Translate this page into:
Cucurbitacin B supplementation reduces inflammatory responses and alveolar bone loss via regulating MPO, COX-2 and RANK/RANKL/OPG signals in a rodent model of ligature-induced periodontitis
⁎Corresponding author. KathrineClineyjg@yahoo.com (Wei Luo)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Cucurbitacin B, a triterpenoid compound found in many Cucurbitaceae species, possesses significant antioxidant and anti-inflammatory activity. This research aims to determine the effects of Cucurbitacin B in experimental periodontitis model. Experimental periodontitis were induced in Sprague Dawley rats by ligature method. Silk suture (4/0) was ligated subgingivally around the mandibular right first molars and placed in position for 11 days. The treatment group animals were administered Cucurbitacin B (12.5 mg, 25 mg, and 50 mg/kg bwt) via oral gavage for 12 days starting two days before ligature. The rats were sacrificed following 11 days after ligature, and the alveolar bone loss in the first molars was determined histometrically. For histological changes and levels of inflammatory mediators (1L-10, 1L-β, COX-2, MPO, and TNF-α), the periodontal tissues were excised and examined. The expressions of RANKL/RANK and OPG were determined. Alveolar bone loss was reduced expressively on Cucurbitacin B treatment. The MPO activity was substantially down-regulated. Cucurbitacin B significantly regulated the expressions of RANK, RANKL, and OPG. This study demonstrated that oral administration of cucurbitacin B significantly decreased loss in alveolar bones via regulating the RANKL/OPG stability and reducing inflammatory responses in experimental periodontitis.
Keywords
Alveolar bone loss
Cucurbitacin B
Osteoprotegerin (OPG)
Periodontitis
Receptor activator of nuclear factor-kappa B ligand (RANKL)
1 Introduction
Periodontitis is one of the predominant oral cavity diseases and the foremost cause of tooth loss in adults (Kassebaum et al., 2014). Clinical manifestations of the disease include inflammation of the tissues surrounding the teeth, periodontal pocketing, and alveolar bone loss, eventually leading to tooth loss. The interaction between the specific anaerobic microbes in the dental plaque and the host immune responses has been well established in periodontitis. Inflammatory responses driven by increased levels of inflammatory mediators against microbial challenge along with impaired endogenous resolution pathways amplifies periodontal tissue destruction (van Dyke, 2011). Regulation of the balance between pro- and anti-inflammatory mediators crucially determines the severity of tissue destruction in periodontitis. Increased activity of osteoclast without a corresponding rise in bone formation is the primary characteristic of inflammatory bone loss in periodontitis. The pro-inflammatory cytokines- including IL-1β, IL-6, and TNF-α increase the recruitment and activity of osteoclasts, the bone-resorbing cells via enhanced production of a crucial osteoclastogenic factor, the Receptor Activator of Nuclear Factor κ B Ligand (RANKL) and favor bone destruction (Hienz et al., 2015).
RANK/RANKL-Osteoprotegerin (OPG) axis plays a crucial role in bone loss in periodontitis (Graves et al., 2011). RANKL, a membrane-bound protein of the TNF ligand superfamily, is responsible for stimulating osteoclast differentiation and bone resorption. OPG, also a member of the TNF family, inhibits the binding of RANKL to RANK and prevents osteoclastogenesis. RANK-L and OPG levels are critical in regulating the bone tissue destruction, as observed in periodontitis. RANK-L/OPG expression patterns at the sites of active bone resorption are different as compared to regions where bone resorption is either absent/negligible or negligible. At regions that exhibited increased RANKL expressions than OPG (RANKL > OPG) presented progressive lesions, while regions with similar RANKL and OPG levels or raised OPG levels presented more potentially stable lesions (Menezes et al., 2008).
