CuO/NiO bimetallic nanocomposite: A facile DNA assisted synthetic approach and evaluation of bio efficacy
⁎Corresponding author. sonagkrishnan@sncwkollam.org (Sona G. Krishnan)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
In this present work, a simple chemical co-precipitation method was used to synthesize deoxyribonucleic acid (DNA) capped CuO-NiO bimetallic nanocomposite. The morphology and structure related properties of the samples were analyzed using Energy Dispersive X-Ray Analysis, X-Ray Diffraction, Transmission Electron Microscopy and Scanning Electron Microscopy. Widely used Agar well diffusion method evaluated the antimicrobial activity of the sample. DPPH and FRAP assays were applied to test the antioxidant activity of the sample. Average crystallite size of 16.218 nm NiO and 15.871 nm CuO were confirmed within CuO- NiO bimetallic oxide nanoparticles by means of XRD technique. EDAX studies confirmed the purity of the sample. The grain size obtained from TEM studies matches with the XRD results. The free radical scavenging activity of sample was higher in lower concentrations, viz. 0.1, 1, 5 and 10 µg/ml of the sample and activity decreased after that. The IC50 value in FRAP assay shows the potential of this mixed metal oxide nanoparticle in radical scavenging. In agar well cut method, the sample showed moderate antibacterial activity against Mycobacterium smegmatis and Salmonella typhimurium. The results also showed that the CuO-NiO bimetallic nanoparticles are biologically compatible, environmentally benign and economical material having potential applications in biomedical industry.
Keywords
Nanocomposites
Capping agents
DNA
Antimicrobial
Antioxidant
- DNA
-
Deoxyribinucleic acid
- DMSO
-
Dimethyl sulphoxide
- XRD
-
X-ray diffraction
- SEM
-
Scanning Electron Microscopy
- TEM
-
Transmission Electron Microscopy
- EDX
-
Energy-dispersive X-ray
- DPPH-1
-
1-diphenyl-2-picryl-hydrazyl
- FRAP
-
Ferric Reducing Antioxidant Power
Abbreviations
1 Introduction
Nanomaterials has always captivated scientific interest over the decades due of its distinctive size dependent physical and chemical properties compared to its bulk counterparts. Increased surface area to volume ratio and quantum size effects in the nano regime (Qin and Szpunar, 2005) play crucial roles in their varied properties leading to a wide range of applications in the diverse fields of catalysis, drug delivery, biosensors, electronics and optoelectronics (Sakib et al.,2019; El-Kemary et al.,2013). In recent years, metallic nanoparticles have opened a wide horizon in the field of biomedical research because of its capability to permeate cell, excellent stability in solutions, low cytotoxicity, electron transfer reactions at lower over potentials (Mody et al.,2010). Reports on the applicability of metal oxide nano particles in the areas of drugs and medications, electronics, energy harvesting (Salata, 2004; Khan et al., 2015; Khan et al., 2019), in-vivo and vitro sensing and targeting studies (Verma and Kumar, 2019) and its biological and caltalytic activities (Rabiee et al., 2020) have elicited researchers to probe further in this area. Bimetallic nanocomposites comprising of two different metals or metal oxides often exhibits stable structures, enhanced properties and offers major biological applications to enrich the environment and human health (Lingaraju et al.,2020; Chaudhary et al.,2019). The electrical and magnetic properties of CuO-MgO nanocomposites studies were reported by Kaviyarasu, et al., 2015 while, Tamizh Selvi et al. (2021) presented the enhancements in dielectric and impedance properties. Another study carried out by Sakib et al., 2019 on various proportions of CuO-ZnO nanocomposites for the photodegradation of textile dye using mechanochemical combustion method exhibited increased photocatalytic activities compared to ZnO metal oxide. CuO and NiO are the two extensively studied metal oxide nano particles because of its varying properties in the nano regime (Sana et al.,2021; Bonomo, 2018). The optical band gaps of Nickel Oxide and Copper Oxide nanoparticles (Nilima and Hande, 2011) are reported as 3.6–4 eV and 1.2 eV respectively. The space group Fm-3 m is associated with cubic NiO and space group C1c1 is associated with monoclinic CuO (Gajendiran et al.,2016). In order to associate this nanocomposite for various application, care must be given to reduce agglomeration and to ensure the uniform arrangement of particles. This can be done by choosing proper synthesis technique. Co-precipitation method is one of the most simple and cost-effective way of preparing metal oxide nanoparticles. It usually involves precipitation of nano particles in hydroxide form from a salt precursor with the help of a base in a solvent followed by filtration, washing and calcination to convert hydroxide into oxides (Cruz et al.,2018; Pereira et al.,2012). Further, Capping agents plays a crucial role in reducing the agglomeration of nanoparticles caused by its high surface energy. It has also been reported that the use of biocompatible capping agents can alter the surface chemistry and size distribution of nanoparticles (Javed et al., 2016; Aisida et al., 2020). To enhance the biomedical functionality of the synthesized nanoparticles in living systems and to alleviate cellular toxicity often bio-degradable, bio-soluble and non-toxic capping agents are preferred. Even though different types of biological capping agents like plant extracts, fruit extracts etc. are used (Jain and Mehata, 2017; Singh et al., 2018), the application of well-known biomaterial Deoxyribonucleic acid (DNA), which transport the genetic code in all living organic structures, as a capping agent is hardly explored. DNA acts as a good bio template to grow inorganic quantum confined structures because of its physicochemical stability and unique structure. The Double helix structure of DNA prevents the aggregation of the nanostructures is reported to be more efficient in controlling nanoparticle size (Nithyaja et al., 2012). Recently, it is reported that DNA molecules can be used as a data storage medium (Liu et al.,2008; Sharmila and Nisha, 2014). Moreover, studies reveal that stearic effects of capping agents plays an inevitable part in inhibiting the over-growth and aggregation of nano particles (Lu et al., 2008, Javed et al., 2020).
