Coumarin derivative, 5′-hydroxy-auraptene, extracted from Lotus lalambensis, displays antifungal and anti-aflatoxigenic activities against Aspergillus flavus
⁎Corresponding author at: Biological Sciences Department, College of Science, King Faisal University, P.O. Box: 380, Al-Ahsa 31982, Saudi Arabia. babdallallah@kfu.edu.sa (Basem M. Abdallah)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Pathogenic fungi are the primary infectious cause of plant diseases during pre- and post-harvest. Nuts, although recognized as a food with health benefits, are frequently contaminated by aflatoxins. In this study, we investigated the antifungal and anti-aflatoxigenic effects of our previously isolated phytochemical coumarin derivative, named (5′-hydroxy-aurapten) (5′-HA) from Lotus lalambensis Schweinf on Aspergillus flavus isolated from nuts. 5′-HA showed higher antifungal potential against A. flavus, by inhibiting its growth (MIC and MFC were 62.5 and 125 µg/l, respectively). 5′-HA also inhibited conidial germination of A. flavus by 60% at concentration 40 µg/ml. 5′-HA (40 µg/ml) displayed anti-aflatoxigenic activity against the production of aflatoxins, AFB1 and AFB2 by 50% and 23.3% respectively in association with the down-regulation of gene expression of early (aflA, alfB and alfC), middle (aflL, aflM and aflN) and late (aflP, aflQ and aflW) stages of aflatoxin biosynthesis pathway. 5′-HA (40 µg/ ml) resulted in the leakage of sugars from the mycelia by 235.8 μg/mL. Additionally, the extracellular conductivity in cells exposed to 5′-HA at 20, 40, and 80 µg/ml increased to values of 26.0 4, 43.2, and 55.1 μs/cm, respectively. Interestingly, 5′-HA significantly stimulated the activities of antioxidant enzymes, CAT (Catalase) and SOD (Superoxide dismutase) by 56.25% and 66.66% respectively. The anti-aflatoxigenic mechanism of 5′-HA was found to be mediated via modulating the oxidative stress process by upregulating the mRNA expression of the stress response transcription factors, atfA and atfB, by 2 and 2.5 folds respectively. In conclusion, our data identified 5′-HA as a novel natural antifungal compound against A. flavus that can be potentially applied to protect foodstuffs against aflatoxigenic fungi in agroindustry.
Keywords
Lotus lalambensis
Coumarin
5′-Hydroxy aurapten
Aspergillus flavus
Antifungal activity
Aflatoxin
Oxidative stress
1 Introduction
Nowadays, food losses are a main concern worldwide particularly with a growing world population. Every year, approximately 1.3 billion tons of food are wasted as identified by the Food and Agriculture Organization of the United Nations (FAO, 2017). The reasons for this huge universal food loss are varied, but microbial contamination, which affects organoleptic food quality plays an important role. Amongst spoilage microorganisms, fungal pathogens are a main subject at every stage of the food chain because of their capability to grow in different environments (Pitt and Hocking, 2009). Aflatoxigenic fungi can result in a reduction in production, a decrease of nutritional value, and a reduction of market value of food products (Ren et al., 2020). One of the most widely distributed mycotoxigenic fungi, which cause risk on human health, is Aspergillus flavus (Sheikh-Ali et al., 2014). A. flavus play a major role in contamination and degradation of both quality and quantity in several agricultural commodities (Mongalo et al., 2018). A. flavus is belonging to ascomycete that influences agriculture, public health as a result of production of extremely toxic, secondary metabolites including aflatoxins in seed crops (Sweany and Damann, 2019).
Nuts are recognized as a food with health benefits such as the prevention of coronary heart disease and diabetes (Diella et al., 2018). However, nuts are frequently contaminated with aflatoxins. 30.97 million tons of seed crops, mostly peanuts and pistachios, were polluted by A. flavus during or after harvesting, storage and transition and the occurrence of aflatoxins, produced by A. flavus, in nuts was confirmed in several studies (Lien et al., 2019). The need for protection of crops and feedstuffs against A. flavus species is commonly recognized and different strategies have reported to limit aflatoxin contamination including biological control or chemical control (Abbas et al., 2017).
The adverse environmental and health effects of some fungicides and preservatives have directed to more natural methods. Although the use of synthetic fungicides is a most effective control method along the agro-food chain, there is a crucial need to develop efficient and harmless non-fungicide means of monitoring microbes principally due to the poisonousness of the artificial fungicide remains which cause health problems (Zhu and Jiang, 2018). Recently, phytochemical control against pathogenic microbes was considered as sustainable alternative to synthetic agrochemical (Holmes et al., 2008).
Coumarin and its derivatives considered as one of the most active groups of compounds having an extensive range of biological actions, including, antimicrobial, anti-inflammatory, antioxidant, anticoagulant, and anticancer activities (Prashanth et al., 2018). In addition, several coumarin derivatives were reported to display antifungal capacity against A. flavus (Kovač et al., 2017).
We have recently isolated and purified a coumarin derivative named 7-(5-hydroxy-3,7-dimethylocta-2,6-dienyloxy)-chromen-2-one (5′-hydroxy-aurapten) for the first time from Lotus lalambensis Schweinf (Abdallah and Ali, 2019).
