Translate this page into:
Curative effects of kaempferide on cadmium-instigated hepatotoxicity in male albino rats
⁎Corresponding author. asmaashraf@gcuf.edu.pk (Asma Ashraf)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Cadmium (Cd) is an environmental and industrial toxicant that possess the ability to cause severe health issues to humans and animals. The flavonol, kaempferide (KF), exhibits multiple pharmacological potentials. Therefore, this investigation was designed to estimate the mitigative effect of KF against Cd instigated hepatic damage in rats. 24 albino rats (male) were kept in 4 different groups. The group-1 was designed as untreated/control group, group-2 was orally provided with Cd (5 mg/kg), group-3 was orally co-administrated with Cd (5 mg/kg) along with KF (20 mg/kg) and group-4 was supplemented with KF (20 mg/kg) during 30 days of trial. Cd administration significantly escalated ALT, ALP as well as AST levels. A substantial decline in SOD, GST, POD, CAT, GSR activities and GSH content was observed due to Cd exposure along with an elevation in TBARS and H2O2 levels. Moreover, Cd administration elevated NF-kB, IL-6, TNF-α as well as IL-1β levels in addition to the activity of COX-2. Furthermore, the levels of Bax, Caspase-9 as well as Caspase-3 were escalated, besides the level of Bcl-2 was decreased after Cd exposure. A significant increase in histopathological damages after Cd administration is a clear indication of liver damage. However, co-treatment of KF with Cd potential recovered all the damages and significantly reduced the biochemical and histological variations in rat liver. Overall results indicated that KF has efficient anti-oxidant properties, which mitigated Cd instigated hepatic damage in rats.
Keywords
Cadmium
Flavonoid
Hepatotoxicity
Kaempferide
Antioxidant
1 Introduction
Increasing human activities significantly disturbed the universal cycle of metalloids and heavy metals i.e., cadmium (Cd) like toxic elements. Cd is among the major environmental and industrial toxicants (Kadry and Megeed, 2018). Its prevalent existence in natural environment and its application in daily life activities (Ma et al., 2019) cause inevitable exposure to humans (Alshatwi et al., 2014). It is profoundly present in products of polyvinyl chloride, tires, photo-cells, heating elements, electrical conductors and automobile radiators (Mead, 2010). According to El-Maraghy and Nassar (2011) cigarette smoking is one of the main source of Cd exposure. Furthermore, the use of Cd containing phosphate fertilizers may elevate Cd level in crops and natural environment (Newbigging et al., 2015).
Cd can potentially accumulate in different body organs particularly in liver (Rahimzadeh et al., 2017). Cd induces functional damages in several organs like lung, pancreas, testes, kidneys, heart and liver at cellular level (Cuypers et al., 2010). Exposure to Cd may cause different hepatic variations (Othman et al., 2014). At lower doses, Cd induces several pathological alterations in hepatic tissues. Several investigations revealed that Cd can change anti-oxidant status, which leads to oxidative damage (El-Habit and Abdel Moneim, 2014). The mechanism through which Cd damages the tissues is the reactive oxygen species (ROS) generation, which react with cellular biomolecules and causes DNA damage, lipid peroxidation, membrane protein injury, apoptosis and reduced anti-oxidant status (Stohs et al., 2000).
Growing evidences suggest that the use of phytochemicals to treat various ailments is getting traction (Flora et al., 2007). An O-methylated flavonol, kaempferide (KF) is a naturally occurring flavone, which is reported in Kaempferia galangal (Jiao et al., 2017). It shows anti-carcinogenic, anti-inflammatory, anti-oxidant (Zhang et al., 2013), anti-bacterial (Eumkeb et al., 2012) and neuroprotective potentials (Wang et al., 2016). Nevertheless, there is no literature available discussing the therapeutic role of KF against liver damage. By keeping these attributes in mind, this research was designed to estimate the mitigative role of KF on Cd instigated hepatic damage in rats.
2 Materials and methods
2.1 Chemicals
Cd was bought from Sigma Aldrich (Germany) and KF was bought from Merck (Germany).
