Translate this page into:
Cross infection with gastro-intestinal tract parasites between domestic goat and endemic Farasan gazelle (Gazella gazella farasani) in Farasan Islands, Saudi Arabia
*Corresponding author. Tel.: +966 1 404 4412 t_wronski@gmx.de (Torsten Wronski)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Host related variations in helminth egg and coccidian oocyst counts were compared between a naturally infected endemic population of Farasan gazelle and domestic goats on the Farasan Islands, Saudi Arabia in April 2009. Both bovid species inhabit and browse in the same area but no cross-infection could be detected. The prevalence and mean intensity quantified as the number of eggs and oocysts per gram of faeces were taken as a measure of parasite burdens. Host related differences in prevalence values of Eimeria spp. were significantly higher in domestic goats than in wild gazelles. A similar trend was observed for nematode prevalence (strongyle-type eggs), with no infection in gazelle and low infection in goats. There was also a significant difference in mean intensity values between different Eimeria spp. found in domestic goats.
Keywords
Wildlife/livestock interface
Cross-infection
Protozoa
Nemathelminthes
Bovidae
1 Introduction
The Farasan gazelle (Gazella gazella farasani, Thouless and Al Bassri, 1991) is endemic to the Farasan Archipelago (16°20′–17°20′ N, 41°30′–42°30′ E), off-shore the Red Sea coast of Saudi Arabia. The IUCN Red List classification of the species is vulnerable (Mallon and Kingswood, 2001). The Farasan gazelle population is still believed to number around 1000 animals (Cunningham and Wronski, 2010), but the population of this subspecies is characterized by an acute restriction in the number of locations (three islands), and is thus threatened by human nuisance, stochastic events and devastating disease outbreaks. The Farasan Islands are designated as a protected area and the gazelle population receives considerable conservation effort since it is considered the last viable population of the species in Saudi Arabia (Dunham et al., 2001; Flamand et al., 1988).
Little is known about the ecology of the gazelles on the archipelago (Habibi, 1992), and an understanding of the parasite fauna of both wildlife and traditional domestic livestock is necessary to assess the potential of two-way parasite exchange and the impact of parasites. Gastro-intestinal parasite fauna is relatively well defined for most domestic animals in Saudi Arabia, including an understanding of parasite ecology and host ranges (Al Issa et al., 1985; Alyousif et al., 1992; Cheema et al., 1984; El-Bihari and Kawasmeh, 1980; Farah et al., 1984; Ghandour, 1988; Hussein and Hussein, 1985; Radwan and Bekairi, 1980). However, there is lack of information related to the parasite fauna of wildlife, the range of hosts and parasite–host specificity (Hussein and Mohammed, 1992; Ibrahim et al., 2008; Mohammed and Hussein, 1992, 1994; Mohammed and Flamand, 1996; Mohammed, 1997, 2002). The possible range of interactions between endemic and introduced fauna in native wildlife is complex. Introduction of parasites to which endemic wildlife species may be susceptible, can result in the displacement of the endemic fauna, hybridization with and introgression into the endemic fauna, or allopatric coexistence with the possibility of cross-infection. A wide range of parasites have been identified that can infect both wild and domestic animals (Grootenhuis 1986, 1999; Kock et al., 2002). The exchange of potentially pathogenic parasites between domestic and wild hosts presents real but incompletely defined risks to the health and vigour of wildlife populations (Coetzer et al., 1994; Grootenhuis 1986, 1999). On the other hand, some case studies reported on parasite introduction and subsequent disease outbreak in domestic livestock after being in contact with game animals or other wildlife species (Bwangamoi, 1968; Ocaido et al., 1996, 2004).