Mechanical and surgical therapies have been employed in the treatment of periodontitis. However, these therapies are not always satisfactory and demand the use of antibiotics and non-steroidal anti-inflammatory drugs which, modulate host inflammatory responses by reducing the activation of mediators as- prostaglandins and cytokines. The use of these drugs is often related to side effects as gastrointestinal bleeding and also renal and hepatic impairments. Thus, the need for identification and development of newer therapeutic agents with negligible side effects is clinically valuable.
In recent years, much research is done on phytochemicals as suitable alternatives to synthetic chemicals. Studies have reported the potential effects of plant extracts in periodontitis and bone repair (Guimarães et al., 2016). Lima et al. (2017) reported that aqueous extract of Calendula officinalis flowers prevented alveolar bone loss in experimental periodontitis. Cucurbitacins are bioactive compounds found in many plant species, majorly present in plants of the Cucurbitaceae family as in cucumber, squash, and zucchini. Cucurbitacins are triterpenes, formally derived from cucurbitane, a triterpene hydrocarbon (Chen et al., 2005). Cucurbitacin B, one of the most potent and frequent members of cucurbitacin, has been reported to have a variety of biological activities, including antitumor, hepatoprotective, anti-inflammatory and antioxidant activities. Cucurbitacin B inhibited the LPS-mediated release of pro-inflammatory cytokines. Cucurbitacin B isolated from Hemsleya endecaphylla (Chen et al., 2008) and Cucurbita andreana (Halaweish and Tallamy, 1993) were reported to exhibit anti-inflammatory, anti-hepatotoxic (Chen et al., 2005). Cucurbitacin B, extracted from Trichosanthes cucumerina L, a Thai medicinal plant has been reported to possess active biological properties including anticancer, antimicrobial and anti-inflammatory. Cucurbitacin B from T. cucumerina Linn. was found to exert cytotoxic effect on breast cancer cell lines SKBR-3 and MCF-7 with an IC50 value of 4.60 and 88.75 μg/mL, respectively. Growth inhibition was attributed to G2/M phase arrest and apoptosis. Cucurbitacin B was also observed to inhibit Wnt signaling. Cucurbitacin B was reported to inhibit proliferation of osteosarcoma cells – U-2 OS cells by downregulating major pathways-MAPK and JAK2/STAT3 (Zhang et al., 2017)
Hua et al. (Hua et al., 2017) reported that intraperitoneal administration of cucurbitacin B at doses 1–5 mg/kg.b wt was found to exert protective effects against acute lung injury in rats. Based on the vast array of biological properties of Cucurbitacin B, we hypothesized that oral supplementation of cucurbitacin B reduced alveolar bone loss and inflammatory responses in experimentally induced periodontitis.
2 Materials and methods
2.1 Animals
The Southern Medical University animal care and the ethical committee (Ethical approval number: TAMY05471) approved this research study, and all experimental and surgical protocols were conducted in accordance with the National Institutes of Health Guide for the Use of Laboratory Animals [30]. Male Sprague-Dawley rats (n = 60; weighing 180–220 g and 7–8 weeks of age) from Guangdong Medical Laboratory Animal Co., China, were used in the study. The rats were held in sterile cages and were sustained on 12 h light/dark cycle with a relative humidity of 50–55% and room temperature at 22° ± 1 °C. Study animals were provided with ad libitum access to standard pellet diet and water. The animals were left to be tolerable and to adapt to the lab conditions for five days before initiation of the study.
2.2 Study design
The animals were divided into five experimental groups, with 12 animals per group. Using ligature method periodontitis was induced. The rats were anesthetized under ketamine (70 mg/kg), and xylazine (10 mg/kg) intramuscularly and a 4/0 silk suture (Teleflex Medical OEM, USA) was placed subgingivally positioned around the right mandibular first molars. The ligatures were observed daily to make sure of the subgingival position. The treatment group animals were administered Cucurbitacin B (12.5 mg/kg, 25 mg/kg, and 50 mg/kg) via oral gavage for 12 days starting 2 days prior ligature. The doses were chosen based on the previous results in our laboratory conducted with varying doses of cucurbitacin B (10 mg/ Kg b.wt to 2 g/Kg b.wt). The effects of 10–100 mg/Kg of cucurbitacin B were assessed for anti-inflammatory studies in our laboratory in other experimental models (data not included). The 3 doses of 12.5 mg/kg, 25 mg/kg, 50 mg/kg were found to exhibit anti-inflammatory effects and thus were chosen for the present study against periodontitis.
