)
2.00* (0.00–2.00)
22.00 *** (16. 00 –25.00)
Hg + Cur (150 ppm)
11.00 # (9.00–13.00)
17.00 ## (10.00–18.00)
35.00 # (29.00–39.00)
21.00 # (13.00–25.00)
4.00 # (2.00–4.00)
3.00 ### (3.00–4.00)
5.00 # (2.00–5.00)
7.00 ## (4.00–9.00)
Hg + Cur (300 ppm)
13.00 ## (9.00–15.00)
21.00 ### (15.00–33.00)
41.00 ## (35.00–41.00)
25.00 ### (15.00–25.00)
6.00 ## (3.00–7.00)
5.00 ### (3.00–6.00)
6.00 ## (3.00–7.00)
9.00 ### (3.00–10.00)
Group
Median number (with ranges) of acts and postures
Wall rears
Rears
Wash
Squat
Digging
Control
22.00 (17.00–30.00)
16.00 (14.00–18.00)
4.00 (3.00–6.00)
1.00 (0.00–2.00)
7.00 (2.00–10.00)
Cur (150 ppm)
18.00 (12.00–25)
13.00 (13.00–18.00)
2.00 (2.00–4.00)
3.00 (2.00–4.00)
4.00 (3.00–12.00)
Cur (300 ppm)
20.00 (15.00–30.00)
15.00 (14.00–18.00)
3. 00 (2.00–5.00)
1.00 (1.00–3.00)
5.00 (2.00–6.00)
Hg (10 ppm)
4.00 *** (3.00–8.00)
2.00 *** (1.00–4.00)
1.00 ** (0.00–2.00)
9.00 *** (8.00–12.00)
2.00 ** (1.00–3.00)
Hg + Cur (150 ppm)
15.00 ### (10.00–16.00)
10.00 ### (8.00–11.00)
2.00 (1.00–2.00)
4.00 ### (2.00–5.00)
3.00 (1.00–4.00)
Hg + Cur (150 ppm)
17.00 ### (12.00–20.00)
12.00 ### (9.00–13.00)
3.00 # (3.00–4.00)
2.00 ### (0.00–3.00)
4.00 # (2.00–5.00)
3.3 Fertility of male and female offspring of mice
Analyses was performed to explore the impact of sperm count, mating index and viable sperm in mercuric chloride exposed animal and control group. The hormone level such as, testosterone and FSH and sperm mortality were significantly reduced in mercuric chloride exposed group than curcumin groups (Table 3, Fig. 3A–F). The impact of curcumin on resisting mercuric chloride toxicity is dose dependent and was statistically significant. Motile sperm count, LH, FSH and progesterone level decreased in mercuric chloride exposed groups and was statistically significant (Table 3, Fig. 4A–D). ** and *** show statistically significant at P < 0.01 and P < 0.001 respectively from the control and Cur groups.#,## and ### show statistically significant at P < 0.05, P < 0.01 and P < 0.001 respectively from Hg group by ANOVA and student's t-test.