Veterinary monitoring is an integral part of wildlife management and forms an empirical basis for eco-system health in protected areas (Gaczyk, 2002). Techniques to monitor the health status of populations differ vastly according to the species under consideration and the habitat type/landscape unit in which the survey is to be conducted (Epstein, 1998). Also monetary and time constraints will need to be considered when monitoring the wildlife/livestock interface. In many cases, this means that monitoring accuracy inevitably will be lower than theoretically possible, simply because limits to the effort spent per survey prevent in-depth monitoring. It lies in the very nature of applied ecosystem health that there is often an immediate need for action, so methods in conservation medicine will need to rely on the best existing data, even if sample sizes are low (Kock, 2004). In the present paper we report on just such a case, where only 19 faecal samples are available allowing for the deduction of a method to test for differences in helminthic and coccidian parasite prevalence and intensity in domestic goats (Capra hircus) and wild Farasan gazelles sharing the same area for foraging, i.e. the former gardens of Miharraq and Al Qisar villages where gazelles browse predominantly at night (app. 20% of female gazelle home ranges; Wronski et al., 2013). We hypothesized that the prevalence of helminthic or protozoan parasites common to both host species (domestic goat and gazelle) would indicate cross-infection in either direction. On the other hand, zero parasite prevalence in either bovid species would rather indicate no parasite exchange or the failure of the parasite to get established in the new host, reach adulthood and to produce patent eggs.
2 Material and methods
Sampling was carried out during a four-week field study on Farasan Kebir, the largest island of the Farasan Archipelago harbouring the endemic Farasan gazelle. The biggest populations live in the vicinity of Seir and Miharraq villages on Farasan Kebir (Cunningham and Wronski, 2010) where they are in close contact with free roaming domestic goats. Faecal samples were collected from domestic goats and wild gazelles browsing in the same habitat (Acacia/Prosopis-thickets in former gardens) near the Miharraq village. Fresh samples were picked from the ground into wide-mouthed, screw-capped plastic containers immediately after the animal had defecated. A few gazelle samples were collected during a systematic search for localized defecation sites in the study area. Samples from such latrines were fresh, originating from one individual (clumped faecal pellets), but could not be assigned to a sex or age class. Faecal samples were preserved in 2.5% (w/v) potassium dichromate (K2Cr202) and transferred to the laboratory at the King Khalid Wildlife Research Centre (KKWRC) in Thumamah, Saudi Arabia. Samples were then subjected to a flotation over saturated sodium chloride solution (Hansen and Perry, 1990).
Three grams of fresh faecal pellets were ground to fine particles using pestle and mortar and mixed with 20 ml of distilled, saturated sodium chloride. The mixture was filtered through a strainer to remove the coarse faecal materials. Well-mixed sub-samples from the filtrate were then transferred into the McMaster egg counting chambers. Helminth eggs and coccidian oocysts floating on the flotation solution were identified and counted. Eggs were counted under the magnification ×10 objective lens while for oocysts a magnification of ×40 objective was used. The number of eggs and oocysts per gram (epg/opg) were then calculated by multiplying the total counts, of each type of parasite egg or oocyst by 50 (Hansen and Perry, 1990).
Samples in which Eimerian oocysts were detected, were ground up in a mortar, thoroughly mixed with 2.5% (w/v) aqueous solution of potassium dichromate, strained with a fine-mesh wire strainer, and suspended in shallow layers of the solution in Petri dishes at room temperature (25 ± 2°C) for sporulation (Mohammed and Hussein, 1992). Measurements of sporulated oocysts were taken using a calibrated ocular micrometre, and photomicrographs were made using a Nikon camera (Nikon Company, Japan) attached to a Zeiss compound micrometre (Carl Zeiss, Jena, Germany). All measurements were depicted in micrometers (μm; mean ± SE). Identification of Eimeria spp. was based on measurements of sporulated oocysts, prevalence or absence of micropyle, micropylar caps, oocyst shape and wall colour, following descriptions and microphotographs provided by Alyousif et al., (1992) and Soulsby, (1982). Identification of helminth eggs followed keys provided by Anonymous,(1977), Hansen and Perry, (1990), Soulsby, (1982) and Bürger and Stoye, (1968).