In this report, we studied the antifungal and anti-aflatoxigenic effects of 5′-HA against A. flavus isolated from nuts in Saudi Arabia.
2 Materials and methods
2.1 Fungal identification and isolation of A. flavus from nuts
Different fungal species were isolated from various types of nut products, which collected randomly from different markets (Al-Hassa, Saudi Arabia) during November and December 2018. Fungal species and identification method were provided in Supplementary M&M and table S1.
2.2 Extraction and purification of 5′-HA
5′-Hydroxy-aurapten (5′-HA) was extracted and purified from Lotus lalambensis Schweinf as previously described by our group (Abdallah and Ali, 2019) (For details, see supplementary M&M). A stock solution of coumarin (100 mg/ml) was prepared in DMSO and then diluted to obtain the concentrations (20–80 µg/ml).
2.2.1 Commercial coumarin compounds
Bergapten, herniarin, xanthotoxin, umbelliferone, and scopoletin (Fluka Chemie AG, Switzerland) were prepared in methanol to give a concentration of 1 mg/mL.
2.3 Antifungal susceptibility
2.3.1 Determination of minimum inhibitory concentration of 5′-HA
MIC was evaluated using broth micro dilution techniques according to CLSI. Compounds with MICs less than or equal to 250 μg/mL were considered.
Amphotericin B (Sigma Chem. Co.) was used as a positive control. Endpoints are known as the lowermost concentration of compound that results in complete growth inhibition when compared to control growth which does not contain antifungal.
2.3.2 MFC determination
Fungicidal activities (minimum fungicidal concentration, MFCs) were determined after 48 h incubation as described (Espinel-Ingroff et al., 2001). The plates were incubated for 72 h and MFCs were determined as the lowermost antifungal concentration which inhibited 99% of the inoculum.
2.3.3 Effect of the 5′-HA on conidial germination of A. flavus
Sporicidal activity of 5′-HA were conducted using spore germination assay according to (Das et al., 2010). A haemocytometer slide was used to count spores production to have approximately 108 spore/mL. 20–80 µg/ml of 5′-HA was prepared in 5 mL of sabouraud dextrose broth medium (Biokar, Beauvais, and France) in 100 mL flask and then 1 mL of the spore suspension were added to each flask. Germinated spores were observed using a light microscope at 400× magnification. Results were expressed in terms of the percentage of spores germinated as compared to the control.
2.3.4 Scanning electron microscopic examinations
The SEM technique was carried out at Electron Microscope Unit, Faculty of agriculture, Cairo Univ., Cairo, Egypt as following: Mycelia of A. flavus grown in Czapek’s broth medium treated without or with 40 µg/ml 5′-HA were fixed in 2.5% glutaraldehyde. The specimens finally sputter coated with gold.
2.4 Effect of 5′-HA on aflatoxin production
2.4.1 Microorganisms and preparation of the spore solution
A. flavus ATCC 22546 was grown on malt extract agar (MEA: Difco Laboratories, Sparks, MD, USA) to induce aflatoxin B1 and B2. Different concentrations of 5′-HA (20–80 µg/ml) were spiked to the corresponding liquid media and cultures were incubated at 25 °C for 5 days under shaking conditions.
2.4.2 HPLC analysis
HPLC was carried out on Hitachi D-7000 interface equipped with L-7485 fluorescence detector, L-7100 pump, L-7200 auto sampler, L-7300 column oven, and post-column photochemical reaction system (Cairo, Egypt).
2.5 Measurement of cellulase and pectinase enzyme activities in A. flavus
Growth of the A. flavus were exposed to 20–80 µg/ml of 5′-HA. One ml spore suspension of A. flavus were inoculated in conical flasks containing either carboxymethyl cellulose or pectin medium mixed with different concentration of 5′-HA. The cellulolase and pectinase activities were determined by the viscometric method (El-naghy et al., 1991).
2.6 Effects of 5′-HA on extracellular conductivity
The extracellular conductivity of A. flavus cells were determined by a conductivity meter at 0, 30, 60, 90 and 120 min of treatment after the addition of the 5′-HA at (20–80 µg/ml). Values were expressed as the amount of extracellular conductivity (μs/cm).
2.7 Measurement of sugar leakage
The leakage of sugars from mycelium of A. flavus was assayed according to previously published method (Li et al., 2018). The total sugars was determined by anthrone-sulfuric acid method (Moshayedi et al., 2013).
2.8 Measurement of antioxidant enzymes
Prior to determination of antioxidant enzymes, the antioxidant activity of 5′-HA was evaluated by measuring DPPH radical scavenging activity according to the method of (Choi et al., 2002). The measurements of the antioxidant enzymes were described in supplementary M&M.
2.9 RNA isolation and real-time PCR analysis
Total RNA was extracted from Fungal mycelia using the RNAeasy Plant Mini Kit (Qiagen, Hilden, Germany) following the manufacturer’s instructions (Chang et al., 2011). QPCR was performed using Fast SYBR® Green Master Mix with Applied Biosystems 7500 Real-Time system (Applied Biosystems, California, USA) according to manufacturer’s instructions (Abdallah and Ali, 2019) with primer sequences in supplementary Table S3 (Baidya et al., 2014). The expression of each target gene was normalization to β-tubulin mRNA expression as reference gene. Relative expression level of each target gene was calculated using a comparative CT method [(1/(2delta-CT) formula.