Several studies on the enhanced optical, magnetic and physical properties of CuO-NiO nanocomposites were reported but not much reports are available on their biological activities in detail (Kumar et al., 2020; Abo Zeid et al., 2020). Recently, (Ramu et al., 2021) have used CuO-NiO nanoparticles for removing nitro compounds from aqueous medium. However, there are no previous research work reported on the synthesis and characterization of CuO-NiO nanocomposites using DNA-aided chemical co-precipitation method. Keeping this on mind, a study has been designed to synthesize, characterize and evaluate the biological activities of the synthesized DNA capped CuO-NiO nanocomposite.
2 Materials and methods
2.1 CuO-NiO nanocomposite preparation
Copper Cholride, Nickel(II)Chloride hexahydrate, Sodium Hydroxide were the precursors used for the synthesis and all are purchased from Merck. The Fish sperm DNA powder was purchased from Sigma-Aldrich and has a minimalist structure called “axoneme”. To prepare CuO-NiO nanocomposites via chemical co-precipitation method, initially equal molarities of NiCl2·6H2Oand CuCl2·2H2O and 1 M NaOH were separately dissolved in 50 ml of deionized water. Under constant stirring these three solutions were added dropwise to the DNA solution. 0.3722 g of fish sperm DNA was used as capping agent. After addition, Cu(OH)2 and Ni(OH)2 were precipitated. The aggregation of nanoparticles by Ostwald ripening and agglomeration is the major problem faced during the synthesis of nanoparticles (Cao, 2004). Capping agents can reduce this type of aggregation. The steric effect of the double-helix structure of DNA was considered as the restricting agent for the further growth of nanostructures. A mesh-like network is formed in the solution due to the long polynucleotide chain of the DNA molecules. The nanoparticles cannot grow because of the limited space in the network (Nithyaja et al.,2012). The solution mixture was then stirred for 5 h. The filtered precipitate was then washed many times using deionized water. Filtrate thus obtained was then dried at 120 0C for 2 h in an oven. TG-DSC studies mentioned in literatures shows that Nickel Hydroxide decomposes to Nickel Oxide at temperature above 300 °C (Kuang et al., ,2009) while Cu(OH)2 decomposes to its oxide form at temperature below 250 °C (Tamaekong et al., 2014; Juma et al., 2017). Finally, the annealing temperature chosen for this study was taken as 500 °C.The obtained powder was calcined at 500 °C for 3 h. The synthesized nanocomposite was black in color.
2.2 Characterization techniques
The crystallinity of synthesized CuO-NiO nanocomposite was determined with the help of a Bruker D8 Advance diffractometer with CuKα radiation of wavelength 1.5406Ao in the range of 20° to 80owith 0.02° step size. Jeol/JEM 2100 instrument with electron beam accelerating voltage 200 kV was used for HRTEM studies. Jeol 6390LA/ OXFORD XMX N instrument working in an accelerating voltage of 0.5 to 30 kV connected to a detector for EDX analysis to determine the surface morphology and composition of the samples. The free radical scavenging property of DNA capped CuO-NiO nanocomposite(CND) was recorded using an UV–Visible spectrophotometer (UV1900, Shimadzu, USA).
2.3 Antioxidant activity assays
2.3.1 DPPH assay
DPPH molecule was used to check the free radical scavenging property of CND as per the previously reported method (Krishna et al.,2015). DPPH stock solutions were prepared in methanol at a concentration of 0.1 mM in methanol. Test Samples were prepared at varying concentrations ranging from 0.1, 1, 5, 10, 25 and 50 µg/µl in methanol for assay. 1 ml of test material was mixed well with equal volume of DPPH and allowed to incubate at room temperature for 30 min in dark. UV–Visible spectrophotometer recorded the absorbance value at 570 nm. Ascorbic acid at varying concentration was used as standard. All readings were recorded in triplicate and blank corrected average value was used to plot the activity curve against concentration. The formula that applied to compute the percentage of inhibition was (Ghasemzadeh et al., 2016).