A total of 19 faecal samples (seven domestic goats, 12 Farasan gazelles) were analysed. All sampled goats were females; while seven male and one female gazelle were included in the analysis, (the sex of four gazelles was unknown). Apart from two subadult gazelles, all other animals were adults. Comparably few animals were infected with either type of parasites (coccidia (Eimeria), helminths). Therefore, the prevalence of eggs or oocysts in the faeces (present or absent in the sample) was used for statistical analyses. We used “host species” (goat or gazelle) as a factor effecting parasite load in a ‘two by two Chi-Square test’ (4-fold contingency table) to test for differences in the prevalence of the two parasite types. Furthermore, we tested whether the prevalence of coccidia (Eimeria) was reflected by a greater intensity of this parasite type amongst different species. The mean parasite intensity for goats (mean count/g for all infected animals) was calculated for each Eimeria species, and tested for significant differences using non-parametric Kruskal Wallis ANOVA on ranks (Sokal and Rohlf, 1981). A post hoc Student-Newman–Keuls test was used to isolate those species that differed from others. Due to the low level of parasite prevalence, intensities for gazelles were not determined.
3 Results
Helminth eggs of a Strongyle-type were present in only one female out of seven domestic goat samples (percentage prevalence: 14.29%), but their abundance was too small to quantify them using McMaster egg count chamber. All 12 gazelle samples were free of helminth eggs. There was a significant difference between the helminth prevalence in domestic goats and that in wild gazelles (x2 = 15.57, df = 1, p = 0.001).
Eimeria spp. was the dominant coccidian parasite in domestic goats living around Miharraq and Al-Qisar village on Farasan Kebir. Six out of the seven sampled goats were infected (percentage prevalence: 85.7%). For the sampled gazelle population, in only one sample out of 12, eimerian oocysts were detected (percentage prevalence: 8.33%). The proportion of single infection in goats was 57.1%, that of mixed infection 28.6%, while 14.3% were not infected at all. There was a significant difference between the Eimerian oocysts prevalence in domestic goats and that of wild gazelles (x2 = 9.61, df = 1, p = 0.01).
Since helminth prevalence in Farasan gazelles was on extremely low level, parasite intensity was only determined for coccidian parasites. Three Eimeria species were determined in domestic goat, i.e. E. carina (29.4 ± 3.36 × 22.8 ± 1.85 μm), E. arliongi (31.27 ± 1.89 × 22.91 ± 1.75 μm) and E. ninakohlyakimovae (16.5 ± 1.5 × 20 ± 1.02 μm).
The mean intensity of Eimeria spp. significantly differed between species (H = 6.09, df = 2, p = 0.047). A post hoc multiple comparison procedure (Student-Newman–Keuls test: p = 0.05) revealed that infection intensity of E. arliongi is distinctively higher (mean ± SE: 481.58 ± 199.61, range: 0–2400 opg) than that of E. ninakohlyakimovae (47.73 ± 33.53, range: 0–600 opg) or E. caprina (36.84 ± 36.84, range: 0–700 opg; Fig. 1). The oocysts’ abundance detected in gazelles was too low to determine intensity or species.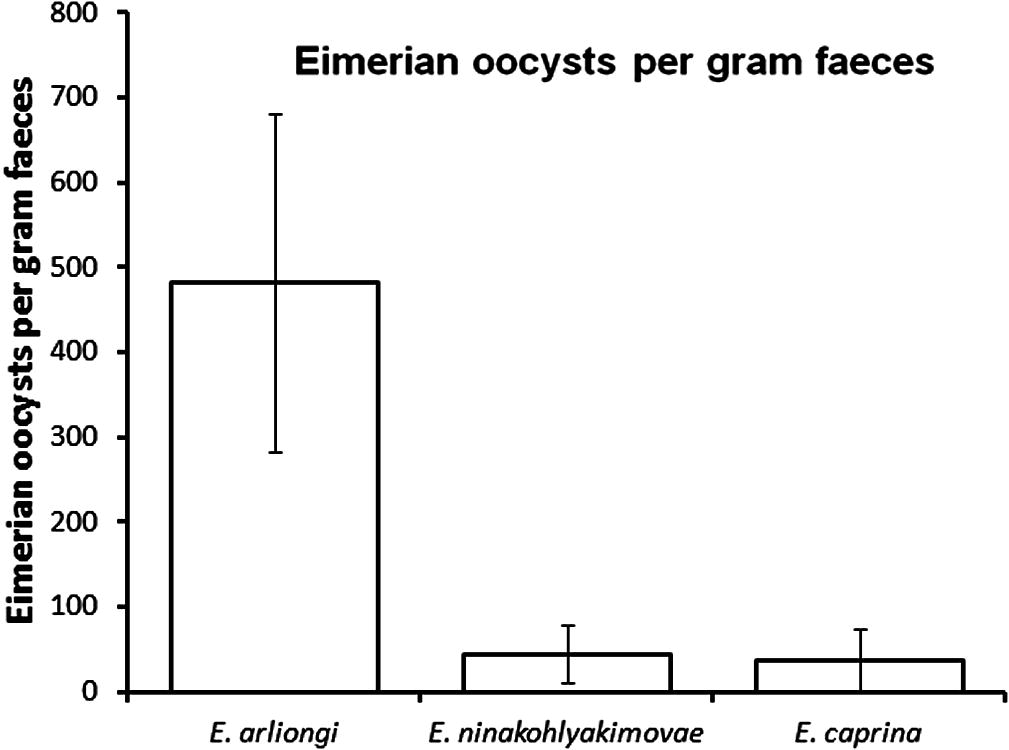
Intensity infection (oocysts per gram faeces; mean ± SE) of domestic goats (Capra hircus) with three Eimeria spp. in Miharraq area on Farasan Kebir.