2.10 Statistical analysis
The data were described as mean ± standard error (SD) of 3 experiments. Power calculation was performed for 2-samples using unpaired Student’s T-test (2-tailed) assuming equal variation in the two groups. Differences were considered statistically significant at *P < 0.05, and **P < 0.005.
3 Results
3.1 Isolation and identification of A. flavus
The data recorded in supplementary table S1, showed the microbiological quality of different nut products gathered from different shops in Al-Hassa, Saudi Arabia.
Interestingly, A. flavus was the most prominent fungus isolated from all the nut samples, it was used in further experiments to investigate mode of action of 5′-HA. A. flavus identified based on molecular techniques to confirm the morphological identification. Amplification of the genomic DNA was carried out using PCR with sets of universal primers for fungi. The nucleotide sequences were determined by the sequencing of the DNA [GenBank: LC096956.1]. After analysis by BLAST, fungus-specific amplicons exhibited 99% identity with A. flavus [GenBank: EU982151.1] and; A. flavus [GenBank: EU982162.1].
3.2 Antifungal activity of 5′-HA
5′-HA (Fig. 1A) and other coumarin compounds showed antifungal activities that retained both fungistatic and fungicidal capabilities (Table 1). Interestingly, 5′-HA was the most effective compound, while herniarin and umbelliferone showed the lowest activity.
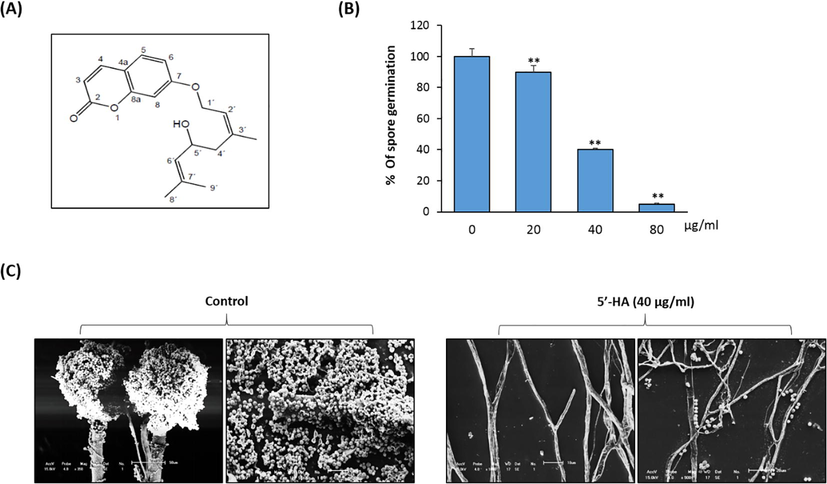
- A Effect of different concentrations of 5′-HA on percentage of spore germination of A. flavus. (A) Chemical structure of 5′-HA. (B) The spore germination rates of A. flavus treated with 5′-HA at different concentrations 20–80 µg/ml. Untreated spores served as the control. Values are the averages of the replicates for all the analyses. In some cases the error bar is obscured by the symbol. (C) Scanning electron microscopy of A. flavus after treatments with 5′-HA at concentration 40 µg/ml. Morphological changes induced in A. flavus were investigated by Scanning Electron Microscope, scale bars = 20 μm. Values are mean ± SD of three independent experiments, (**p < 0.005 compared to control without 5′-HA).
| Compound or extract | Reference drug | ||||||
|---|---|---|---|---|---|---|---|
| Fungus | 5′-HA * | Herniarin | Bergapten | umbelliferone | Scopoletin | xanthotoxin | Amphotericin |
| Aspergillus flavus | 62.5/125 | >1000 | 125/250 | >1000 | 500/500 | >1000 | 0.025 |
| Aspergillus niger | 125/250 | >1000 | 500/500 | >1000 | >1000 | >1000 | 0.125 |
| Penicillium chrysogenum | 250/250 | >1000 | >1000 | >1000 | >1000 | >1000 | 0.5 |
| Penicillium citrinium | 62.5/125 | >1000 | 125/250 | >1000 | 500/500 | >1000 | 0.075 |
| Alternaria alternate | 125/250 | >1000 | 500/500 | >1000 | 500/500 | 750 | 0.125 |
| Fusarium moniliforme | 500/500 | >1000 | >1000 | >1000 | >1000 | >1000 | 0.5 |
| Rhizopus oryzae | 500/500 | >1000 | >1000 | >1000 | >1000 | >1000 | 1 |
5′-HA *: Purified phytochemical coumarin from root of Lotus lalambensis.
5′-HA reduced the percentage of spore germination in a dose-dependent manner (40% inhibition of spores at 40 µg/ml) (Fig. 1B). Colonies grown on solid media in the presence 40 µg/ml of 5′-HA displayed morphological changes as compared with the controls. Examination by SEM (Fig. 1C) revealed the suppression of A. flavus conidiophore production. Our results revealed, that hyphae were the main targets of the 5′-HA. SEM showed that in the presence of 40 µg/ml 5′-HA, treated A. flavus had shorter hyphae and wrinkling of the cell surface and emptying of the cytoplasmic content, which were not detected on untreated hyphae (Fig. 1C).