2.2 Animals
Twenty-four male albino rats having weight 180 ± 20 g (6–8 weeks old) were housed in the animal house at the University of Agriculture, Faisalabad (UAF). The animals were placed under controlled conditions at 25 ± 2 °C for 12 hrs. light/dark periods. Throughout the experimental duration, the rats were given tap water and standard diet. Rats were acclimatized for 7 days before the start of the experiment. Rats were treated and handled in compliance with the instructions of the European union of animal care and experimentation (CEE Council 86/ 609).
2.3 Experimental layout
The animals were allocated into 4 equal groups and housed in separate cages. Animals of group-1 (control) were provided with tap water. Group-2 was intoxicated with Cd (5 mg/kg). Group-3 was treated with Cd (5 mgkg−1) and KF (20 mgkg−1) orally for about 30 days. Group-4 orally received the dose of KF (20 mgkg−1). After 30 days, the animals were made unconscious and beheaded. Blood was gathered in heparinized tubes for further assessment. The liver was excised and divided into 2 equal parts, one half was stored in zipper bags for biochemical observation at −80 °C. The homogenization of hepatic tissues was performed with the chilled phosphate buffer saline (25 mM; pH: 7.4) at 11,000 g for 20 min by using homogenizer. Laterally, the homogenate was used to estimate anti-oxidative enzymes & inflammatory markers. The second half was preserved in 10 percent formalin for histological evaluation.
2.4 Biochemical evaluation
CAT activity was evaluated calorimetrically by means of dichromate-acetate acid reagent (Sinha (1972). The activity of POD was measured through a standard technique exemplified by Chance and Maehly (1955). The action of SOD was determined using the technique of kakkar et al. (1984). GST activity was computed by the technique of Habig et al., 1974. The evaluation of GSH content was appraised via the protocol of Jollow et al. (1974). GSR content was determined through the procedure of Carlberg and Mannervik (1975). The technique of Jiang et al. (1992) was used to evaluate the TBARS level·H2O2 level was appraised via the procedure of Pick and Keisari (1981).
2.5 Hepato-serum markers analysis
The levels of ALP, AST and ALT were analyzed by using the commercially available kits, bought from Wiesbaden (Germany).
2.6 Inflammatory biomarkers analysis
Inflammatory markers (IL-1β, TNF-α, IL-6, NF-κB levels and COX-2 activity) were estimated with ELISA kits (Cusabio Technology Llc, USA) in accordance with the company’s instructions.
2.7 Apoptotic markers assessment
The levels of apoptotic markers i.e., Caspase-9, Bax, Bcl-2 along with Caspase-3 were estimated by ELISA kits (Cusabio Technology Llc, USA) as directed by the manufacturer.
2.8 Histopathological observations
Fukuzawa et al. (1996) technique was used for histopathological examination of liver. In first step, samples were rinsed for 24 h in 10% formalin for the fixation of the tissue samples and then passed through different grades of alcohol to dehydrate, cleaned with xylene and embedded in paraffin wax. 5 µm thin sections were sliced by using microtome and then stained with Hematoxylin & Eosin stain. Lastly, the slides were examined using light microscope (Nikon Labophot, Japan) with attached camera (Canon-EOS 200D).
2.9 Statistical analysis
Data were presented as Mean ± SEM. Values were estimated through the application of one-way ANOVA and comparative measurements between treatments were estimated by applying Tukey’s test. Data estimation was performed using Graph Pad Prism software. Statistics were considered significant at p < 0.05.
3 Results
3.1 Curative effect of KF on anti-oxidant enzymes
Cd administrated rats demonstrated a notable (p < 0.05) reduction in anti-oxidants i.e., CAT, POD, SOD, GST, GSR activities & GSH content when matched to the control rats. However, the administration of Cd + KF notably improved anti-oxidant enzymes activities & GSH content in contrast to Cd intoxicated rats. Furthermore, in KF only supplemented group the activity of anti-oxidant enzymes was comparable to untreated rats (Table 1). Values exhibited unlike letters are notably different from other groups.