4 Discussion
The effect of host species on the prevalence and intensity of gastrointestinal tract parasitic infections in domestic goats and wild Farasan gazelles was analysed. Small x2 values obtained from ‘two by two Chi-Square test’ indicate that prevalence of gastrointestinal tract parasites (coccidia and helminths) in domestic goats is distinctively higher than that of wild gazelles. That suggests a cross infection with gastrointestinal tract parasites in either direction (i.e., domestic goat to Farasan gazelles, or vice versa) could not be verified and is therefore, highly unlikely. This is even more surprising when considering that goats and gazelles have similar food preferences. A study on food preferences in the Ibex Reserve in central Saudi Arabia, where re-introduced gazelles browse together with domestic goats, indicates that both species use Acacia tortilis as their main food source (gazelle: 76.6%, goat: 88.8%; Wronski, unpubl. data).
Only one animal (adult female goat) was infected with strongyle nematodes, whereas Eimeria spp. was the most frequently occurring parasite in both host species. This low rate of infection may be attributed to the application of the flotation method which is suspected to not capture all eggs/oocysts in faecal samples. The FLOTAC has been recently developed as a novel multivalent faecal egg count (FEC) technique to measure the prevalence and intensity of helminth soil-transmitted infections for epidemiological surveys in humans (Knopp et al., 2009; Utzinger et al., 2008; Gringoli, 2004, 2006). For future analysis it would be therefore, advisable to use the FLOTAC method recommended by Gringoli, (2004, 2006). Amongst the different Eimeria species found in the faeces of domestic goat, E. arliongi was most frequent and distinctively more prevalent than E. caprina or E. ninakohlyakimovae. While Eimeria species are transmitted through water and (wet) soil, the risk of infection with strongyles and nematodes is lower, since infectious larval stages are ingested with the food during foraging. Infectious larvae need optimal conditions, i.e. persistent moisture on the vegetation in order to be able to reach edible parts of food plants before complete evaporation of moisture. Moisture is likely to positively influence the survival of free-living infectious nematode stages but less so the survival of sporulated oocysts, which persist in tiny puddles or moist soil long after the surrounding vegetation, has completely dehumified. Moreover, Eimeria spp. produces millions of oocysts per specimen, while strongyle nematodes shed comparatively few eggs (Soulsby, 1982). Thus, coccidian parasites may spread faster than the nematode parasites during the wet season. Such ecological conditions may explain the low prevalence of helminth infections in both the domestic goats and wild gazelles on Farasan Kebir.
The mean prevalence of infection by coccidian parasites observed in goats during this study was 71.43% and proves to be low compared to those reported by Alyousif et al., (1992) for goats from the central parts of Saudi Arabia. Most prevalent was E. arliongi in the study of Alyousif et al., (1992), which conforms to the findings of this study, with E. arliongi reaching the highest intensity of coccidian infection amongst domestic goats (Fig. 1). Compared to captive mountain gazelle (Gazella gazella) at the King Khalid Wildlife Research Centre in Saudi Arabia, the percentage prevalence of these parasites in Farasan gazelles was extremely low, which may possibly be due to some intrinsic factors related to the host and the patency of such parasites in these hosts. Mohammed, (2002) reported a mean prevalence of 34% E. idmii infections in captivity, while that observed on Farasan Kebir was only 8.3% for all Eimeria species.