3.3 5′-HA inhibits aflatoxin production by A. flavus
As shown in Fig. 2A&B, 5′-HA inhibited the production of aflatoxin, AFB1 and AFB2 by A. flavus in a dose-dependent manner. The highest inhibition of aflatoxins production (50 and 23.3% for AFB1 and AFB2, respectively) observed at 40 µg/ml 5′-HA while, 80 µg/ml 5′-HA resulted in complete inhibition of aflatoxins production in A. flavus (Fig. 2A&B).
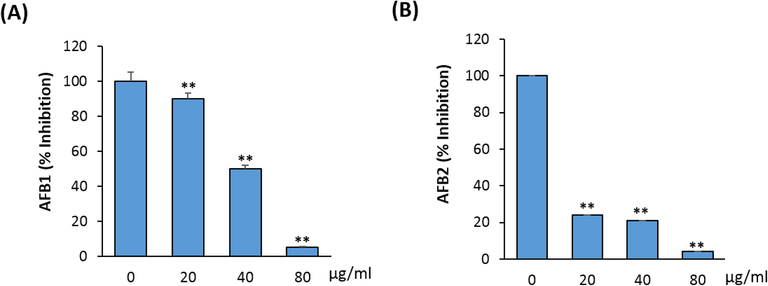
- Effect of different concentrations of 5′-HA on Aflatoxin production (µg/g dry mass). Influence of 5′-HA on A. favus aflatoxin production (A) AFB1 and (B) AFB2, in the culture filtrates during 168 h of incubation at 29° C. Control (0) was induced with DMSO instead of 5′-HA. Aflatoxin concentration is determined in µg aflatoxin per g mycelial dry weight. Values are mean ± SD of three independent experiments, (**p < 0.005 compared to control without 5′-HA).
3.4 Effect of 5′-HA on the membrane permeability of A. flavus
As shown in Fig. 3A, 5′-HA stimulates the leakage of sugars from the mycelia in dose-dependent manner. The maximum amount of leakage of sugars (235.8 μg/mL) was achieved with 40 µg/ ml 5′-HA while, 80 µg/ml 5′-HA resulted in complete inhibition of growth of A. flavus.
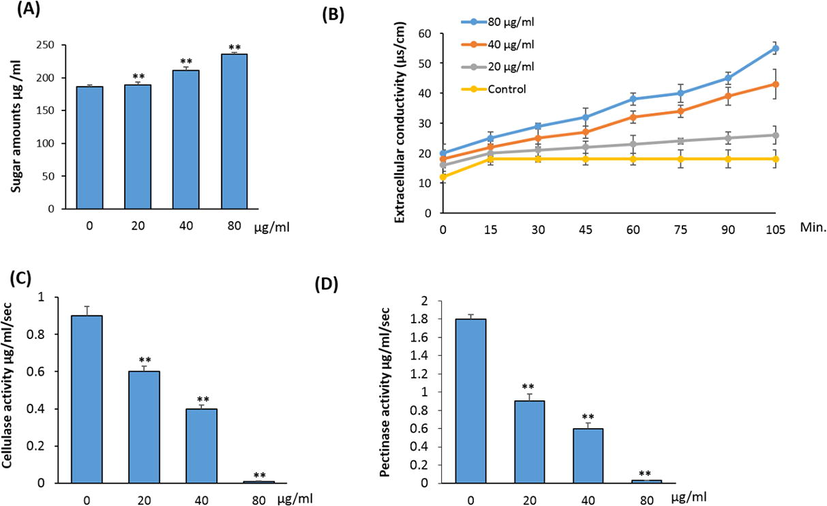
- Effect of 5′-HA on membrane permeability of A. flavus. Effect of different concentrations of 5′-HA on (A) sugar leakage (μg/ml); (B) extracellular conductivity (µs/ cm); C) cellulase activity (µg/ml/sec); (D) pectinase activity (µg/ml/sec) of A. flavus. A. flavus was cultured in basal medium mixed with different concentrations of 5′-HA (20–80 µg/ml) or in basal media without 5′-HA as the control at 25 °C. Control (0) was induced with DMSO instead of 5′-HA. The absorbance recorded at 540 nm. The activity of enzyme is expressed as mmol of the released glucose per sec-1 mL-1 of culture filtrate as enzyme solution. Values are mean ± SD of three independent experiments, (**p < 0.005 compared to control without 5′-HA).
In addition, an immediate and considerable increase in the extracellular conductivity was observed upon treatment with different 5′-HA concentrations (Fig. 3B). The extracellular conductivity increased as exposure time increased. After 105 min of treatment, conductivity in cells exposed to 5′-HA at 20, 40, and 80 µg/ml increased, to values of 26.0 4, 43.2, and 55.1 is/cm, respectively (Fig. 3B).
3.5 Effect of 5′-HA on cellulase and pectinase activities of A. flavus
Enzymes that attack pectic and cellulosic substances in the plant cell wall play a major role in pathogenicity. 5′-HA significantly decreased the cellulase and pectinase activities of A. flavus and the A. flavus failed to grow with 80 µg/ml 5′-HA (Fig. 3C&D).
