Translate this page into:
Corrosion behaviour of metal complexes of antipyrine based azo dye ligand for soft-cast steel in 1 M hydrochloric acid
⁎Corresponding author. devikabg2018@gmail.com (B.G. Devika)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
In this communication the corrosion behaviour of three metal [Co(II), Ni (II) and Fe (III)] complexes of Antipyrine based azo dye ligand [5-[(1,5-dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-yl)diazenyl]-6-hydroxy-1,4-dimethyl-2-oxo-1,2-dihydropyridine-3-carbonitrile](L) for soft-cast steel in 1 M hydrochloric acid solution was discussed by experimental and theoretical methods. The study reveals that the ligand and their metal complexes show good inhibition efficiency. Here Ni complex shows significant inhibition efficiency at an optimized concentration of 25 mg/L. The quantum studies strengthen the experimental results of capacity of the ligand and their metal complexes acts as corrosion inhibitors for soft-cast steel in 1 M hydrochloric acid.
Keywords
Inhibition
Ligand
Zindo
Soft-cast steel
1 Introduction
Soft-cast steel is a significant alloy of iron because of its superior mechanical and thermal stability. Therefore, it can be used in construction and industrial fields. Unfortunately, soft-cast steel undergoes corrosion when came into contact with acids or alkalis in various industrial applications. The addition of inhibitors is the convenient practical method to develop the corrosion inhibitors especially in acid solutions (Issaadi et al., 2011; Nathan, 1997). Furthermore, electron donating atoms such as nitrogen, oxygen, sulphur and the presence of π electrons associated with heterocyclic rings in the molecules were reported as effective corrosion inhibitors for soft-cast steel in hydrochloric acid solution (Behpour et al., 2009; Behpor et al., 2008). From the scientific literature, the inhibitive capacity of the molecule is attributed caused by the adsorption on surface of the metal from bulk solution. Therefore, the corrosion study of metal complexes of ligand for soft-cast steel in acidic solution appeared in the earlier works are particularly inadequate (Rangelov and Mircheva, 1996; Khaled et al., 2006).
The present investigation is the study of corrosion inhibition effect of ligands and their metal complexes as corrosion inhibitors for soft cast steel in 1 M HCl was evaluated by experimental techniques. Therefore, experimental used for this study are electrochemical impedance spectroscopy and Tafel’s polarization measurements. Hence, due to the lack of information in earlier works on corrosion inhibitive study of ligands and their metal complexes, there is needed to explicate a probable mechanism for the corrosion inhibition investigation. Quantum chemical parameters are deciding the probability of inhibitive effect of corrosion inhibitors by theoretically through its molecular orbitals, molecular geometry optimization, and energy calculations.
2 Experimental
2.1 Materials
The corrosion parameters for the selected inhibitors (ligand and their metal complexes) for different steel strips were used for weight loss measurements. And the same steel strip, which is used in weight loss measurement, was used for electrochemical measurements with an uncovered area of 1 cm2 (the remaining area was shielded by epoxy resin). The soft-cast steel strips were clean and rubbed by SiC emery paper (grade no 2000), and washed with deionised water. The analytical grade 1 M hydrochloric acid is used to prepare the corrosive media.
The novel ligand (L) and their metal complexes such as [Co(L)2(H2O)2], [Ni(L)2(H2O)2] & [Fe(L)2(H2O)2] are tested as corrosion inhibitors and their molecular structures are shown in Figs. 1–4. The inhibitors were dissolved in 1 M Hydrochloric acid as inhibited solution with the concentration range 5–25 mg/L.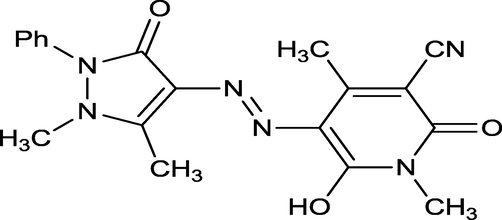
Molecular structure of the ligand (L).
![Molecular Structure of the [Co (L)2(H2O)2].](/content/185/2020/32/1/img/10.1016_j.jksus.2019.04.007-fig2.png)
Molecular Structure of the [Co (L)2(H2O)2].