The low level of infection by coccidian parasites observed in Farasan gazelles was similar compared to those reported for other browsing wild herbivores (Apio et al., 2006a). One possible explanation is that gazelles have exceptionally effective immune response mechanisms against these parasites. The ability of wild herbivores to overcome gastrointestinal tract infections and to tolerate light parasite burdens has been described by various authors (Boomker et al. 1984, 1986, 1987; Pullan et al., 1971). However, a more plausible explanation for the low degree of infection is that the foraging habits of gazelles, i.e. browsing at higher vegetation levels, keep the risk of infection to a minimum (Apio et al., 2006b). Surface water as a potential substrate for infectious parasites can be excluded since most time of the year no free water (except after a few days of heavy rain) is available on the Farasan Islands.
Although considerable parasite–host specificity has been reported for wild ungulates and their gastro-intestinal parasites (e.g. gazelles; Hussein and Mohammed, 1992; Mohammed, 2002; Mohammed and Hussein, 1992), the exchange of potentially pathogenic parasites between domestic and wild hosts presents a considerable risk to the health of wildlife populations (Coetzer et al., 1994; Grootenhuis 1986, 1999). On the other hand, several studies reported on disease transmission from wildlife species to domestic livestock (Bwangamoi, 1968; Ocaido et al., 1996, 2004) reducing the tolerance of livestock farmers towards wildlife. At least for the transmission of gastro-intestinal tract parasitic infections between domestic goats and gazelles on Farasan Island this seems to be not the case. However, a word of caution is required regarding the precision of parasite prevalence based on small sample sizes and mono-seasonal sampling as employed in this study. It must be emphasized that this survey represents a preliminary study to refute claims of local livestock keepers that Farasan gazelles are responsible for high parasite prevalence in domestic goats. The comparability of our results may therefore be limited, but given the immediate threat to the survival of Farasan gazelles, there is an urgent need to establish a cost- and time-effective survey method that will provide data for the management of domestic livestock and Farasan gazelles on the islands and that will disprove farmer’s claims. For future surveys it will be imperative to obtain larger sample sizes of both wildlife and domestic live stock species and to use more sensitive techniques such as FLOTAC in order to detect most of the eggs/oocysts present in the samples. This will allow robust management decisions such as a ban of domestic goats from the gazelle habitat and the removal of camels and feral donkeys from the protected area.
Acknowledgements
Our gratitude extends to His Highness Prince Bandar Bin Saud bin Mohammed Al Saud, President of the Saudi Wildlife Authority, Riyadh, Saudi Arabia for his continued support towards conservation efforts in Saudi Arabia and for permitting research in the Farasan Islands Protected Area. Our appreciation goes to Dr Ernest Robinson (former Director, King Khalid Wildlife Research Centre) for commenting on an earlier draft of this paper.
References
- Al Issa, M.A., Al-Zaftawi, F.M., Mustafa, N.M., 1985. Incidences of disease in slaughtered animals in Saudi Arabia. In: Proceedings of the 8th Symposium on the Biological Aspects of Saudi Arabia, King Faisal University – Al–Hassa, 12–14 March 1985, pp. 141–142.
- Coccidia of the domestic goat (Capra hircus) in Saudi Arabia. Int. J. Parasitol.. 1992;22:807-811.
- [Google Scholar]
- Anonymous, 1977. Manual of veterinary parasitological techniques, 2nd ed. Technical Bulletin 18 – Ministry of Agriculture, Fisheries and Food. Her Majesty’s Stationary Office.
- Patterns of gastrointestinal parasitic infections in the bushbuck Tragelaphus scriptus from the Queen Elizabeth National Park. Uganda. J. Helminthol.. 2006;80:213-218.