Groups
CAT (U/mg protein)
POD (U/mg protein)
SOD (U/mg protein)
GST (nM/min/mg protein)
GSH (μM/g tissue)
GSR (nM NADPH
oxidized/min/mg tissue)
Control
8.98 ± 0.11a
7.11 ± 0.05a
5.60 ± 0.12a
24.71 ± 0.76a
15.30 ± 0.73a
3.85 ± 0.08a
Cd (5 mg/kg)
4.69 ± 0.09b
3.59 ± 0.05b
3.11 ± 0.06b
10.77 ± 0.67b
8.76 ± 0.38b
1.49 ± 0.08b
Cd (5 mg/kg) + KF (20 mg/kg)
8.01 ± 0.09a
6.80 ± 0.08a
5.22 ± 0.08a
20.20 ± 0.58ac
14.55 ± 0.60c
3.18 ± 0.09a
KF (20 mg/kg)
8.71 ± 0.13a
7.10 ± 0.07a
5.41 ± 0.18a
22.85 ± 0.91c
15.51 ± 0.76a
3.48 ± 0.15a
3.2 Curative effect of KF on hepatic serum markers
Liver serum markers were assessed to determine the state of live damage. A remarkable (p < 0.05) escalation was detected in AST, ALP & ALT levels in Cd exposed rats when matched to untreated rats. But the level of these markers was notably lowered in Cd + KF co-administrated rats in comparison to Cd exposed rats. Furthermore, hepatic serum markers in KF only treated group did not differ significantly than control rats (Table 2). Values exhibited unlike letters are notably different from other groups.
Groups
ALT(U/L)
AST (U/L)
ALP (U/L)
Control
40.26 ± 1.17a
56.66 ± 2.72a
73.25 ± 1.13a
Cd (5 mg/kg)
179.9 ± 6.07b
199.4 ± 7.35b
135.6 ± 2.93b
Cd (5 mg/kg) + KF (20 mg/kg)
78.69 ± 4.24c
63.77 ± 2.02a
86.24 ± 2.00a
KF (20 mg/kg)
45.57 ± 2.63a
61.21 ± 3.37a
75.93 ± 2.30a
3.3 Curative effect of KF on oxidative stress markers
TBARS as well as H2O2 levels were noticeably (p < 0.05) increased in Cd exposed rats in contrast to control. Nevertheless, a notable reduction in H2O2 and TBARS levels was observed in Cd + KF co-treated rats relative to Cd exposed rats. Moreover, the treatment of only KF displayed these levels comparable to control rats (Table 3). Values exhibited unlike letters are notably different from other groups.
Groups
TBARS (nm TBARS/min/mg tissue)
H2O2 (nM/min/mg protein)
Control
14.55 ± 0.46a
1.55 ± 0.05a
Cd (5 mg/kg)
22.46 ± 0.72b
5.03 ± 0.09b
Cd (5 mg/kg) + KF (20 mg/kg)
14.90 ± 0.12a
1.99 ± 0.06a
KF (20 mg/kg)
13.46 ± 0.37c
1.83 ± 0.05a
3.4 Curative effect of KF on inflammatory biomarkers
After Cd exposure a notable (p < 0.05) increase in inflammatory indices (IL-1β, TNF-α, IL-6 and NF-κB levels in addition to COX-2 activity) was noticed relative to control rats. Conversely, Cd and KF co-administration remarkably brought down the levels of these biomarkers in contrast to Cd exposed rats. Besides, in only KF treated group inflammatory marker levels were near to control rats (Table 4). Values exhibited unlike letters are notably different from other groups.