![Molecular Structure of the [Ni (L)2(H2O)2].](/content/185/2020/32/1/img/10.1016_j.jksus.2019.04.007-fig3.png)
Molecular Structure of the [Ni (L)2(H2O)2].
![Molecular structure of the [Fe (L)2(H2O)2].](/content/185/2020/32/1/img/10.1016_j.jksus.2019.04.007-fig4.png)
Molecular structure of the [Fe (L)2(H2O)2].
2.2 Weight loss measurements
The weight loss analyses were carried out by immersing different soft-cast steel strips in 1 M hydrochloric solution by dissolving different concentrations of the inhibitors over 4 h immersion period. Afterwards, the steel strips were taken outside, wash away with deionised water and dried at room temperature. The clean and dry steel strips were weighed accurately by high précised weighing balance and same procedure is used to get the average values.
2.3 Electrochemical measurements
The corrosion parameters of selected inhibitors for soft cast steel in 1 M HCl were calculated by electrochemical measurements by using electrochemical workstation CHI608D with 3 electrode system. The electrode system consists a working electrode (soft-cast steel strip), a counter electrode (platinum) and a reference electrode (SCE) were used for the present investigations. The Tafel polarisation measurements were carried out with a potential sweep rate of 1 mV/s in specified potential range. Under these conditions, potential-current curves were recorded to measure the corrosion parameters. For electrochemical impedance spectra was measured by AC signals with amplitude of 3 mV at OCP at the frequency range of 100 k Hz–10 k Hz.
2.4 Quantum chemical parameters
The quantum chemical study was investigated for ligand and their metal complexes to elucidate their electronic structure, which favors to corrosion parameters by using the Zindo/1 method in Hyperchem professional software.
2.5 Scanning electron microscopic (SEM) studies
The surface morphology of the soft-cast steel in the absence and presence of inhibitors in 1 M hydrochloric acid solution was recorded by scanning electron microscopic (SEM) method.
3 Results and discussions
The corrosion parameters of ligand and their metal complexes were studied for soft-cast steel in 1 M hydrochloric acid by physical, electrochemical, surface & theoretical measurements.
3.1 Physical study
3.1.1 Weight loss measurement
The different soft-cast steel strips were immersed in various containers, which contain 100 cm3 of 1 M hydrochloric acid including the inhibitors with concentration range of 5–25 mg/L over the immersion period of about 4 h. During the measurements, soft-cast steel strips were weighed previously and subsequently the soaking period to record the weight difference. The inhibition efficiency (
) found from weight loss measurement in various solution concentrations are given in Table 1 and the fallowing equation is used to calculate the inhibition efficiency,
Ligand/Metal complexes
Corrosive medium (mg/L)
Corrosion rate (gm/cm2 hr)
Inhibition efficiency (ηw) (%)
L
Blank
0.280
–
5
0.190
32.14
10
0.178
36.40
15
0.130
53.57
20
0.110
60.71
25
0.070
75.00
[Co (L)2(H2O)2]
Blank
0.320
–
5
0.130
59.37
10
0.125
60.93
15
0.112
65.00
20
0.090
71.87
25
0.060
81.25
[Ni(L)2(H2O)2]
Blank
0.450
–
5
0.198
56.00
10
0.150
66.66
15
0.130
71.11
20
0.098
78.22
25
0.048
89.33
[Fe(L)2(H2O)2]
Blank
0.535
–
5
0.255
52.33
10
0.210
60.74
15
0.175
67.28
20
0.140
73.83
25
0.110
79.43
The corrosion parameters by weight loss measurements were recorded in Table 1. The weight loss of steel strips was significantly observed with the increasing concentration of inhibitors in 1 M HCl. This is attributed because of the addition of inhibitors covers surface of steel by the inhibitor molecules. The inhibitors get adsorbed on the surfaces of steel, which reduces in corrosion rate of metal (Prasanna et al., 2014). Hence, the addition of inhibitor significantly increases the inhibition efficiency of the inhibitors as a result of the adsorption process. In this investigation, ligand and their metal complexes show good inhibition efficiency for steel in 1 M HCl solution. The order of corrosion inhibition efficiency for the steel in 1 M HCl by weight loss method was reported as [Ni(L)2(H2O)2] > [Fe(L)2(H2O)2] > [Co (L)2(H2O)2], Among those metal complexes, Ni complex shows high inhibition efficiency of about 89.33% at the concentration of 25 mg/L. This is attributed due to the strong adsorption of Ni metal complexes of ligand on the surfaces of steel from the bulk of solution (Mahdavian and Attar, 2009)
3.2 Electrochemical measurements
3.2.1 Tafel polarisation measurements
The ligand and their metal complexes were subjected into corrosion studies by polarisation measurements for soft cast steel in 1 M hydrochloric acid solution. The Tafel polarisation plots were reported in Fig. 5 and corrosion parameters such as corrosion potential (Ecorr), corrosion current density (icorr) and percentage of inhibition efficiency (ηp) were reported in Table 2. The ηp was computed by the following expression,
![Tafel plots of (i) L, (ii) [Co (L)2(H2O)2], (iii) [Ni(L)2(H2O)2], (iv) [Fe(L)2(H2O)2] for corrosion for soft cast steel in 1 M HCl.](/content/185/2020/32/1/img/10.1016_j.jksus.2019.04.007-fig5.png)
Tafel plots of (i) L, (ii) [Co (L)2(H2O)2], (iii) [Ni(L)2(H2O)2], (iv) [Fe(L)2(H2O)2] for corrosion for soft cast steel in 1 M HCl.