Gender
Parameters
Dose (ppm)
Control
Cur (1 5 0)
Cur (3 0 0)
Hg (10)
Hg + Cur (1 5 0)
Hg + Cur (3 0 0)
Male
Sperm count (×106/ml)
42.50 ± 1.91
35.21 ± 1.24
40.12 ± 1.31
13.64*** ± 1.18
28.41### ± 1.42
32.51### ± 2.13
Sperm motility (%)
89.21 ± 3.2
77.3 ± 1.4
81.24 ± 2.1
31.20*** ± 1.7
60.28### ± 1.1
71.12### ± 1.5
Viable sperm (%)
79.94 ± 4.6
63.15 ± 2.1
71.13 ± 3.1
36.41*** ± 1.5
52.19# ± 1.2
60.17## ± 1.7
Mating index (%)
96.0 ± 5.1
81.37 ± 3.8
89.21 ± 2.9
40.21*** ± 2.1
65.17## ± 3.1
79.71### ± 2.7
FSH
821.4 ± 35.6
789.24 ± 13.2
801.21 ± 11.9
342.3*** ± 14.3
512.67### ± 15.2
614.57### ± 24.7
Testosterone
28.4 ± 1.2
21.9 ± 1.6
25.12 ± 2.1
2.8*** ± 1.7
10.24### ± 2.3
18.27### ± 2.7
Female
Viable embryo/litter
10.0 ± 1.7
8.0 ± 1.5
9.0 ± 1.4
3.0*** ± 14
5.0 ± 1.3
7.0## ± 1.5
FSH
951.4 ± 31.8
851.3 ± 28.4
907 ± 25.7
431.1*** ± 14.3
617.2### ± 18.4
818.7### ± 21.3
LH
68.8 ± 9.7
53.9 ± 8.1
62.1 ± 5.7
28.4*** ± 3.9
41.5## ± 4.5
50.1### ± 6.1
Progesterone
20.16 ± 1.9
16.12 ± 1.3
18.21 ± 1.7
1.6*** ± 1.6
11.1### ± 1.8
13.4### ± 1.4
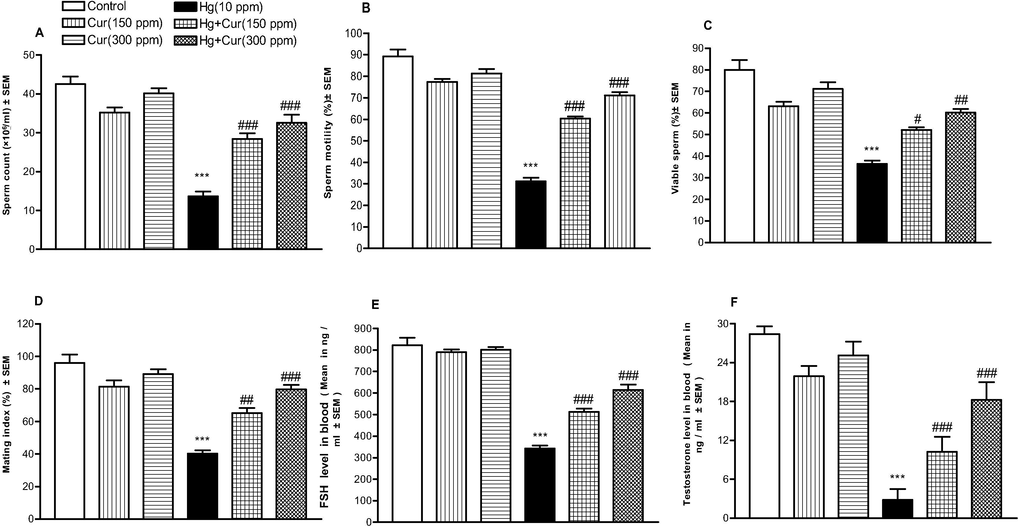
A–F. Effect of perinatal HgCl2 exposure on male mice offspring fertility. A, Sperm count; B, Sperm motility; C, viable sperm; D, mating index; E, FSH; F, testosterone. *** shows statistically significant at P < 0.001 from the control and Cur groups. #, ## and ### shows statistically significant at P < 0.001 respectively from the Hg group by ANOVA and student's t-test.
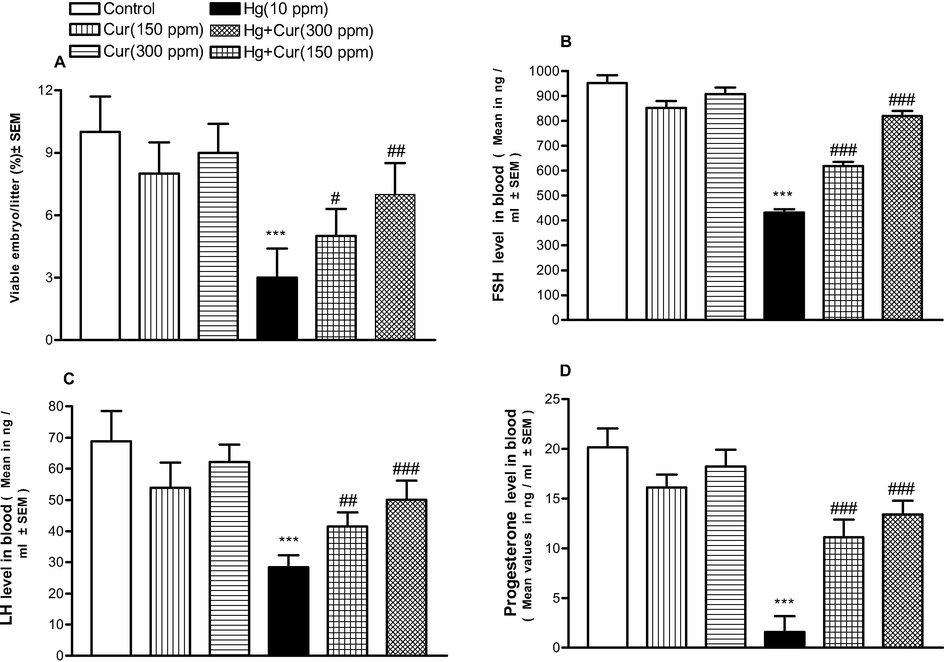
A–D Effect of perinatal HgCl2 exposure on female mice offspring fertility. A, Viable embryo/litter; B, FSH; C, LH; D, progesterone. *** shows statistically significant at P < 0.001 from the control and Cur groups. #,## and ### shows statistically significant at P < 0.05, P < 0.01 and respectively from Hg group by ANOVA and student's t-test.