3.6 Antioxidant activity of 5′-HA
Interestingly, 5′-HA showed higher antioxidant activity (IC50 = 72.45 μg/mL), than L-ascorbic acid (IC50 = 52.65 μg/ mL) and the free radical scavenging activity of the 5′-HA was found to be concentration-dependent. However, the L-ascorbic acid displayed higher antioxidant activity than 5′-HA at a lower 161 concentration (supplementary table S2). We further, studied the effect of 5′-HA on the activities of the antioxidant enzymes; catalase (CAT) and superoxide dismutase (SOD). The results revealed the stimulatory effect of 5′-HA of the activities of CAT and SOD enzymes by 56.25% and 66.66% respectively (Fig. 4A&B). These data suggested that 5′-HA modulates the oxidative stress by increasing the activity of the antioxidant enzymes CAT and SOD.
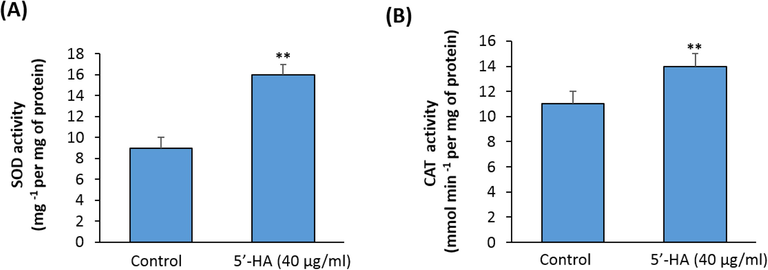
- Effect of 5′-HA on antioxidant enzymes activities. Effect of 5′-HA (40 µg/ ml) on antioxidant enzymes activities: (A) cytosolic superoxide dismutase activity; (B); total catalase activity Absorbance was measured at 540 and 450 nm for catalase and superoxide dismutase assays respectively. Control was induced with DMSO instead of 5′-HA. Values are mean ± SD of three independent experiments, (**p < 0.005 compared to control without 5′-HA).
3.7 5′-HA down-regulates the aflatoxins biosynthesis cluster genes
The aflatoxin biosynthesis pathway was reported to be controlled by 27 clustered genes (Georgianna and Payne, 2009). As shown in Fig. 5A, 5′-HA (40 µg/ml) significantly downregulated the transcriptional factors aflR and aflS, which are involved in the activation and regulation of aflatoxin biosynthesis by 36% and 44% respectively. The inhibition of aflatoxin production by 5′-HA was associated with a dramatic suppression of the early genes involved in polyketide formation in the biosynthesis cascade including aflA, alfB and alfC, by 73%, 77% and 72%, respectively. In addition, 5′-HA significantly downregulated the expression of genes involved in the middle (aflL, aflM and aflN) and late (aflP, aflQ and aflW) stages of the aflatoxin biosynthesis pathway.
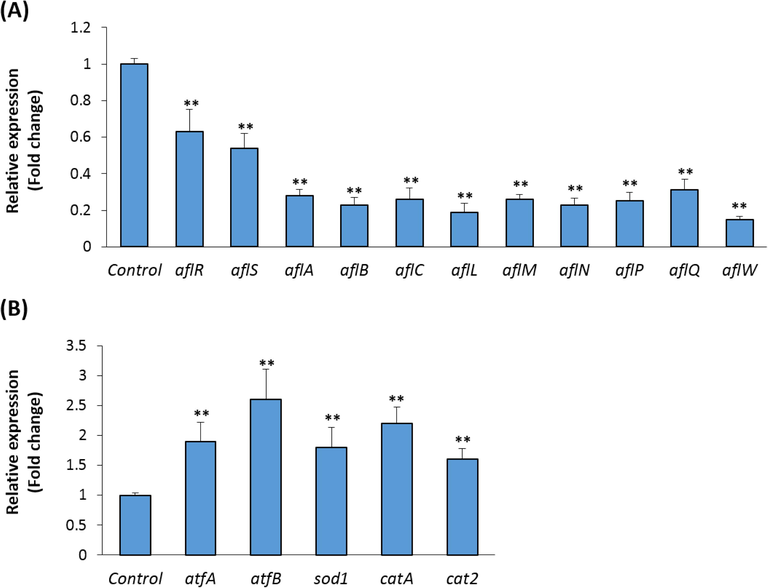
- Effect of 5′-HA on mRNA expression of aflatoxin biosynthesis cluster genes and antioxidant-related genes. (A) Quantitative real time RT-PCR (qPCR) analysis of the mRNA expression of aflatoxin biosynthesis cluster genes and (B) antioxidant-related genes in A. flavus after exposure to 5′-HA (40 µg/ml). Control was induced with DMSO instead of 5′-HA. mRNA expression of each target gene was represented as fold change over control after normalization to reference genes as described in M&M. Values are mean ± SD of three independent experiments, (**p < 0.005 compared to control without 5′-HA).
3.8 5′-HA modulates the expression of oxidative stress-related genes
The addition of 5′-HA to the culture medium of A. flavus upregulated the mRNA expression of genes coding for the stress response transcription factors, atfA and atfB, which are known to couple the oxidative stress response and aflatoxin biosynthesis (Hong et al., 2013) (Fig. 5B). Consistent with our biochemical data, 5′-HA increased the expression levels of genes encoding for the antioxidant enzymes catalase (catA and cat2) and superoxide dismutase (sod1) by factors of 2.3, 1.6 and 1.7, respectively, as assessed by qPCR analysis. These data suggested that the inhibitory effect of 5′-HA on aflatoxin biosynthesis in A. flavous is mediated at least in part by modulating the oxidative stress process.