The control group rats were not induced nor received cucurbitacin B. Separate group of rats that were induced experimental periodontitis and not treated with cucurbitacin B served as an experimental control. The rats were sacrificed following 11 days after ligature under anesthesia and the mandibles were excised and split into two equal pieces in at the incisor teeth by dissection. The right piece of the mandible was removed and fixed in formalin for 24 h and used for analysis, and the alveolar bone loss in the first molars were determined histometrically. Periodontal tissues were excised and examined for histological changes, and the expressions of COX-2, RANKL/RANK, and OPG were determined. MPO activity in the tissues was assessed. The inflammatory mediators (1L-10, 1L-β, and TNF-α) levels were measured.
2.3 Histometrical analysis
The right mandible samples were fixed for 24–72 h in 10% formalin and were decalcified and then integrated in paraffin. Mesio-distal serial sections of 5 μm thickness were made and subjected to hematoxylin and eosin (HE) staining. First and last sections were excluded for observation, and three equally distant sections of each molar were taken. The distance between the alveolar bone crest and the cemento-enamel junction was measured too. Evaluation was done using an image analysis system connected to a light microscope (Olympus BX51, Tokyo, Japan) at 40x magnification. Six linear measurements were noted on the mesial and distal sides (three on each side), and the mean was calculated.
2.4 Histopathological analysis
The excised maxillae along with ligature were formalin (10%) fixed, demineralized in 10% formic acid, dehydrated and were paraffin embedded. The tissues were sectioned (4 μm) in a mesiodistal plane and were stained with HE and were observed and analyzed under light microscopy. The sections were taken from the area where the ligature was made between the first and second molars. Parameters as inflammatory cell influx, alveolar bone, and cementum integrity were determined. The level of inflammation was scored from 0 to 3, as previously standardized (Lima et al., 2000). Score 0 – absence of or inflammatory cell infiltration restricted to the marginal gingiva and preserved alveolar process and cementum; score 1- moderate cellular infiltration observed all over the insert gingiva and minor alveolar process resorption with intact cementum; score 2 – severe infiltration of inflammatory cells into the gingiva and periodontal ligament, degradation of the alveolar process, and partial destruction of cementum; score 3 indicates severe cellular infiltration with complete resorption of the alveolar process and intense destruction of cementum.
2.5 Immunohistochemical analysis
Immunohistochemical analysis was performed to evaluate the expressions of COX-2, RANKL, RANK, and OPG. 4 µm sections of the periodontal tissue were made and were transferred to slides that were coated with gelatin. The tissue sections were then deparaffinized, rehydrated, and washed with Triton X-100 (0.3%) in phosphate buffer. The sections were then incubated with hydrogen peroxide (3%) to reduce endogenous peroxidase reactions and then incubated overnight with specific primary antibodies at 4 °C for COX-2, RANK-L, RANK, and OPG (1:1000 dilution; Santa Cruz Biotechnology, CA, USA). The slides were washed with phosphate buffer and further incubated with secondary antibodies (streptavidin-HRP-conjugated; Biocare Medical, CA, USA) for 40 min at room temperature. Immunoreactivity was measured using TrekAvidin- HRP Label + Kit (Biocare Medical).
2.6 Determination of TNF-α, 1L-10, and IL-1β
The gingival tissues that were excised and stored at −70 °C were used for the assay. The tissues sections were processed as previously described (Safieh-Garabedian et al., 1995). The levels of TNF-α, IL-1β, and 1L-10 - the inflammatory mediators were assayed using commercially available ELISA kits from R&D Systems in accordance with the instructions provided.