2.3.2 FRAP assay
FRAP assay was also used to check the antioxidant potential of CND using the protocol reported previously (Benzie and Strain, 1996; Nilima and Hande, 2011). Assay measures the antioxidant activity of the sample by reducing the colourless complex Fe3+-TPTZ to a blue coloured Fe2+-TPTZ under the control of electron producing antioxidants. The change in complex formation is monitored by the colour variation and is measured as absorbance at 593 nm. The reduction process was measured and blank correction was made against reagent blank. Reagent blank was prepared by dissolving 3.995 ml FRAP reagent with 5 µl distilled water. The value, extrapolated as percentage of activity in radical scavenging, was plotted against concentration.
2.4 Antibacterial assay by agar well diffusion method
The microbial strains used in the study were obtained from Microbial Type Culture Collection (MTCC) Chandigarh and included Mycobacterium smegmatis (MTCC No. 6) and Salmonella typhimurium (MTCC No. 3231). The culturing of the microorganisms was as per the recommendations provided by MTCC.
Widely accepted Agar well diffusion method evaluated antimicrobial activity of the test sample and the protocol for this study is reported previously (Rahdar et al., 2017). The test organism was distributed uniformly on the surface of the plates using sterile cotton swab. Four wells having 20 mm separation and 9 mm diameter were punched aseptically with a sterile cork borer in each plate (Mol et al., 2018). Test sample was added into the wells T1 & T2. In the positive well (+) Gentamycin was added and in the negative well (-) the solvent used for the sample dilution was added. Under aerobic conditions the plates were kept for 24 h at 36 °C ± 1 °C. The plates were observed and zone of bacterial growth inhibition around the wells was measured in mm after incubation.
3 Results
3.1 3.1Morphological studies
The X-Ray diffractogram of the CuO-NiO nanocomposite capped using DNA after annealing at 500 °C is given in Fig. 1.
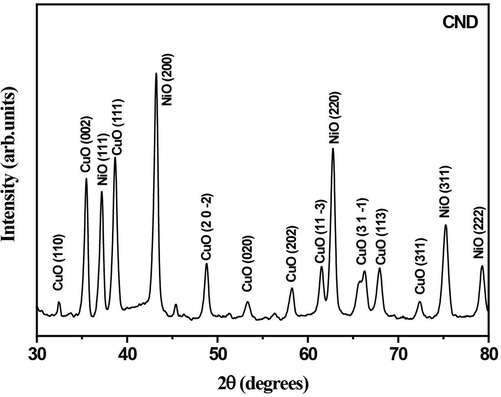
- XRD pattern of CuO-NiO bimetallic nanocomposite capped using DNA.
The diffraction peaks corresponding to CuO and NiO are well resolved after annealing at 500 °C. XRD peaks obtained for nanocomposite shows that it is a mixture of individual binary oxide phases. The CuO peaks appear at diffraction angles of 32.40, 35.40, 38.60, 48.70, 53.30, 58.10, 61.50, 67.90, 72.30 corresponding to reflection from (1 1 0), (0 0 2), (1 1 1), (20–2), (0 2 0), (2 0 2), (11–3), (1 1 3) and (3 1 1) planes confirms the formation of monoclinic structured CuO and the lattice parameters obtained as a = 0.46927 nm b = 0.34283 nm c = 0.51370 nm and plane angles as α = γ = 900 β = 99.5460 (COD File No. 00–901-6326).The calculated volume of the cell is ∼ 81(106pm3).The NiO peaks appear at diffraction angles of 37.10,43.10,62.70,75.20,79.20 corresponds to (1 1 1), (2 0 0), (2 2 0), (3 1 1), (2 2 2) respectively, belongs to the cubic phase of NiO with COD File No.00–432-0493.
The diffraction peak’s intensity and the concentration of the component producing that are proportional to each other. The relative intensity ratio depends on the volume fractions,
In X-ray diffraction, line broadening is due to the variation in crystallite size (D) and microstrain (Madhu et al., 2013). According to the Scherrer equation.