4 Discussion
Phytochemicals extracted from plants are natural alternatives of interest to be used in food as bio preservatives tools to control fungal damage. In this report, we studied the antifungal effect of 5′-HA isolated from Lotus lalambensis against A. flavus. We were the first to extract 5′-HA from the roots of Lotus lalambensis (Abdallah and Ali, 2019). The genus Lotus, belonging to family Fabaceae, contains more than 100 species distributed throughout the world (Abdel-Kader, 2013) with antimicrobial activities against Gram-positive and Gram-negative bacteria and fungi (Mahasneh, 2002).
The antifungal activity of coumarin derivatives has been established in several studies (supplementary table S4) (Kovač et al., 2017, Zhu and Jiang, 2018). 5′-HA showed to exert an inhibitory effect against A. flavus growth by 60% at concentration of 40 µg/ml. Similarly, Kovač et al. (2017) revealed that a caumarin derivative, 2-(2-((4-methyl-2-oxo-2H-chromen-7-yl) oxy) acetyl)-N-(2, 4, 6-trichlorophenyl) hydrazine-1-carbothioamide, exhibited the highest antifungal activity of 45.86% against A. flavus at concentration 10 µg/ml (Kovač et al., 2017). Moon et al. (2017) reported that 4-hydroxy-7-methoxy-3-phenylcoumarin and 4-hydroxy-6, 7-dimethylcoumarin displays approximately 50% inhibition on the A. flavus growth at the concentration of 100 µg/mL (Moon et al., 2017). 6-Hydroxycoumarin showed MIC values higher than 500 μg/ml against A. fumigatus (Montagner et al., 2008). Guerra et al. (2018) studied the effect of 4-acetatecoumarin (Cou-UMB16) on A. fumigatus ATCC 46913 and A. flavus ATCC 16013, concentrations of Cou-UMB16 at 16 g/mL (MIC), and 32 g/mL (MIC × 2) showed to inhibit mycelial growth of normal strains when compared to the control (Guerra et al., 2018). In addition, Shaikh et al. (2018) tested the antifungal activity of some 2,3‐Dihydro Flavone Coumarin against Candida albicans, Aspergillus flavus and Aspergillus niger, they found that the compounds have displayed potent in vitro power with low MIC values ranges from 0.4 to 3.12 μg/mL (Shaikh et al., 2018). The antifungal effect of coumarin compounds was reported to be due to the inhibitory effect of coumarin on cellular enzymes that rely on the dissemination rate of the substance in the cell, or it may be triggered by changes in the permeability of the cell membrane (Ayine-Tora et al., 2016). For example, 4-acetatecoumarin (Cou-UMB16) showed antifungal activity against A. flavus by forming pores in the cell wall, with consequent release of cytoplasmic contents and cell death (Guerra et al., 2015). In consistent, our data demonstrated the potent antifungal properties of 5′-HA against A. flavus by affecting membrane permeability and decreasing cellulase and pectinase enzyme activities in the cell wall. Other mechanisms were also reported for mediating the antifungal activity of coumarin including, the alteration of the fungal mitochondrial cell morphology, the induction of apoptosis, and the activation of ROS accumulation and mitochondrial dysfunction (Jia et al., 2019).
Our data showed the inhibitory effect of 5′-HA on the production of aflatoxigenic AFB1 and AFB2 by A. flavus. Similarly, Kovač et al. (2017) showed that N-(4-chlorophenyl)-2-(2-((4-methyl-2-oxo-2H-chromen-7-yl) oxy) acetyl) hydrazine-1-carbothioamide, completely inhibits aflatoxin production at the concentration of 10 µg mL-1 (Kovač et al., 2017). In addition, (Moon et al., 2017) showed that 4-hydroxy-7-methoxy-3-phenylcoumarin displayed powerful inhibitory actions on AFB1 and AFB2 production at a concentration of 1 µg/mL while 4-hydroxy-6,7-dimethylcoumarin displayed powerful inhibitory effects on AFB1 and AFB2 production at a concentration of 10 µg/mL (Moon et al., 2017). It is uncertain whether the inhibition of aflatoxins production by coumarin is triggered by its effect on growth or on aflatoxins biosynthesis. The structural similarity of coumarins and aflatoxin intermediates; resulted in competitive inhibition of the biosynthesis of aflatoxin enzymes (Kurt et al., 2017). Our data demonstrated that, the anti-aflatoxigenic properties of 5′-HA were found to be mediated at the transcriptional level by inhibiting the expression of genes involved in the aflatoxin biosynthesis pathway and the oxidative stress activation. The aflatoxin biosynthesis pathway in fungi is controlled by 27 clustered genes encoding for enzymes along with two positive-acting master transcriptional regulators, aflR and aflS (Ehrlich et al., 2005, Georgianna and Payne, 2009). Our data demonstrated, that the exposure of A. flavus to 5′-HA resulted in down-regulation of the expression of the two main transcriptional activator genes, aflR and aflS, and consequently the downregulation of the entire genes of aflatoxin biosynthesis pathway. A similar molecular inhibitory mechanism preventing the biosynthesis of aflatoxin in A. flavus has been described for the coumarin derivative; 4-hydroxy-7-methyl-3-phenyl coumarin (Moon et al., 2017), as well as other compounds, such as the aqueous extract of hyssop (El Khoury et al., 2017), eugenol and 4-allyl-2-methoxyphenol (Caceres et al., 2016).