2.7 Myeloperoxidase assay
The level of concentration of neutrophils into the tissues was determined by measuring myeloperoxidase (MPO) activity. The gingival samples (n = 6) from all the experimental groups were removed and stored at −70 °C. The tissues were homogenized and centrifuged (2000xg for 20 min). The supernatant was collected and assayed for MPO by the colorimetric method as described by Souza et al. (2003). The absorbance was measured at 450 nm using a Beckmann DU-65 spectrophotometer, and the results were presented as units of MPO/mg of tissue.
2.8 Statistical analysis
Statistical analysis of the obtained data was done using SPSS (Statistical Package for the Social Sciences) version 22.0 (SPSS, IBM, IL, USA). One-way analysis of variance was performed, followed by Duncan’s Multiple Range Test (DMRT) post hoc analysis for multiple group comparisons. Values at p < 0.05 were regarded statistically important.
3 Results
3.1 Cucurbitacin B reduces alveolar bone loss
The histometrical analysis revealed alveolar bone loss in rats presenting periodontal disease (Fig. 1) while no bone loss was seen in control animals. The observation indicated that administration of cucurbitacin B at all the three tested doses (12.5 mg/kg, 25 mg/kg, and 50 mg/kg) effectively prevented alveolar bone loss. The highest tested dose of 50 mg was said to be the most efficient dose in reducing a bone loss compared to lower doses. The results indicate the efficacy of cucurbitacin B supplementation in preventing alveolar bone loss.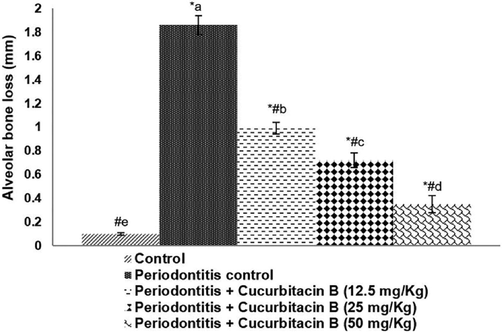
Cucurbitacin B reduced alveolar bone loss. Values are represented as mean ± SD, n = 6. * represents p < 0.05 vs. control; # represents p < 0.05 vs. Periodontitis control. a-e represents a significant difference (p < 0.05) between mean values of different experimental groups as determined by one-way ANOVA followed by DMRT analysis.
3.2 Cucurbitacin B reduces inflammatory cell influx and improves histology
Histopathologic analysis of the excised maxillae from rats that were induced experimental periodontitis exhibited severe inflammatory cell infiltration, alveolar bone loss, severely damaged cementum, presenting scores between 2 and 3. The periodontium of rats administered with cucurbitacin B showed considerably preserved cementum, alveolar process, and reduced cellular influx (Fig. 2a and b). 50 mg/kg cucurbitacin B treated animals revealed almost near normal histology with negligible infiltration of inflammatory cells and well-preserved collagen and cementum.![Cucurbitacin B reduced inflammation following ligature-induced periodontitis (a) and histopathological analysis (b) Values are represented as mean ± SD, n = 6. * represents p < 0.05 vs. control; # represents p < 0.05 vs. Periodontitis control. a-e represents a significant difference (p < 0.05) between mean values of different experimental groups as determined by one-way ANOVA followed by DMRT analysis [a-Control; b-Periodontitis + Cucurbitacin B (50 mg/Kg)].](/content/185/2020/32/3/img/10.1016_j.jksus.2020.01.028-fig2.png)
Cucurbitacin B reduced inflammation following ligature-induced periodontitis (a) and histopathological analysis (b) Values are represented as mean ± SD, n = 6. * represents p < 0.05 vs. control; # represents p < 0.05 vs. Periodontitis control. a-e represents a significant difference (p < 0.05) between mean values of different experimental groups as determined by one-way ANOVA followed by DMRT analysis [a-Control; b-Periodontitis + Cucurbitacin B (50 mg/Kg)].