Groups
NF-κB (ng/g tissue)
TNF-α (ng/g tissue)
IL-1β (ng/g tissue)
IL-6 (ng/g tissue)
COX-2 (ng/g tissue)
Control
13.37 ± 0.67c
7.52 ± 0.31c
21.09 ± 1.37c
5.19 ± 0.55c
24.39 ± 0.54c
Cd (5 mg/kg)
62.57 ± 0.95a
16.60 ± 1.68a
85.44 ± 1.18a
24.18 ± 1.60a
65.82 ± 1.58a
Cd (5 mg/kg) + KF (20 mg/kg)
27.59 ± 1.23b
12.24 ± 0.41b
33.76 ± 1.59b
12.17 ± 0.56b
34.49 ± 1.22b
KF (20 mg/kg)
13.19 ± 0.58c
7.42 ± 0.31c
21.05 ± 1.37c
5.11 ± 0.59c
24.05 ± 0.59c
3.5 Curative effect of KF on apoptotic markers
A substantial (p < 0.05) escalation in the Bax, Caspase-9 in addition to Caspase-3 levels was noticed following the Cd exposure, whereas the level of Bcl-2 was lowered relative to control rats. Nevertheless, Bax, Caspase-9 along with Caspase-3 levels were considerably reduced and the level of Bcl-2 was escalated in Cd + KF co-treated rats relative to Cd treated rats. Furthermore, the levels of these markers in only KF treated rats were comparable to control rats (Table 5). Values exhibited unlike letters are notably different from other groups.
Groups
Bax (pg/mL)
Bcl-2 (ng/mL)
Caspase-3 (pg/mL)
Caspase-9 (pg/mL)
Control
1.69 ± 0.88c
17.43 ± 0.65 a
1.39 ± 0.08c
2.83 ± 0.14c
Cd (5 mg/kg)
9.48 ± 0.26a
6.11 ± 1.16b
13.43 ± 0.63a
14.14 ± 1.09a
Cd (5 mg/kg) + KF (20 mg/kg)
3.92 ± 0.11b
14.84 ± 0.75a
2.98 ± 0.08b
5.44 ± 0.16b
KF (20 mg/kg)
1.67 ± 0.08c
17.51 ± 0.69a
1.36 ± 0.08c
2.78 ± 0.14c
3.6 Curative effect of KF on histopathology
Normal structural pattern including central veins and standard sinusoids of liver was observed in control rats. The administration of Cd resulted in deterioration, increased fat deposition and dilated sinusoid. Nevertheless, KF supplementation markedly alleviated these severe histological abnormalities and decreased the sinusoids dilation as well as necrotic cells. The morphological studies revealed that the administration of KF restored all the structural injuries in rat liver. Moreover, the histopathological profile of only KF treated rats was same as in the control rats (Fig. 1).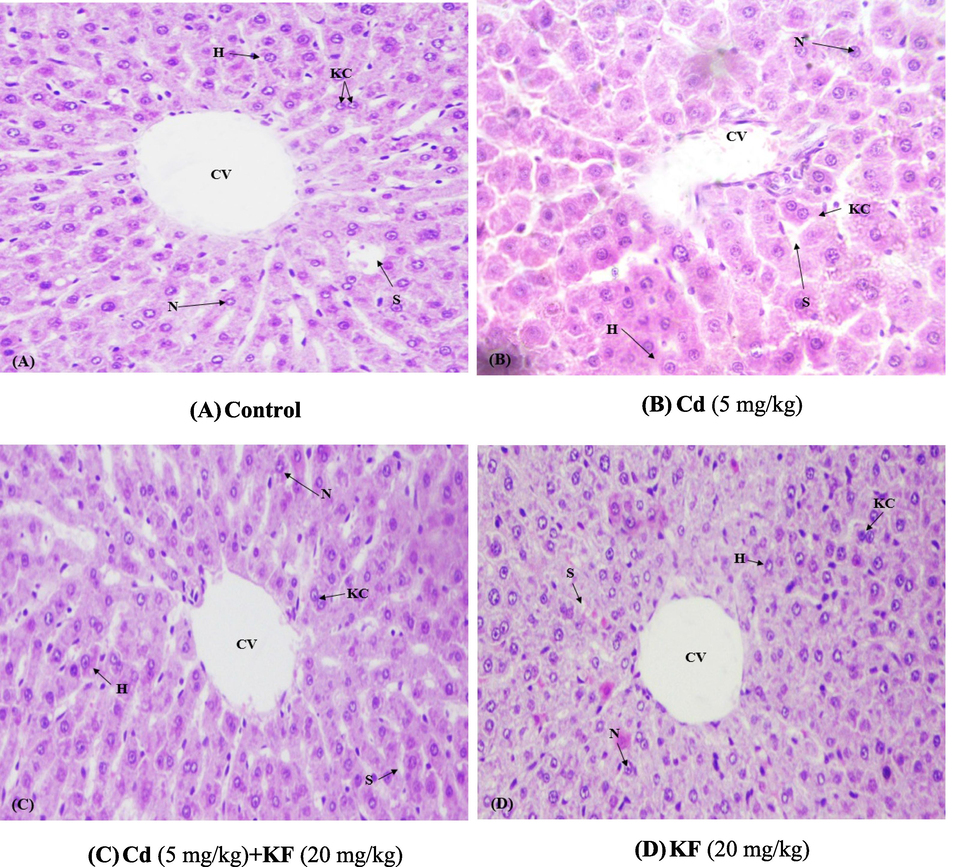
Protective role of KF on histopathology of liver (400X /H&E). (A) Control group (B) Cd administered group (5 mg/kg). (C) Cd + KF co-treated group (D) KF administrated group (20 mg/kg).
4 Discussion