Several lines of evidence have suggested a positive correlation between levels of oxidative stress and the production of aflatoxin in different Aspergillus species (Hong et al., 2013, Reverberi et al., 2008). Stimulation of anti-oxidation processes in fungi (Reverberi et al., 2008) and applying naturally occurring anti-oxidant were demonstrated to inhibit aflatoxin production (Patil et al., 2010). For example, piperine, a major component of black and long peppers was reported to exert its anti-aflatoxigenic effect on A. flavus by modulating the oxidative stress response at both the biochemical and the molecular levels (El Khoury et al., 2017). Our data showed that the antioxidant activity of 5′-HA is associated with stimulation of the mRNA expression levels of the stress-related transcription factors atfA and atfB. Both atfA and atfB, that belong to the ATF/CREB family and are involved in coupling response to oxidative stress with the secondary metabolite production in fungi. Several genes related to antioxidant activity including cat and sod genes as well as the aflatoxin production genes were found to have CRE-binding motifs for atfA and atfB in their promoter regions (Hong et al., 2013). Similarly, we demonstrated the upregulation of the expression levels of sod1, catA and cat2. Thus, the upregulation of both atfA and atfB expressions and their antioxidant targets by 5′-HA could be directly linked to its inhibitory effect on aflatoxin.
Our data demonstrated cell membrane disruption of A. flavus by 5′-HA is due to its effect on increasing the leakage of sugars and the extracellular conductivity. Increasing the leakage of carbohydrates and electrolytes from the fungal hyphae deprives them of the vital metabolites required for regular fungal growth (Shievly and Mathre, 1971). In consistence, synthesized coumarin was found to increase the lipophilicity of the fungal cell membrane and consequently to exert an antifungal effect (Al-Amiery et al., 2012).
5 Conclusion
Our results identified 5′-HA as safe, effective, and inexpensive natural product that reduces the presence of aflatoxigenic A. flavus isolated from nuts. The antifungal effect of 5′-HA was found to possess potent anti-aflatoxigenic and antioxidant activities against A. flavus. Biochemical and molecular studies revealed that the mode of action of 5′-HA is mediated through repression of oxidative stress in A. flavus and the dramatic downregulation of the expression of the entire aflatoxin biosynthesis cluster genes. Thus, 5′-HA is a novel identified phytochemical with antifungal and anti-aflatoxigenic potentials, that can be developed into promising natural preservatives to protect nuts and other foodstuffs against A. flavus infection.
Conflict of interest
The authors declare that they have no competing interests.
Author contributions
EMA conceived the project, designed the study, performed experiments, analyzed data and wrote the manuscript. MAA and MFA performed experiments and analyzed data. BMA designed the study, performed experiments, and revised and edit the manuscript.
Funding
This work was funded by the Deanship of Scientific Research at King Faisal University, Saudi Arabia, under Nasher Track [Grant no. 186010].
Acknowledgments
The Authors acknowledge the Deanship of Scientific Research at King Faisal University, Al-Ahsa for the financial support under Nasher Track [Grant no. 186010].
References
- Biological control of aflatoxin contamination in U.S. crops and the use of bioplastic formulations of Aspergillus flavus biocontrol strains to optimize application strategies. J. Agric. Food Chem.. 2017;65:7081-7087.
- [Google Scholar]
- 5′-hydroxy Auraptene stimulates osteoblast differentiation of bone marrow-derived mesenchymal stem cells via a BMP-dependent mechanism. J. Biomed. Sci.. 2019;26:51.
- [Google Scholar]
- Two isoflavans and a 3-arylcoumarin from the roots of Lotus lalambensis growing in Saudi Arabia. Rev. Latinoam. Quim.. 2013;41:61.
- [Google Scholar]
- Coumarin antifungal lead compounds from Millettia thonningii and their predicted mechanism of action. Molecules. 2016;21
- [Google Scholar]
- VeA is associated with the response to oxidative stress in the aflatoxin producer Aspergillus flavus. Eukaryot. Cell. 2014;13:1095-1103.
- [Google Scholar]
- Deciphering the anti-aflatoxinogenic properties of eugenol using a large-scale q-PCR approach. Toxins (Basel). 2016;8:123.
- [Google Scholar]
- Loss of msnA, a putative stress regulatory gene, in Aspergillus parasiticus and Aspergillus flavus increased production of conidia, aflatoxins and kojic acid. Toxins (Basel). 2011;3:82-104.
- [Google Scholar]
- Antioxidant activity and free radical scavenging capacity between Korean medicinal plants and flavonoids by assay-guided comparison. Plant Sci.. 2002;163:1161-1168.
- [Google Scholar]
- Das, K., Tiwari, R., Shrivastava, D., Bilaspur, B.C. 2010. Techniques for evaluation of medicinal plant products as antimicrobial agent: Current methods and future trends.
- Aflatoxin contamination in nuts marketed in Italy: preliminary results. Ann. Ig.. 2018;30:401-409.