3.3 Cucurbitacin B regulates the expressions of RANK/RANKL and OPG following induction of periodontitis
RANK and RANKL expression in the periodontal tissues of rats with ligature-induced periodontitis was elevated following when related to control group (Fig. 3a and b). OPG expression, however, was reduced (p < 0.05) to 63.11% in rats presenting periodontitis vs. control rats. Cucurbitacin B treatment significantly (p < 0.05) reduced RANK and RANKL expression while enhancing OPG levels to 94.01% with 50 mg/kg dose. The expression of RANK reduced from 169.10% to 103%, and RANKL levels reduced from 177.09% to 107.02% upon administration of 50 mg/kg cucurbitacin B.![Cucurbitacin B down-regulated RANK/RANKL and OPG expressions (a) Cucurbitacin B down-regulated RANK/RANKL and OPG expressions and (b) Immunohistochemical analysis Values are represented as mean ± SD, n = 6. * represents p < 0.05 vs. control; # represents p < 0.05 vs. Periodontitis control. a-e represents a significant difference (p < 0.05) between mean values of different experimental groups as determined by one-way ANOVA followed by DMRT analysis. [a-Control; b-Periodontitis + Cucurbitacin B (12.5 mg/Kg); c-Periodontitis + Cucurbitacin B (50 mg/Kg)].](/content/185/2020/32/3/img/10.1016_j.jksus.2020.01.028-fig3.png)
Cucurbitacin B down-regulated RANK/RANKL and OPG expressions (a) Cucurbitacin B down-regulated RANK/RANKL and OPG expressions and (b) Immunohistochemical analysis Values are represented as mean ± SD, n = 6. * represents p < 0.05 vs. control; # represents p < 0.05 vs. Periodontitis control. a-e represents a significant difference (p < 0.05) between mean values of different experimental groups as determined by one-way ANOVA followed by DMRT analysis. [a-Control; b-Periodontitis + Cucurbitacin B (12.5 mg/Kg); c-Periodontitis + Cucurbitacin B (50 mg/Kg)].
3.4 Cucurbitacin B reduces the levels of inflammatory mediators
Cytokines are well documented to play a crucial role in periodontitis. The levels of inflammatory cytokines (TNF-αand IL-1β) were found to be higher in animals with ligature-induced periodontitis vs. normal control (Fig. 4a). MPO activity was also observed to be markedly (p < 0.05) increased in ligature-induced rats in comparison to control (Fig. 4b). MPO activity reduced from 21.75 nmol/g tissue to 5.10 nmol/g tissue on treatment with 50 mg cucurbitacin B. However, the levels of IL-10 were observed to be slightly raised in animals that were induced periodontitis. The increase, however, was non-significant. The levels were brought down to almost near normal from 270 pg/mL to 265 pg/mL, 263 pg/mL and 259 pg/mL on treatment with 12.5 mg/kg, 25 mg/kg and 50 mg/kg respectively. Cucurbitacin B treatment at 12.5 mg, 25 mg/kg and 50 mg/kg doses were found to reduce the levels of TNF- α and IL-1β in the gingival tissues. Interestingly, IL-10 levels were found to be near normal and not much altered on cucurbitacin B administration.![Effect of Cucurbitacin B on inflammatory mediators (a) Cucurbitacin B reduced the levels of inflammatory cytokines-IL-10, IL-1β and TNF-α (b) MPO activity (c-d) Relative expressions of COX-2 expression (d) Immunohistochemical analysis were significantly downregulated by cucurbitacin B. Values are represented as mean ± SD, n = 6. * represents p < 0.05 vs. control; # represents p < 0.05 vs. Periodontitis control. a-e represents a significant difference (p < 0.05) between mean values of different experimental groups as determined by one-way ANOVA followed by DMRT analysis [a-Control; b-Periodontitis + Cucurbitacin B (12.5 mg/Kg); c-Periodontitis + Cucurbitacin B (50 mg/Kg)].](/content/185/2020/32/3/img/10.1016_j.jksus.2020.01.028-fig4.png)
Effect of Cucurbitacin B on inflammatory mediators (a) Cucurbitacin B reduced the levels of inflammatory cytokines-IL-10, IL-1β and TNF-α (b) MPO activity (c-d) Relative expressions of COX-2 expression (d) Immunohistochemical analysis were significantly downregulated by cucurbitacin B. Values are represented as mean ± SD, n = 6. * represents p < 0.05 vs. control; # represents p < 0.05 vs. Periodontitis control. a-e represents a significant difference (p < 0.05) between mean values of different experimental groups as determined by one-way ANOVA followed by DMRT analysis [a-Control; b-Periodontitis + Cucurbitacin B (12.5 mg/Kg); c-Periodontitis + Cucurbitacin B (50 mg/Kg)].