and the microstrain contribution to line broadening is given by
Here, λ = 1.5406 Å -wavelength of the X-rays,θ- angle of diffraction, k = 0.9,shape factor,
In Uniform Deformation Model (UDM), the microstrain is taken to be equal in all crystallographic directions. The Wiliamson-Hall equation for this model is given by
By plotting
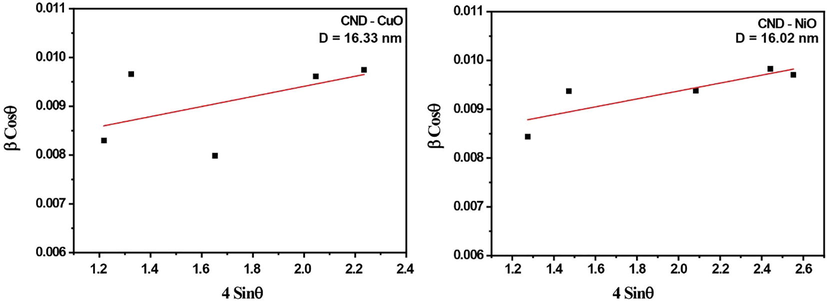
- Wiliamson-Hall plot of NiO and CuO for CND sample assuming UDM.
| Sample Name | Oxide | Crystal System | a | b | c | Crystallite size(nm) | |
|---|---|---|---|---|---|---|---|
| W-H(nm) | Scherrer(nm) | ||||||
| CND | CuO | Monoclinic | 4.6927 Å | 3.4283 Å | 5.1370 Å | 16.33 | 16.106 |
| NiO | Cubic | 4.1872 Å | 4.1872 Å | 4.1872 Å | 16.02 | 15.722 | |
SEM-EDS analysis was done to verify the content and homogeneity of the components in the sample. The stoichiometry and composition of elements in the samples were studied using EDS. The EDAX of CND (Fig. 3) reveals high purity of the sample. Surface morphology of the synthesized sample shows that they are non-homogeneous in nature (Fig. 4). The prepared mixed metal oxide nanocomposites contain only CuO and NiO nanoparticles. The mass percentage and weight percentage of Oxygen Copper, and Nickel are shown in Table 2.
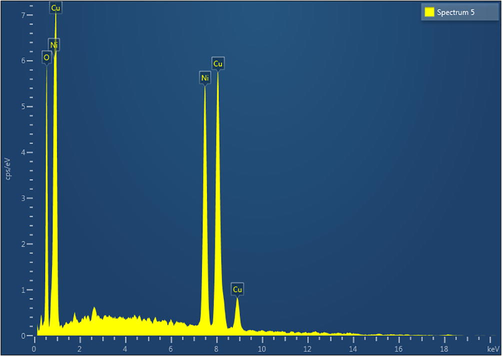
- EDAX Spectrum of sample CND showing the elemental composition Cu, Ni and O.
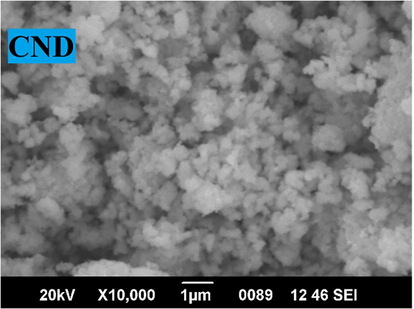
- SEM image of CND showing non homogeneous distribution.
| Element | Line Type | Weight% | Atomic% |
|---|---|---|---|
| O | K series | 13.45 | 37.35 |
| Ni | K series | 37.27 | 28.2 |
| Cu | K series | 49.28 | 34.25 |
| Total | 100 | 100 | |
The TEM images and electron diffraction pattern of CuO-NiO nanocomposite prepared using DNA is presented in Fig. 5. A non-uniform surface morphology of CND was obtained from TEM. Crystalline nature of CND sample was shown in TEM image. The grain size obtained from the TEM image matches with the XRD result.
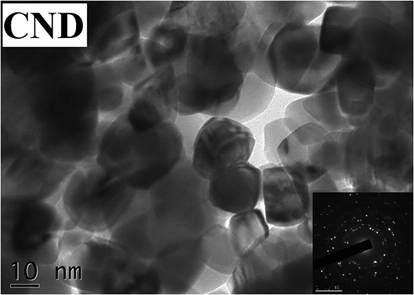
- TEM Image and SAED pattern showing the crystalline nature of CuO-NiO nanocomposite.
3.2 Antioxidant activity
3.2.1 DPPH assay
Antioxidant behavior of CND was tested using DPPH and FRAP assay at varying concentrations in controlled experimental setting. Results showed that CND was very active in free radical scavenging as shown in Fig. 6. The effect was higher in lower concentrations, viz. 0.1, 1, 5 and 10 µg/ml of CND and activity decreased after that. The effect might be due to the low solubility of compounds in methanol, as well as to the conditions favoring precipitation. This will reduce the availability of free compounds for scavenging free radicals. Ascorbic acid was used as positive control (Fig. 6) which showed enhanced radical scavenging effect compared to CND.