- [Google Scholar]
- Aflatoxin biosynthesis gene clusters and flanking regions. J. Appl. Microbiol.. 2005;99:518-527.
- [Google Scholar]
- Degradation of cellulosic materials by Sporotrichum thermophile culture filtrate for sugar production. Int. Biodeterioration. 1991;27:75-86.
- [Google Scholar]
- Identification of the anti-aflatoxinogenic activity of Micromeria graeca and elucidation of its molecular mechanism in Aspergillus flavus. Toxins (Basel). 2017;9
- [Google Scholar]
- In vitro antifungal activities of voriconazole and reference agents as determined by NCCLS methods: review of the literature. Mycopathologia. 2001;150:101-115.
- [Google Scholar]
- Genetic regulation of aflatoxin biosynthesis: from gene to genome. Fungal Genet. Biol.. 2009;46:113-125.
- [Google Scholar]
- A new coumarin derivative, 4-acetatecoumarin, with antifungal activity and association study against Aspergillus spp. Brazilian J. Microbiol.. 2018;49:407-413.
- [Google Scholar]
- Evaluation of antifungal activity and mode of action of new coumarin derivative, 7-hydroxy-6-nitro-2H-1-benzopyran-2-one, against Aspergillus spp. Evid. Based Complement. Alternat. Med.. 2015;2015:925096
- [Google Scholar]
- Diverse inhibitors of aflatoxin biosynthesis. Appl. Microbiol. Biotechnol.. 2008;78:559-572.
- [Google Scholar]
- Evidence that a transcription factor regulatory network coordinates oxidative stress response and secondary metabolism in aspergilli. Microbiologyopen. 2013;2:144-160.
- [Google Scholar]
- Antifungal activity of coumarin against Candida albicans is related to apoptosis. Front. Cell. Infect. Microbiol.. 2019;8
- [Google Scholar]
- Antifungal and antiaflatoxigenic activities of coumarinyl thiosemicarbazides against Aspergillus flavus NRRL 3251. Arh Hig Rada Toksikol. 2017;68:9-15.
- [Google Scholar]
- Synthesis and biological evaluation of novel coumarin-chalcone derivatives containing urea moiety as potential anticancer agents. Arabian J. Chem. 2017
- [Google Scholar]
- Ethyl p-coumarate exerts antifungal activity in vitro and in vivo against fruit Alternaria alternata via membrane-targeted mechanism. Int. J. Food Microbiol.. 2018;278:26-35.
- [Google Scholar]
- Assessing aflatoxin exposure risk from peanuts and peanut products imported to taiwan. Toxins (Basel) 2019:11.
- [Google Scholar]
- Screening of some indigenous Qatari medicinal plants for antimicrobial activity. Phytother. Res.. 2002;16:751-753.
- [Google Scholar]
- Antifungal, anti-oxidant activity and cytotoxicity of South African medicinal plants against mycotoxigenic fungi. Heliyon. 2018;4:e00973
- [Google Scholar]
- 4-Hydroxy-7-methyl-3-phenylcoumarin suppresses aflatoxin biosynthesis via downregulation of aflK expressing versicolorin B synthase in Aspergillus flavus. Molecules. 2017;22
- [Google Scholar]
- Moshayedi, S., Shahraz, F., Schaffner, D., Khanlarkhani, A., Shojaee-Aliabadi, S., Shahnia, M., Khaksar, R. 2013. In vitro control of Enterococcus faecalis by Zataria multilfolira Boiss, Origanum vulgare L and Mentha pulegium essential oils.
- Antiaflatoxigenic and antioxidant activity of an essential oil from Ageratum conyzoides L. J. Sci. Food Agric.. 2010;90:608-614.
- [Google Scholar]
- Fungi and Food Spoilage. Springer; 2009.
- Prashanth T, A Begum B, Fatima Khanum N, Khanum S. 2018. Synthesis and characterization of coumarin analogs: evaluation of antimicrobial and antioxidant activities.
- Control of aflatoxigenic molds by antagonistic microorganisms: inhibitory behaviors, bioactive compounds, related mechanisms, and influencing factors. Toxins (Basel). 2020;12:24.
- [Google Scholar]
- Modulation of antioxidant defense in Aspergillus parasiticus is involved in aflatoxin biosynthesis: a role for the ApyapA gene. Eukaryot. Cell. 2008;7:988-1000.
- [Google Scholar]
- Synthesis of 2, 3-dihydro flavone coumarins as a class of potent antifungal and anti-inflammatory agents. ChemistrySelect. 2018;3:3451-3458.
- [Google Scholar]
- The potential hazards of Aspergillus sp. in foods and feeds, and the role of biological treatment: a review. J. Microbiol.. 2014;52:807-818.
- [Google Scholar]
- Mode of action of oxathin systemic fungicides. IV. Effect of carboxin on solute leakage from hyphae of Rhizoctonia solani. Can. J. Microbiol.. 1971;17:1465-1470.
- [Google Scholar]
- Influence of neighboring clonal-colonies on aflatoxin production by Aspergillus flavus. Front. Microbiol.. 2019;10:3038.
- [Google Scholar]
- Pharmacological and nutritional effects of natural coumarins and their structure-activity relationships. Mol. Nutr. Food Res.. 2018;62:1701073.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2020.10.013.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