![Effect of Cucurbitacin B on inflammatory mediators (a) Cucurbitacin B reduced the levels of inflammatory cytokines-IL-10, IL-1β and TNF-α (b) MPO activity (c-d) Relative expressions of COX-2 expression (d) Immunohistochemical analysis were significantly downregulated by cucurbitacin B. Values are represented as mean ± SD, n = 6. * represents p < 0.05 vs. control; # represents p < 0.05 vs. Periodontitis control. a-e represents a significant difference (p < 0.05) between mean values of different experimental groups as determined by one-way ANOVA followed by DMRT analysis [a-Control; b-Periodontitis + Cucurbitacin B (12.5 mg/Kg); c-Periodontitis + Cucurbitacin B (50 mg/Kg)].](/content/185/2020/32/3/img/10.1016_j.jksus.2020.01.028-fig5.png)
Effect of Cucurbitacin B on inflammatory mediators (a) Cucurbitacin B reduced the levels of inflammatory cytokines-IL-10, IL-1β and TNF-α (b) MPO activity (c-d) Relative expressions of COX-2 expression (d) Immunohistochemical analysis were significantly downregulated by cucurbitacin B. Values are represented as mean ± SD, n = 6. * represents p < 0.05 vs. control; # represents p < 0.05 vs. Periodontitis control. a-e represents a significant difference (p < 0.05) between mean values of different experimental groups as determined by one-way ANOVA followed by DMRT analysis [a-Control; b-Periodontitis + Cucurbitacin B (12.5 mg/Kg); c-Periodontitis + Cucurbitacin B (50 mg/Kg)].
Immunohistochemical analysis reveals a significant increase (p < 0.05) in the expression levels of the COX-2 enzyme in the animals following ligature (Fig. 4 c-d). The expressions were found to increase from 2.1 folds to 1.05 Cucurbitacin B administration prior and after ligature resulted in a significant reduction in tissue COX-2 level compared to ligature-induced animals. 50 mg/kg cucurbitacin B exhibited the highest anti-inflammatory effect as compared to were doses of 12.5/kg and 25 mg/kg.
4 Discussion
The exacerbated inflammatory response has been documented to be the primary cause of tissue destruction and tooth loss (Pihlstrom et al., 2005). Ligature-induced periodontitis is widely used study model system for periodontitis (Bezerra et al., 2000). Also, the method has been effectively employed to study the inflammatory responses in periodontal tissues and for measurement of bone resorption. In this study, ligature-induced periodontitis rodent model was used to analyze the effects of oral administration of cucurbitacin B on alveolar bone loss, inflammatory responses, and RANK/RANKL/OPG expressions.