Cd is a toxic heavy metal, which is broadly reported in the environment. Major sources of Cd distribution are industrial and agricultural fertilizers. Cd accumulation in the tissues for longer time period may cause oxidative stress, which leads to certain pathological conditions such as severe liver impairments (Bagchi et al., 2000). Cd induced toxicity may be attenuated via anti-oxidant supplementation (Karbownik et al., 2001). KF, a flavonoid derived from plants, displays promising pharmacological properties, making it useful for nutraceutical and medicinal applications (Qiu et al., 2022). KF is reported to hold the anti-inflammatory, antiviral in addition to anti-oxidant potentials (Jiao et al., 2017).
Antioxidants have abilities to protect the cellular mechanisms from oxidative injuries (El-Demerdash et al., 2004; Ishtiaq et al., 2022). In Cd treated rats, SOD, CAT, POD, GST as well as GSR activities & GSH content were substantially lowered due to accumulation as well as production of free radicals i.e., hydroxyl and superoxide anions (Mira et al., 2002). CAT as well as SOD are considered as the vital components of anti-oxidant defense mechanism and they are crucial for eliminating oxidative stress. Cd is responsible for direct inhibition of CAT and SOD activities due to the direct interaction with these enzymes, which lead to discomposure of enzymes (Obioha et al., 2009). Previous investigation revealed that Cd exposure imbalances the defense system by lowering the level of anti-oxidant enzymes i.e., CAT, SOD & GSH content (Seif et al., 2019). However, co-treated (Cd + KF) rats displayed improvement in anti-oxidants enzymatic activity & GSH content, which shows its potential to reduce oxidative stress by eliminating the free radicals. The levels of TBARS as well as H2O2 were remarkably escalated in Cd intoxicated rats. The exposure to Cd elevated these markers, which represents the abnormalities and damages in liver tissues. The increased TBARS level is due to the raised lipid-peroxidation, which is consecutively linked with the decreased GSH content (Zhao et al., 2014). However, concurrent treatment of Cd + KF reduced TBARS and H2O2 levels owing to its anti-oxidant property.
Cd exposure prompted a significant escalation in ALP, AST along with ALT serum levels. Previous studies showed that the Cd exposure causes liver injuries, which ultimately increases the leakage of function markers into blood stream that resulted in a remarkable elevation of ALP, AST and ALT in serum or plasma (Ijaz et al., 2023). However, the supplementation of KF ameliorated the adverse effects of Cd by decreasing the tissue injuries, which ultimately resulted in decreased level of ALP, AST and ALT in plasma, that shows hepatoprotective nature of KF.