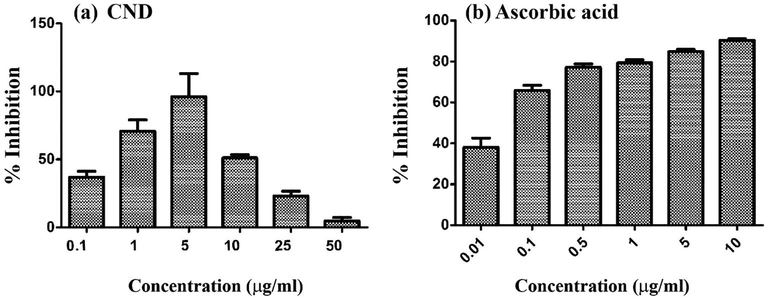
- DPPH radical scavenging effect of (a) CND and (b) ascorbic acid at various concentrations.
In this study, CND shows high activity at 5 µg/ml concentration and the activity was comparable to ascorbic acid at 5 µg/ml concentration. On the basis of the calculations obtained from the activity curve, to scavenge 50% of free radicals (IC50 value) the minimum concentration of CND sample needed is 0.349 µg/ml and while that of ascorbic acid is 0.052 µg/ml.
3.2.2 FRAP assay
The anti-oxidant activity of CND was also probed using FRAP assay. Presence of potent antioxidants will lead to the generation of this colored molecule. Here, the sample CND showed a trend in activity similar to DPPH scavenging. The activity reduced after a concentration of 5–10 µg/ml concentration (Fig. 7). The IC50 value of CND in FRAP assay is 9.648 ± 0.991, which shows the potential of this mixed metal oxide nanoparticle in radical scavenging.
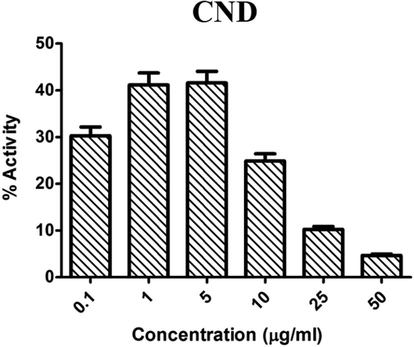
- Antioxidant activity of CND using FRAP assay at various concentrations.
3.3 Anti-bacterial activity
The sample CND was checked for anti-bacterial activity against Salmonella typhimurium and Mycobacterium smegmatis at two concentrations. In agar well cut method, CND showed good antibacterial activity at 800 µg of compound load per well. The potential of this compound to inhibit bacterial growth was measured as the diameter of clearance zone around wells (Table 3). The positive control used for this study was Gentamycin and negative control wells were loaded with vehicle alone which failed to produce any inhibition zone (Fig. 8).
| Sample Name | Organism | Inhibition Zone (mm) | |||
|---|---|---|---|---|---|
| Standard Gentamycin (160 mcg) | Negative control | T1 (40 µl from 10 mg/ml) | T2 (80 µl from 10 mg/ml) | ||
| CND | M. smegmatis | 27 | – | 9 | 20 |
| S. typhimurium | 26 | – | – | 21 | |
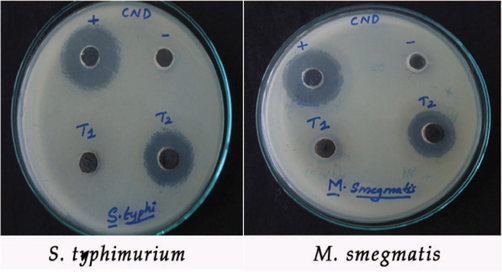
- Zone of Inhibition for CuO-NiO nanocomposite against S.typhimurium and M.smegmatis.
4 Discussion
The present study was performed to synthesize CuO-NiO nanocomposites with the help of DNA as capping agent and to check the antioxidant and antibacterial activities of it. Even though DNA assisted synthesis of nanoparticles have already been reported for various metal oxides (Malu et al., ,2017; Jyothi et al., 2020). This is the first report on using DNA as capping agent in the synthesis of CuO-NiO bimetallic oxide nanocomposites. XRD structural analysis using Scherrer method and W-H plot gives almost the same crystallite size for DNA capped CuO-NiO nano particles. This may be due to the effective capping of the DNA. There is a difference in volume fractions of the two metal oxides. This may be attributed to the different nanoparticle precipitation ability of two oxides as reported earlier (Juma et al., 2017).