In the study, intense inflammatory cell infiltration raised levels of pro-inflammatory mediators- IL-1β and TNF-α along with elevated alveolar bone loss were observed following induction of experimental periodontitis. However, the levels of anti-inflammatory cytokine – IL-10 were closer to the normal range. Histopathological analysis revealed intense damage to the periodontium and severe alveolar process resorption and destruction of the collagen fibers. The raised levels of inflammatory cell influx and pro-inflammatory cytokines are a hallmark feature of periodontitis. These exuberated inflammatory responses could have contributed to tissue destruction, as observed. Studies have reported that over-expression of TNF-α and IL-1β increases the damage of connective tissue and alveolar bone by stimulating osteoclastogenesis and inhibiting osteoblasts function (Nanes, 2003). Also, IL-10 plays a vital role in β-cell proliferation and differentiation. Thus, suggesting IL-10 as a crucial regulator of both cell and humoral responses.
The early immune responses involve the migration of inflammatory cells as neutrophils to the site. The cells release enzymes as MPO that promote the inflammatory responses. Further, cytokines as TNF-α and IL-1β play a significant role in promoting extravasation of leucocytes (Newton and Dixit, 2012). Thus, the raised levels of TNF-α and IL-1β observed in part would have increased cellular influx into the periodontal tissues contributing to the elevated levels of MPO. The decrease in MPO activity following administration of cucurbitacin B could be either due to the direct action of the compound or could be partly attributed to the reduced levels of cytokines TNF-α and IL-1β and reduced number of inflammatory cells in the site. Also, IL-1β and TNF-α are found to exert critical roles leading to pathological bone loss in periodontitis. This suggests that cucurbitacin B- mediated decrease in alveolar bone loss in the study may be contributed by the reduced levels of IL-1β and TNF-α observed. Further, a reduction in the inflammatory response by cucurbitacin B is evidenced by significant down-regulation in the expression of COX-2, one of the major enzyme that is involved in the inflammatory process. Souza et al. (2003) reported carvedilol exerted protective effects in experimental periodontitis via reducing the levels of inflammatory mediators.
IL-1β and TNF-α - pro-inflammatory cytokines are known to stimulate the RANKL and decrease OPG expressions, subsequently leading to bone resorption and osteoclastogenesis (Wei et al., 2005). IL-1β is found to promote osteoclastogenesis and bone resorption via the upregulation of RANK and RANKL, while TNF-α acts through RANKL.
Thus, the regulation of RANKL expressions is regarded as vital for osteoclast differentiation. The effects of RANKL are countered by OPG, its soluble decoy receptor possessing structural homology to RANK. OPG consequently inhibits osteoclast differentiation and activation of matrix osteoclasts. While increased RANKL expression relative to OPG leads to binding of RANKL to RANK, promoting osteoclast differentiation and activation resulting in bone resorption (Cochran, 2008). Thus, RANKL-OPG balance is critical in bone resorption and formation.
Also, in periodontitis, osteoblasts, B-cells, T-cells, and epithelial cells have been reported as sources of RANKL (Liu et al., 2003). The gingival epithelial cells upon contact with pathogens in the periodontal tissues produce several pro-inflammatory cytokines and also RANKL, thus promoting osteoclastogenesis (Zhao et al., 2012). Gingival epithelial cells are the first to contact with bacterial stimulus and, can produce RANK-L when stimulated by TNF-α (Fujihara et al., 2014). These observations reflect the efficacy of cucurbitacin B in reducing inflammatory responses, and as well restoring RANKL-OPG balance prevents tissue destruction and alveolar bone loss.
5 Conclusion
The study’s findings reveal that regular administration of cucurbitacin B effectively decreased inflammation by down-regulating the levels of pro-inflammatory cytokines and inflammatory mediators in periodontitis-induced rats. Cucurbitacin B supplementation also suppressed alveolar bone loss by regulating the RANK/RANKL/OPG balance. The findings suggest cucurbitacin B as a potential candidate in the treatment of periodontitis, however further studies are needed to examine the molecular events involved and to assess the bioavailability of the compound.
Data availability statement
All data generated or analyzed during this study are included in this published article. If any specific data required, then available on request from the authors.
Funding support
The study was supported by the Guangdong Medical Research Foundation (Health and Family Planning Commission of Guangdong Province) B2014034.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Selective cyclooxygenase-2 inhibition prevents alveolar bone loss in experimental periodontitis in rats. J. Periodontol.. 2000;71:1009-1014.