- [Google Scholar]
- Foraging height levels and the risk of gastro-intestinal tract parasitic infections of wild ungulates in an African savannah eco-system. Helminthologia. 2006;43:134-138.
- [Google Scholar]
- The helminths of various antelope species from Natal. Onderstepoort J. Vet. Med.. 1984;51:253-256.
- [Google Scholar]
- The helminth parasites of various artiodactylids from some South African nature reserves. Onderstepoort J. Vet. Med.. 1986;53:93-102.
- [Google Scholar]
- Parasites of South African wildlife. I. Helminths of bushbuck, Tragelaphus scriptus, and grey duiker, Sylvicapra grimmia, from the Weza State Forest. Natal. Onderstepoort J. Vet. Med.. 1987;54:131-134.
- [Google Scholar]
- Parasitologische Diagnostik: Teil 2, Eizählung und Larven-Differenzierung. Therapogen Praxisdienst. 1968;3:1-22.
- [Google Scholar]
- Helminth parasites of domestic and wild animals in Uganda. Bull. Epiz. Dis. Afr.. 1968;16:429-454.
- [Google Scholar]
- Onchocerciasis in camels (Camelus dromedarius) in Saudi Arabia. J. Helminthol.. 1984;58:279-285.
- [Google Scholar]
- Infectious Diseases of Livestock. Oxford: University Press; 1994. Vol. I and II
- Twenty years monitoring gazelles on Farasan Islands, Saudi Arabia: a review. Oryx. 2010;45:50-55.
- [Google Scholar]
- Dunham, K.M., Williamson, D.T., Joubert, E., 2001. Saudi Arabia, in: Mallon, D.P., Kingswood, S.C. (Eds.), Antelopes, Part 4: North Africa, the Middle East, and Asia. Global Survey and Regional Action Plan, IUCN, Gland, pp. 55–62.
- El-Bihari, S., Kawasmeh, Z.A., 1980. Occurrence and seasonal variation of some gastro-intestinal helminthes of the dromedary, Camelus dromedarius in Saudi Arabia. In: Proceedings of the 4th Symposium on the Biological Aspects of Saudi Arabia. Riyad University, 10–13 March 1980, pp. 297–304.
- Integrating health surveillance and environmental monitoring. In: Rapport D., Costanza R., Epstein P.R., Gaudet C., Levins R., eds. Ecosystem Health. Oxford: Blackwell Science; 1998. p. :154-177.
- [Google Scholar]
- The prevalence of some helminthic parasites and hepatic disorders in sheep, cattle and camels in Bureida. Proc. Saudi Biol. Soc.. 1984;7:337-339.
- [Google Scholar]
- Status of the gazelles of the Farasan Islands, Saudi Arabia. Mammalia. 1988;52:608-610.
- [Google Scholar]
- Hazards to rangelands in Saudi Arabia: importance of diseases via livestock. Fauna of Saudi Arabia. 1988;9:468-477.
- [Google Scholar]
- Zoonotic infections and conservation. In: Aguirre A.A., Ostfeld R.S., Tabor G.M., House C., Pearl M.C., eds. Conservation Medicine: Ecological Health in Practice. New York: Oxford University Press; 2002. p. :220-228.
- [Google Scholar]
- FLOTAC, a novel apparatus for a multivalent faecal egg count technique. Parasitologia. 2006;48:381-384.
- [Google Scholar]
- Grootenhuis, J.G., 1999. Twenty five Years of Wildlife Disease Research in Kenya. Kenya Agricultural Research Institute, Nairobi.
- Grootenhuis, J.G., 1986. Trypanosomiasis, East Coast fever and some other tick-borne diseases at the wildlife/livestock interface, In: Macmillan, S. (Ed.), Wildlife/livestock Interfaces on Rangelands. Inter-African Bureau for Animal Resources, Nairobi, pp. 6–62.
- Reproductive strategies of the Farasan gazelle. Gazella gazelle farasani. J. Arid Environ.. 1992;23:351-353.
- [Google Scholar]
- The epidemiology, diagnosis and control of gastrointestinal parasites of ruminants in Africa. Int. Lab. Res. Anim. Dis.. 1990;1:121.