The mass percentage and weight percentage of Copper, Nickel and Oxygen from the EDAX data shows that it obeys stoichiometry. From SEM analysis, one can conclude the formation of aggregates of semi-spherical structure with almost uniform distribution. XRD studies show that DNA can be used as a potent biological capping agent to reduce the size of mixed metal oxide nanoparticle. The crystallite size obtained from XRD studies was confirmed by TEM. The antioxidant activities of the sample reveals that the minimum inhibitory concentration of it is almost same as that of standard ascorbic acid. Anti-oxidant activities of metal oxides are reported previously from many studies (Das et al., 2013; Dobrucka, 2018; Chahardoli et al., 2020). Copper Oxide and Nickel Oxide nano particles are molecules of high application in military and industrial sector (Handy et al., 2008). In a recent study conducted with CuO nanoparticles, the researchers did oral administration of compounds to hypouricemic BALB/c mice to see the antioxidant and histopathological changes. In histopathological analysis, no significant changes in tissues were reported (Kiyani et al., 2021). Not many studies are available on the effect of metal oxides with ferric reducing effect. In one report, the CuO nano particles, synthesized through natural process were tested for antioxidant activity using FRAP assay. Results showed that the material used for test that synthesized copper nanoparticles showed increased reducing potential compared to control (Ramasamy and Selvam, 2015). In yet another study, silver nanoparticles extracted from natural sources showed potent antioxidant activity in FRAP assay (Govindappa et al., 2016). Metal oxide nanoparticles exhibit antibacterial activity and are evidenced by various studies (Ahamed et al., 2014; Sabouri et al., 2021; Peddi et al., 2021; Sathiyaraj et al., 2021). The CuO-NiO nanocomposite exhibits antibacterial activity against M. smegmatis and S. typhimurium and is comparable with the standard at 80 µl. Similar study results were reported with copper oxide nanomaterials also (Nithiyavathi et al., 2021). The higher surface charge density of the nanocomposite enhances the affinity with the negatively charged bacteria membrane, which is mostly responsible for its bacterial activity. Charge, properties of the counter anion, configuration in geometrical space and the oxidation state of the central metal ion are the main factors affecting the biological activities of metal complexes. CuO-NiO metal oxide nanocomposite interacts with the cell generates superoxide (• O2) and hydroxyl radicals. These Reactive Oxygen Species (ROS) cause cytotoxic reactions by inhibiting DNA synthesis and destructing cell viability (Malu et al., ,2017). The mode of action of cytotoxicity is through inducing plasma membrane leakage, generating ROS inside cells and leading to oxidative stress or through slowing down the release of antibacterial drugs (Singh et al., 2021). Zeta potential is formed when the particle approaches near the membrane. This zeta potential is important for the stability of nanoparticles in suspension and is also the major factor in the initial adsorption of nanoparticles onto the cell membrane. Bacterial cell surface is negatively charged and is the target site of the polycation. Therefore, the polycationic nanocomposites with higher surface charge density provide higher affinity to bacteria cells.
5 Conclusion
CuO-NiO bimetallic oxide nanoparticles were synthesized with the help of DNA as capping agent. The cubic phase of NiO with average crystallite size of 16.218 nm and monoclinic phase of CuO with crystallite size of 15.871 nm in the synthesized mixed metal oxide nanoparticles were confirmed by the XRD studies. SEM studies reveal semispherical structure of the sample. TEM studies revealed that the grain size of the synthesized particles was within 20 nm, which is in agreement with the XRD results. The composite shows activity against Salmonella typhimurium and M. Smegmatis. Antioxidant activity of the sample was comparable with the standard ascorbic acid at 5 µg/ml concentration. Considering the uses of metal oxides in commercial arena as well as military applications, it is worth considering to make use of CND in biomedical industry where its multi-potential can be utilized. To improvise the activity spectrum, more detailed experiments have to be conducted that can increase the solubility of compounds that can improve the bioavailability.
Acknowledgements
The authors acknowledge SAIF-STIC, Cochin for XRD, EDAX, SEM and TEM imaging. Authors also extend their sincere appreciation to the Researchers supporting project number (RSP 2021/11), King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Mixed oxides CuO-NiO fabricated for selective detection of 2-Aminophenol by electrochemical approach. J. Mater. Res. Technol.. 2020;9(2):1457-1467.
- [Google Scholar]
- Synthesis, characterization, and antimicrobial activity of copper oxide nanoparticles. J. Nanomater.. 2014;2014:1-4.
- [CrossRef] [Google Scholar]
- Calcination induced PEG-Ni-ZnO nanorod composite and its biomedical applications. Mater. Chem. Phys.. 2020;255:123603.
- [CrossRef] [Google Scholar]
- The ferric reducing ability of plasma (FRAP) as measurement of “antioxidant power” The FRAP assay. Anal Biochem.. 1996;239:70-76.
- [Google Scholar]
- Synthesis and characterization of NiO nanostructures: a review. J. Nanopart. Res.. 2018;20(8):222.
- [Google Scholar]
- Cao, G.,2004. Nanostructures and nanomaterials: synthesis, properties and applications. journal of the american chemical society.126,14679–14679.
- Effects of engineered aluminum and nickel oxide nanoparticles on the growth and antioxidant defense systems of Nigella arvensis L. Sci Rep.. 2020;10(1)
- [CrossRef] [Google Scholar]
- Synthesis and biological function of Nickel and Copper nanoparticles. Heliyon.. 2019;5(6):e01878.