- [Google Scholar]
- Cucurbitacins and cucurbitane glycosides: Structures and biological activities. Nat. Prod. Rep.. 2005;22:386-399.
- [Google Scholar]
- Octanorcucurbitane and Cucurbitane triterpenoids from the tubers of Hemsleya endecaphylla with HIV-1 inhibitory activity. J. Nat. Prod.. 2008;71:153-155.
- [Google Scholar]
- Inflammation and bone loss in periodontal disease. J. Periodontol.. 2008;79:1569-1576.
- [Google Scholar]
- Tumor necrosis factoralpha enhances RANKL expression in gingival epithelial cells via protein kinase A signaling. J. Periodontal Res.. 2014;49:508-517.
- [Google Scholar]
- Review of osteoimmunology and the host response in endodontic and periodontal lesions. J. Oral Microbiol. 2011:3.
- [Google Scholar]
- Dry extract of Matricaria recutita L. (Chamomile) prevents ligature-induced alveolar bone resorption in rats via inhibition of tumor necrosis factor-α and interleukin-1β. J. Periodontol.. 2016;87:706-715.
- [Google Scholar]
- A new cucurbitacin profile for Cucurbita andreana: a candidate for cucurbitacin tissue culture. J. Chem. Ecol.. 1993;19:1135-1141.
- [Google Scholar]
- Protective effects of cucurbitacin b on acute lung injury induced by sepsis in rats. Med. Sci. Monit.. 2017;23:1355-1362.
- [Google Scholar]
- Global burden of severe periodontitis in 1990–2010: a systematic review and meta-regression. J. Dent. Res.. 2014;93:1045-1053.
- [Google Scholar]
- The effect of Calendula officinalis on oxidative stress and bone loss in experimental periodontitis. Front. Physiol.. 2017;8:440.
- [Google Scholar]
- Effects of chlorpromazine on alveolar bone loss in experimental periodontal disease in rats. Eur. J. Oral Sci.. 2000;108:123-129.
- [Google Scholar]
- Expression of RANKL and OPG mRNA in periodontal disease: possible involvement in bone destruction. Int. J. Mol. Med.. 2003;11:17-21.
- [Google Scholar]
- Differential patterns of receptor activator of nuclear factor kappa B ligand/osteoprotegerin expression in human periapical granulomas: possible association with progressive or stable nature of the lesions. J. Endod.. 2008;34:932-938.
- [Google Scholar]
- Tumor necrosis factor-alpha: molecular and cellular mechanisms in skeletal pathology. Gene. 2003;321:1-15.
- [Google Scholar]
- Signaling in innate immunity and inflammation. Cold Spring Harb. Perspect. Biol.. 2012;4:a006049
- [Google Scholar]
- Contribution of interleukin-1 beta to the inflammation-induced increase in nerve growth factor levels and inflammatory hyperalgesia. Br. J. Pharmacol.. 1995;115:1265-1275.
- [Google Scholar]
- Decreased gastric tone and delayed gastric emptying precede neutrophil infiltration and mucosal lesion formation in indomethacin-induced gastric damage in rats. Braz. J. Med. Biol. Res.. 2003;36:1383-1390.
- [Google Scholar]
- Pro-resolving lipid mediators: potential for prevention and treatment of periodontitis. J. Clin. Periodontol.. 2011;38:119-125.
- [Google Scholar]
- Cucurbitacin B inhibits cell proliferation and induces apoptosis in human osteosarcoma cells via modulation of the JAK2/STAT3 and MAPK pathways. Exp. Ther. Med.. 2017;14(1):805-812.
- [Google Scholar]
- Effect of Porphyromonas gingivalis and Lactobacillus acidophilus on secretion of IL1B, IL6, and IL8 by gingival epithelial cells. Inflammation. 2012;35:1330-1337.
- [Google Scholar]