Our results confirmed that the levels of inflammatory indices were escalated in the liver of rats exposed to Cd. Due to oxidative stress, NF-κB gets activated and translocates into the nucleus, triggering the release of inflammatory indices i.e., TNF-α, IL-6, IL-1β & COX-2 activity (Wang et al., 2018). Because of its ability to regulate multiple phases of inflammatory response simultaneously, NF-κB possess significant role in inflammation. It is reported that NF-κB also activates COX-2, which results in inflammation as well as tissue damage (Ijaz et al., 2021; Lee et al., 2004). Nevertheless, the supplementation of KF notably reduced inflammatory indices, proving that it might act as an anti-inflammatory agent.
In the current study apoptosis was estimated by measuring Bax, Caspase-3, Bcl-2 & Caspase-9 levels. The results revealed that Cd treatment decreased Bcl-2 level, but increased Bax, Caspase-9 and Caspase-3 levels. Bcl-2 plays a crucial role in inhibiting apoptosis (Shaikh et al., 2015). In contrast, Bax is primarily responsible for promoting apoptosis and combined with Bcl-2 it controls apoptosis in cells (Alvi et al., 2022; Feng et al., 2016). An augmentation in Bax level and a reduction in Bcl-2 level causes cytochrome C to be evicted from mitochondrial membrane into the cytoplasm and stimulates Caspase-9 (V'egran et al., 2011). Then Caspase-9 triggers Caspase-3, which leads to apoptosis (Cain et al., 2002). By inhibiting Caspase-3 activation, apoptosis can be prevented, as it is the key molecule in apoptosis (Grippa et al., 2015). However, KF alleviated apoptosis in hepatocytes of rats by lowering and elevating apoptotic & anti-apoptotic markers, respectively. It is evident from our study that KF exhibits anti-apoptotic properties against Cd induced toxicity in rat’s hepatocytes.
The histopathological analysis revealed that Cd caused hepatic injuries, which is additionally confirmed by the level of serum markers of liver. In liver tissues, Cd exposure increased the lipid peroxidation that resulted in morphological changes. Various hepatic injuries including formation of large biomass, disruption of hepatic veins, obstruction and clotting, swelling of supportive as well as connective tissues, inflammatory cell infiltration, nucleus aggregation and necrosis were reported in Cd treated groups (Al-Harbi et al., 2014). However, these serious injuries were mitigated by KF administration. This observation revealed that KF might also have anti-oxidant properties, because of which lipid peroxidation decreases and ultimately resulting in reduced tissue damage.
5 Conclusion
Present findings showed that the exposure of Cd induce oxidative damage, elevation of hepatic serum markers level, apoptotic markers and inflammatory markers along with decreased anti-oxidant enzymes activity. Moreover, histopathological damages were detected in Cd intoxicated rats. The ameliorative effects of KF displayed terrific curative property against oxidative damage. KF administration recovered the levels of hepatic serum markers, oxidative stress markers, apoptotic markers in addition to inflammatory indices and decreased hepatic histopathological anomalies. This hepatoprotective effect of KF may be attributed to its anti-oxidant as well as ROS scavenging nature.
Acknowledgment
This work was funded by Researchers Supporting Project number (RSP2023R26), King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Ameliorative effect of selenium and curcumin on sodium fluoride-induced hepatotoxicity and oxidative stress in male mice. J. Chem. Pharm. Res.. 2014;6:984-998.
- [CrossRef] [Google Scholar]
- Delineating the anti-cytotoxic and anti-genotoxic potentials of catechin hydrate against cadmium toxicity in human peripheral blood lymphocytes. Environ. Toxicol. Pharmacol.. 2014;38:653-662.
- [CrossRef] [Google Scholar]
- Nephroprotective Effects of Delphinidin against Bisphenol A Induced Kidney Damage in Rats. Pak. Vet. J.. 2022;43(1):189-193.