- [CrossRef] [Google Scholar]
- Cruz, Inês F.,2018. Magnetic Nanostructured Materials || Multifunctional Ferrite Nanoparticles: From Current Trends Toward the Future. , 59–116.
- Synthesis and evaluation of antioxidant and antibacterial behavior of CuO nanoparticles. Colloids Surf B Biointerfaces.. 2013;1(101):430-433.
- [CrossRef] [Google Scholar]
- Antioxidant and catalytic activity of biosynthesized CuO nanoparticles using extract of Galeopsidis herba. J. Inorg. Organomet. Polym.. 2018;28(3):812-819.
- [Google Scholar]
- Nickel oxide nanoparticles: Synthesis and spectral studies of interactions with glucose. Mater. Sci. Semicond. Process.. 2013;16(6):1747-1752.
- [Google Scholar]
- Gajendiran, J., Ramamoorthy, C., Prabhu Sankar,K.C., Rajkumar Sam Kingsly, T., Kamalakannan,V., Krishnamoorthy,T.,2016. Optical and Luminescent properties of NiO-CuO Nanocomposite by the precipitation method. Journal of Advanced Chemical Sciences. 2,227-229.
- Variation in secondary metabolite production as well as antioxidant and antibacterial activities of Zingiber zerumbet (L.) at different stages of growth. BMC Complementary and Alternative Medicine.. 2016;16(1)
- [CrossRef] [Google Scholar]
- Mycosynthesis of silver nanoparticles using extract of endophytic fungi, Penicillium species of Glycosmis mauritiana, and its antioxidant, antimicrobial, anti-inflammatory and tyrokinase inhibitory activity. Adv. Nat. Sci: Nanosci. Nanotechnol.. 2016;7(3):035014.
- [CrossRef] [Google Scholar]
- The ecotoxicology of nanoparticles and nanomaterials: current status, knowledge gaps, challenges, and future needs. Ecotoxicol.. 2008;17(5):315-325.
- [Google Scholar]
- Medicinal plant leaf extract and pure flavonoid mediated green synthesis of silver nanoparticles and their enhanced antibacterial property. Sci. Rep.. 2017;7(1):15867.
- [Google Scholar]
- Effect of capping agents: structural, optical and biological properties of ZnO nanoparticles. Appl. Surf. Sci.. 2016;386:319-326.
- [Google Scholar]
- Role of capping agents in the application of nanoparticles in biomedicine and environmental remediation: recent trends and future prospects. Journal of Nanobiotechnology. 2020;18(1):172.
- [Google Scholar]
- Synthesis and characterization studies of MgO:CuO nanocrystals by wet-chemical method. Spectrochim. Acta Part A Mol. Biomol. Spectrosc.. 2015;142:405-409.
- [Google Scholar]
- Khan, I., Saeed, K., Khan, I., 2019. Nanoparticles: properties, applications and toxicities.Arabian Journal of Chemistry 12(7): 908-931.
- Graphene based metal and metal oxide nanocomposites: synthesis, properties and their applications. J. Mater. Chem. A. 2015;3(37):18753-18808.
- [Google Scholar]
- Evaluation of antioxidant activity and histopathological changes occurred by the oral ingestion of CuO nanoparticles in monosodium urate crystal-induced hyperuricemic BALB/c Mice. Biol Trace Elem Res. 2022;200(1):217-227.
- [CrossRef] [Google Scholar]
- Effect on oxidative stress, glucose uptake level and lipid droplet content by Apigenin 7, 4'-dimethyl ether isolated from Piper longum.L. J Food Sci Technol.. 2015;52:3561-3570.
- [Google Scholar]
- Kuang, D., Lei,B.,Pan Y.,Yu,X.,Su,C,2009.Fabrication of novel Hierarchial-Ni(OH)2 and NiO Microspheres via an easy Hydrothermal Process, J. Phys. Chem., 113,5508-5513.
- Synthesis and characterization of CuO–NiO nanocomposite: highly active electrocatalyst for oxygen evolution reaction application. J Mater Sci: Mater Electron. 2020;31:11286-11294.
- [Google Scholar]
- Biosynthesis of Nickel oxide Nanoparticles from Euphorbia heterophylla (L.) and their biological application. Arabian J. Chem.. 2020;13(3):4712-4719.
- [Google Scholar]
- Growth of the oxidized Nickel nanoparticles on a DNA template in aqueous solution. Mater. Lett.. 2008;62(15):2315-2317.
- [Google Scholar]
- Synthesis and characterization of magnetic Co nanoparticles: A comparison study of three different capping surfactants. J. Solid State Chem.. 2008;181(7):1530-1538.
- [Google Scholar]
- Microstrain in nanostructured nickel oxide studied using isotropic and anisotropic models. Physica B. 2013;421:87-91.