4 Discussion
This experiment was designed to explore the impact of mercuric chloride on fertility and mating behavior of female and male offspring. The findings indicated the toxic effect of mercuric chloride to offspring during lactation and gestation. Results revealed that reduced relative and absolute body weight and total body weight also, the relative weight of various organs including, liver, brain and reproductive organs were decreased significantly. Mercuric chloride treated mice showed unusual sexual behavior than control group. The fertility factors in female and male offspring were decreased significantly than control. Mercuric chloride change the spermatogenic germinal layers in animals. Mercuric poisoning was characterized in the presence of degenerating cells and disruption of germinal epithelium in the seminiferous tubules. Mercuric compounds also damage DNA and maximize the action of germ cell apoptosis (Boujbiha et al., 2009). Exposure of mercury cause reduction in spermatogonia, primary and secondary spermatocytes and spermatogonia. Mercury exposure affected serum testosterone level in experimental animal. Leydig cells are predominant to produce testosterone in testis, the treatment of mercury affected Leydig cell function to affect the production of sex steroids (Shino et al., 2001; Boujbiha et al., 2009).
In the present study curcumin showed positive impact to repair cells cause by mercury toxicity. Curcumin has significant ameliorating effect on the mercury induced infertility, sexual behavior and reproductive disorders. The experimental animal treated with only curcumin did not show any negative impact to the animal. This clearly shows the beneficial and non toxic effect of curcumin. Administration of curcumin after the exposure of mercuric chloride restored some extent of biochemical and behavioral changes. Stajn et al. (1997) reported antioxidant properties of curcumin and also showed anti-inflammatory activity (Motterlini et al., 2000). Curcumin also has chemoprotective properties and has been reported by Ray (2005). The biochemical changes cause by mercury and ameliorating potential of curcumin in the present investigation may be due to the fact that curcumin has various health benefits, however much work is needed to analyze the ameliorating properties.
5 Conclusion
In this study, perinatal exposure of mercuric chloride on behavior and fertility of female and male mice was studied. The present findings showed significant disruption in fertility of female and male and changes in sexual behavior due to mercuric chloride toxicity. Exposure of mercury poses serious threat to fetus and neonates and easily passes through milk or placenta. Based our finding, curcumin is a key to protect the animals from mercury poisoning.
Declaration of Competing Interest
The author declared no conflict of interest in this manuscript.
References
- Protective effect of curcumin on anxiety, learning behavior, neuromuscular activities, brain neurotransmitters and oxidative stress enzymes in cadmium intoxicated mice. J. Behavior. Brain Sci.. 2013;3(01):74.
- [Google Scholar]
- Neuroprotective effect of ischemic preconditioning via modulating the expression of cerebral miRNAs against transient cerebral ischemia in diabetic rats. Neurol. Res.. 2016;38(11):1003.
- [Google Scholar]
- Anti-diarrhoeal activity of friedelin isolated from Azima tetracantha Lam. in Wistar rats.South Ind. J. Biol. Sci.. 2015;1:34-37.
- [Google Scholar]
- Green synthesis of Silver nanoparticles using aqueous extract of Taraxacum officinale and its antimicrobial activity. South Indian Journal Of Bioogical Sciences. 2015;2:115-118.
- [Google Scholar]
- Smilax chinensis Linn. (Liliaceae) root attenuates insulin resistance and ameliorate obesity in high diet induced obese rat. South Ind. J. Biol. Sci.. 2015;1:47-51.
- [Google Scholar]
- Testicular toxicity in mercuric chloride treated rats: Association with oxidative stress. Repro. Toxicol.. 2009;28:81-89.