- [CrossRef] [Google Scholar]
- Free radicals and grape seed proanthocyanidin extract: importance in human health and disease preven-tion. Toxicology. 2000;148:187-197.
- [CrossRef] [Google Scholar]
- The Apaf-1 apoptosome: A large caspaseactivating complex. Biochimie. 2002;84:203-214.
- [CrossRef] [Google Scholar]
- Purification and characterization of the flavoenzyme glutathione reductase from rat liver. J. Biol. Chem.. 1975;250:5475-5480.
- [CrossRef] [Google Scholar]
- Cadmium-induced changes in lipid peroxidation, blood hematology, biochemical parameters and semen quality of male rats: protective role of vitamin E and beta-carotene. Food Chem. Toxicol.. 2004;42:1563-1571.
- [CrossRef] [Google Scholar]
- Testing the genotoxicity, cytotoxicity, and oxidative stress of cadmium and nickel and their additive effect in male mice. Biol. Trace Elem. Res.. 2014;159:364-372.
- [CrossRef] [Google Scholar]
- Modulatory effects of lipoic acid and selenium against cadmium-induced biochemical alterations in testicular steroidogenesis. J. Biochem. Mol. Toxicol.. 2011;25:15-25.
- [CrossRef] [Google Scholar]
- Synergistic activity and mode of action of flavonoids isolated from smaller galangal and amoxicillin combinations against amoxicillin-resistant Escherichia coli. J. Appl. Microbiol.. 2012;112:55-64.
- [CrossRef] [Google Scholar]
- Chlorogenic acid protects D-galactose-induced liver and kidney injury via antioxidation and anti-inflammation effects in mice. Pharm. Biol.. 2016;54:1027-1034.
- [CrossRef] [Google Scholar]
- Essential metal status, prooxidant/antioxidant effects of MiADMSA in male rats: age-related effects. Biol. Trace Elem. Res.. 2007;120:235-247.
- [CrossRef] [Google Scholar]
- Evaluation of glomerular lesion and abnormal urinary findings in OLETF rats resulting from a long-term diabetic state. J. Lab. Clin. Med.. 1996;128:568-578.
- [CrossRef] [Google Scholar]
- The seipin complex Fld1/Ldb16 stabilizes ER–lipid droplet contact sites. J. Cell Biol.. 2015;211:829-844.
- [CrossRef] [Google Scholar]
- The first enzymatic step in mercapturic acid formation. J. Biol. Chem.. 1974;249:7130-7139.
- [CrossRef] [Google Scholar]
- Orientin Attenuates Cisplatin-Induced Renal Toxicity by Reducing Oxidative Stress and Inflammation. Pak. Vet. J.. 2021;41(4):574-578.
- [CrossRef] [Google Scholar]
- Tectochrysin Attenuates Cisplatin-induced Hepatotoxicity by Restoring Biochemical, Inflammatory and Histological Profile in Rats. Pak. Vet. J. 43 (2): 366–370. 2023
- [CrossRef] [Google Scholar]
- Therapeutic Effect of Oroxylin A Against Bisphenol A-induced Kidney Damage in Rats: a Histological and Biochemical Study. Pak. Vet. J.. 2022;42(4):511-516.
- [CrossRef] [Google Scholar]
- Ferrous ion oxidation in the presence of xyleneol orange for detection of lipid peroxidation in low density lipoprotein. Anal. Biochem.. 1992;202:384-389.
- [CrossRef] [Google Scholar]
- Kaempferide prevents titanium particle induced osteolysis by suppressing JNK activation during osteoclast formation. Sci. Rep.. 2017;7:1-12.
- [CrossRef] [Google Scholar]
- Bromobenzene-induced liver necrosis: protective role of glutathione and evidence for 3,4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology. 1974;11:151-169.
- [CrossRef] [Google Scholar]
- Probiotics as a complementary therapy in the model of cadmium chloride toxicity: crosstalk of β-catenin, BDNF, and StAR signaling pathways. Bio. Trace Elem. Res.. 2018;185:404-413.