- [Google Scholar]
- Malu, S., Nancy, J., Nisha,J.T.,2017.DNA-assisted synthesis of chitosan/α-Fe2O3 nanocomposites for antioxidant and antimicrobial activities. Bull. Mater. Sci.40,1463–1469.
- Experimental and theoretical spectroscopic analysis, chemical reactivity and fungicidal activity study on benalaxyl along with quantum chemical computation on metalaxyl and furalaxyl. Chem. Data Collect. 2018
- [CrossRef] [Google Scholar]
- Estimation of phytochemical content and antioxidant activity of some selected traditional indian medicinal plants. Indian J Pharm Sci.. 2011;73:146-151.
- [Google Scholar]
- Studies on CdS nanoparticles prepared in DNA and bovine serum albumin based biotemplates.J. Appl. Phys.. 2012;112(6):064704.
- [CrossRef] [Google Scholar]
- synthesis, characterization, antioxidant, antibacterial, and photocatalytic activity of Suaeda maritima (L.) Dumort aqueous extract-mediated copper oxide nanoparticles. J Genet Eng Biotechnol.. 2021;19(1)
- [CrossRef] [Google Scholar]
- Superparamagnetic MFe2O4 (M = Fe Co, Mn) Nanoparticles: Tuning the Particle Size and Magnetic Properties through a Novel One-Step Coprecipitation Route. Chem. Of Materials.. 2012;24(8):1496-1504.
- [Google Scholar]
- synthesis of nanoceria, its size dependent structural and optical properties for optoelectronic applications. Bull. Mater. Sci.. 2020;43(1)
- [CrossRef] [Google Scholar]
- Origin of lattice strain in nanocrystalline materials. Philos. Mag. Lett.. 2005;85(12):649-656.
- [Google Scholar]
- Biosynthesis of copper oxide nanoparticles with potential biomedical applications. Int J Nanomedicine.. 2020;15:3983-3999.
- [Google Scholar]
- CuO-NiO nano composites: synthesis, characterization, and cytotoxicity evaluation. Nanomed Res J.. 2017;2:78-86.
- [Google Scholar]
- Green synthesis of copper nanoparticles from Hibiscus rosasinensis and their antimicrobial, antioxidant activities. Res. J. Pharm. Biol. Chem. Sci.. 2015;6:1183-1190.
- [Google Scholar]
- A facile and green synthesis of CuO/NiO nanoparticles and their removal activity of toxic nitro compounds in aqueous medium. Chemosphere. 2021;271:129475.
- [CrossRef] [Google Scholar]
- Gum mediated synthesis and characterization of CuO nanoparticles towards infectious disease-causing antimicrobial resistance microbial pathogens. Journal of Infection and Public Health 2021
- [CrossRef] [Google Scholar]
- Green-based bio-synthesis of nickel oxide nanoparticles in Arabic gum and examination of their cytotoxicity, photocatalytic and antibacterial effects. Green Chem. Lett. Rev.. 2021;14(2):404-414.
- [Google Scholar]
- Synthesis of CuO/ZnO nanocomposites and their application in photodegradation of toxic textile dye. J. Compos. Sci.. 2019;3(3):91.
- [CrossRef] [Google Scholar]
- Salata, O. V., 2004. Applications of nanoparticles in biology and medicine. Journal of nanobiotechnology 2(1): 3-3.
- Biogenesis and application of nickel nanoparticles: a review. Curr. Pharm. Biotechnol.. 2021;22(6):808-822.
- [Google Scholar]
- DNA assisted synthesis, characterization and optical properties of zinc oxide nanoparticles. Int. J. Mater. Sci. Eng.. 2014;2:147-151.
- [Google Scholar]
- Green’ synthesis of metals and their oxide nanoparticles: applications for environmental remediation. J. Nanobiotech.. 2018;16(1):84.
- [Google Scholar]
- Potentialities of bioinspired metal and metal oxide nanoparticles in biomedical sciences. RSC Adv.. 2021;11(40):24722-24746.
- [Google Scholar]
- Sivaji Sathiyaraj, Gunasekaran Suriyakala, Arumugam Dhanesh Gandhi, Ranganathan Babujanarthanam, Khalid S.Almaary, Tse-Wei Chen, K.Kaviyarasu. Biosynthesis, characterization, and antibacterial activity of gold nanoparticles. 2021. Journal of Infection and Public Health. https://doi.org/10.1016/j.jiph.2021.10.007.
- Enhanced electrical and magnetic properties of CuO/MgO nanocomposites. Chem. Phys. Lett.. 2021;765:138320
- [Google Scholar]
- Synthesis and biomedical applications of copper oxide nanoparticles: an expanding horizon. ACS Biomater. Sci. Eng.. 2019;5(3):1170-1188.
- [Google Scholar]