- [Google Scholar]
- Hussein, S.H., Hussein, M.F., 1985. The prevalence and pathology of Haemonchus longistipes infection in Saudi Arabian camels (Camelus dromedarius). In: Proceedings of the 8th Symposium on the Biological Aspects of Saudi Arabia, King Faisal University – Al–Hassa, 12–14 March 1985, pp. 247–258.
- Eimeria rheemi sp. n. (Apicomplexa: Eimeriidae) from Saudi Arabian Sand Gazelle, Gazella subgutturosa marica (Artiodactyla: Bovidae) in Saudi Arabia. J. Helminthol. Soc. Wash.. 1992;59:190-194.
- [Google Scholar]
- Helminths community of veterinary importance of livestock in relation to some ecological and biological factors. Turk. J. Parasitol.. 2008;32:42-47.
- [Google Scholar]
- A single FLOTAC in more sensitive than triplicate Kato-Katz for the diagnosis of low intensity soil-transmitted helminth infections. Trans. R. Soc. Trop. Med. Hyg.. 2009;103:347-354.
- [Google Scholar]
- Kock, R.A., 2004. What is this infamous “Wildlife/Livestock interface?” A review of current knowledge on the subject. Proceedings of the AHEAD Workshop, World Parks Congress, Durban.
- Wildlife and pastoral society – shifting paradigms in disease control. Ann. NY Acad. Sci.. 2002;969:24-33.
- [Google Scholar]
- Mallon, D.P., Kingswood, S.C., 2001. Antelopes. Part 4: North Africa, the Middle East, and Asia. Global Survey and Regional Action Plan, IUCN.
- Mohammed, O.B., 1997. Parasites of Arabian gazelles. In: Abu-Zinada, A., Nader, I.A., Habibi, K. (Eds.), The Gazelles of Arabia. National Commission for Wildlife Conservation and Development, Riyadh, pp. 192–207.
- Mohammed, O. B., 2002. Control of gazelle parasites at King Khalid Wildlife Research Centre (KKWRC), Saudi Arabia. In: Proceedings of the World Association of Wildlife Veterinarians. 27th World Veterinarian Congress, Tunis, 26 September 2002, pp. 15–18.
- Experimental infection of Arabian sand gazelle, Gazella subgutturosa marica with Eimeria rheemi. J. Parasitol.. 1996;82:356-357.
- [Google Scholar]
- Eimeria idmii sp. N. (Apicomplexa: Eimeriidae) from the Arabian Mountain Gazelle, Gazella gazella, in Saudi Arabia. J. Helminthol. Soc. Wash.. 1992;59:120-124.
- [Google Scholar]
- Antibody prevalence of toxoplasmosis in Arabian gazelles and Oryx in Saudi Arabia. J. Wildl. Dis.. 1994;30:560-562.
- [Google Scholar]
- Disease surveillance in mixed livestock and area around Lake Mburo National Park in Uganda. S. Afr. J. Wildl. Res.. 1996;26:133-135.
- [Google Scholar]
- Helminth risks associated with mixed game and livestock interactions in and around Lake Mburo National Park. Uganda. Afr. J. Ecol.. 2004;42:42-49.
- [Google Scholar]
- Some helminths of bushbuck, waterbuck and sitatunga in Busoga district. Uganda. Bull. Epiz. Dis. Afr.. 1971;19:123-125.
- [Google Scholar]
- Outbreaks of coenurosis in sheep and goats in the Central Province of Saudi Arabia. Proc. Saudi Biol. Soc.. 1980;4:317-324.
- [Google Scholar]
- Biometry, the Principles and Practice of Statistics in Biological Research. New York: W. H. Freeman and Company; 1981.
- Soulsby, E.J.L., 1982. Helminths, Arthropods and Protozoa of Domestic Animals. Bailiére, Tindall, Philadelphia.
- FLOTAC: a new sensitive technique for the diagnosis of hook worms infections in humans. Trans. R. Soc. Med. Hyg.. 2008;102:84-90.
- [Google Scholar]
- Sex-difference in the communicatory significance of localized defecation sites in Arabian gazelles (Gazella arabica) J. Ethology 2013
- [CrossRef] [Google Scholar]