- [Google Scholar]
- Quercetin prevents 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin-induced testicular damage in rats. Andrologia.. 2012;44(3):164-173.
- [Google Scholar]
- Protective effect of curcumin on immune system and body weight gain on rats intoxicated with 2, 3, 7, 8-Tetrachlorodibenzo-p-dioxin (TCDD) Immunopharmacol. Immunotoxicol.. 2010;32(1):99-104.
- [Google Scholar]
- Environmental friendly synthesis of TiO2-ZnO nanocomposite catalyst and silver nanomaterials for the enhanced production of biodiesel from Ulva lactuca seaweed and potential antimicrobial properties against the microbial pathogens. Journal of Photochemistry and Photobiology B: Biology.. 2019;193:118-130.
- [Google Scholar]
- Neurotoxicological effects of cinnabar (a Chinese mineral medicine, HgS)in mice. Toxicol. Appl. Pharmacol.. 2007;224:192-201.
- [Google Scholar]
- In vitro regeneration of a rare antidiabetic plant Epaltes divaricata L. South Ind. J. Biol. Sci.. 2015;1:52-59.
- [Google Scholar]
- Multiple biological activities of curcumin: a short review. Life Sci.. 2006;78(18):2081-2087.
- [Google Scholar]
- Curcumin, an antioxidant and anti-inflammatory agent, induces heme oxygenase-1and protects endothelial cells against oxidative stress. Free Radic. Biol. Med.. 2000;28:1303-1312.
- [Google Scholar]
- Parsons M.B., Percival J.W., eds. A brief history of mercury and its environmental impact. In: Mercury Sources, Measurements, Cycles and Effects. Mineralogical Association of Canada Short Course Series. Vol 34. 2005. p. :1-16.
- A novel mechanism for the modulation of luteinizing hormone receptor mRNA expression in the rat ovary. Mol. Cell Endocrinol.. 2005;233:65-72.
- [Google Scholar]
- Biochemical characterization and modulation of LH/CG-receptor during human trophoblast differentiation. J. Cell Physiol.. 2007;212:26-35.
- [Google Scholar]
- Kaviyarasu K. Synthesis of titanium oxide nanoparticles using Aloe barbadensis mill andevaluation of its antibiofilm potential against Pseudomonas aeruginosa PAO1. Journal of Photochemistry & Photobiology, B: Biology 2019
- [CrossRef] [Google Scholar]
- Hepatoprotective activity of ethanolic extract of Alysicarpus vaginalis against nitrobenzene-induced hepatic damage in rats. South Ind. J. Biol. Sci.. 2015;1:60-65.
- [Google Scholar]
- Cancer preventive role of selected dietary factors. Ind. J. Cancer.. 2005;42:11-20.
- [Google Scholar]
- Impairment of spermatogenesis in rats by methylmercury: involvement of stage- and cell-specific germ cell apoptosis. Toxicology.. 2001;169:25-35.
- [Google Scholar]
- Sokal, R.R., Rohlfe, F.J. 1981. Biometry: The principles and practice of statistics in biological research. W.H.Freeman, San Francisco. Starling, J.A. 1975.
- Effect of cadmium and selenium on the antioxidant defense system in rat kidneys. Comp. Biochem. Physiol.. 1997;117C:167-172.
- [Google Scholar]
- Rapid biosynthesis and characterization of silver nanoparticles from the leaf extract of Tropaeolum majus L. and its enhanced in-vitro antibacterial, antifungal, antioxidant and anticancer properties. Journal of Photochemistry & Photobiology, B: Biology. 2019;191:65-74.
- [Google Scholar]
- Valsalam S, Agastian P, Esmail. GA., Ghilan AKM, Al-Dhabi NA, Arasu MV. Biosynthesis of silver and gold nanoparticles using Musa acuminata colla flower and its pharmaceutical activity against bacteria and anticancer efficacy. Journal of Photochemistry & Photobiology, B: Biology: 2019b. doi.org/10.1016/j.jphotobiol.2019.111670
- WHO Protocol MB-50A. 1983. Method for examining the effect of a plant extract administered orally on the fertility of male rats. AP/PP 9914E.
- The distribution. In: Yamane T., ed. Statistics, and introductory analysis (3rd ed.). London: Harper and Row Publishers; 1973. p. :647-650.
- [Google Scholar]