- [CrossRef] [Google Scholar]
- A modified spectrophotometric assay of superoxide dismutase. Indian J. Biochem. Biophys.. 1984;21:130-132.
- [Google Scholar]
- Induction of lipid peroxidation in hamster organs by the carcinogen cadmium: amelioration by melatonin. Cell Biol. Toxicol.. 2001;17:33-40.
- [CrossRef] [Google Scholar]
- Spinal NF-kB activation induces COX-2 upregulation and contributes to inflammatory pain hypersensitivity. Eur. J. Pharmacol.. 2004;19:3375-3381.
- [CrossRef] [Google Scholar]
- Effects of environmental contaminants on fertility and reproductive health. J. Environ. Sci.. 2019;77:210-217.
- [CrossRef] [Google Scholar]
- Cadmium confusion do consumers need protection. Environ. Health Perspect.. 2010;118:528-534.
- [CrossRef] [Google Scholar]
- Interaction of flavonoids with iron and copper ions, a mechanism for their antioxidant activity. Free Radic. Res.. 2002;36:1199-1208.
- [CrossRef] [Google Scholar]
- Cadmium in soybeans and the relevance to human exposure. J. Environ. Sci. (China). 2015;37:157-162.
- [Google Scholar]
- Hepatoprotective potentials of onion and garlic extracts on cadmium-induced oxidative damage in rats. Biol. Trace Elem. Res.. 2009;129:143-156.
- [CrossRef] [Google Scholar]
- Effect of Physalis peruviana L. on cadmium-induced testicular toxicity in rats. Biol. Trace Elem. Res.. 2014;159:278-287.
- [CrossRef] [Google Scholar]
- Superoxide anion and hydrogen peroxide production by chemically elicited peritoneal macrophages-induction by multiple nonphagocytic stimuli. CellImmunol.. 1981;59:301-318.
- [CrossRef] [Google Scholar]
- Metabolic division in an Escherichia coli coculture system for efficient production of kaempferide. ACS Synth. Biol.. 2022;11:1213-1227.
- [CrossRef] [Google Scholar]
- Cadmium toxicity and treatment: an update. Caspian. J. Intern. Med.. 2017;8:135-145.
- [CrossRef] [Google Scholar]
- Hepato-renal protective Effects of Egyptian purslane extract against experimental cadmium toxicity in rats with special emphasis on the functional and histopathological changes. Toxicol. Rep.. 2019;6:625-631.
- [CrossRef] [Google Scholar]
- Alteration in testicular morphology and sperm count due to Glycowithanolides treatment during aging. Ajpcr. 2015;8:72-77.
- [Google Scholar]
- Oxidative mechanisms in the toxicity of chromium and cadmium ions. J. Environ. Pathol. Toxicol. Oncol.. 2000;19:201-213.
- [CrossRef] [Google Scholar]
- A short caspase-3 isoform inhibits chemotherapy-induced apoptosis by blocking apoptosome assembly. PLoS One. 2011;6:e29058.
- [Google Scholar]
- Alleviation of cadmium-induced oxidative stress by trehalose via inhibiting the Nrf2-Keap1 signaling pathway in primary rat proximal tubular cells. J. Biochem. Mol. Toxicol.. 2018;32:e22011.
- [Google Scholar]
- Neuroprotective effects of Kaempferide-7-O-(4 ″-O-acetylrhamnosyl)-3-O-rutinoside on cerebral ischemia-reperfusion injury in rats. Eur. J. Pharmacol.. 2016;788:335-342.
- [CrossRef] [Google Scholar]
- Protective effect of flavonoid-rich extract from Rosa laevigata Michx on cerebral ischemia–reperfusion injury through suppression of apoptosis and inflammation. Neurochem. Int.. 2013;63:522-532.
- [CrossRef] [Google Scholar]
- Grape seed proanthocyanidin extract prevents DDP-induced testicular toxicity in rats. FoodFunct.. 2014;5:605-611.
- [Google Scholar]