Ligand/Metal complexes
Inhibitor Concn mg/L
Corrosion Potential (Ecorr) V
Corrosion current density (icorr) A cm−2
Inhibition Efficiency (ηp) %
Polarization Resistance (Rp) Ωcm2
Double Layer Capacitence (Cdl) µF cm−2
Inhibition Efficiency (ηz) %
L
Blank
−0.487
0.170
–
6.80
687
–
5
−0.418
0.065
61.76
14.40
297
52.77
10
−0.458
0.063
62.94
20.70
290
67.14
15
−0.448
0.049
71.17
25.50
284
73.33
20
−0.468
0.048
71.76
26.20
123
74.04
25
−0.425
0.036
78.82
30.28
131
77.54
[Co (L)2(H2O)2]
Blank
−0.417
0.468
–
6.11
555
–
5
−0.520
0.148
68.37
12.22
377
50
10
−0.522
0.119
74.57
12.66
126
51.73
15
−0.409
0.114
75.64
15.52
58
60.63
20
−0.439
0.099
78.84
20.94
55
70.82
25
−0.489
0.080
82.90
25.57
47
76.10
[Ni(L)2(H2O)2]
Blank
−0.487
0.486
–
2.40
243
–
5
−0.487
0.210
56.79
8.35
135
71.25
10
−0.459
0.190
60.90
10.20
120
76.47
15
−0.498
0.165
66.04
10.35
119
76.81
20
−0.479
0.120
75.30
13.50
115
82.22
25
−0.496
0.058
88.00
16.88
113
85.78
[Fe(L)2(H2O)2]
Blank
−0.479
0.506
–
3.20
650
5
−0.469
0.205
59.48
3.60
540
11.11
10
−0.489
0.160
68.37
9.55
458
66.49
15
−0.484
0.140
72.33
10.20
409
68.62
20
−0.494
0.122
75.88
12.20
215
73.77
25
−0.505
0.105
79.24
16.50
165
80.60
The results from polarisation measurements indicate that, icorr decreases considerably with the addition of inhibitor. Hence, there is a rise in ηp because of the adsorption of inhibitor molecules on surface of soft-cast steel (Solmaz et al., 2008). There is a fall trend of anodic and cathodic current densities without and with the various concentrations of inhibitors in 1 M HCl. Which indicates that the inhibitor addition retards both anodic (i.e metal dissolution) and cathodic (i.e hydrogen liberation) reactions. Therefore, a small change in Ecorr value of around 60 mV relating to blank is a sign of inhibitor acts as mixed type.
The order of inhibition efficiency was reported as [Ni(L)2(H2O)2] > [Fe(L)2(H2O)2] > [Co (L)2(H2O)2], This is because of Ni complexes shows minimum corrosion current density than that of the ligand and other metal complexes. Hence Ni metal complex strongly adsorbed on the metal surface which shows maximum inhibition efficiency of about 85.80% at 25 mg/L concentration (Nassar et al., 2015).
3.2.2 Electrochemical impedance spectroscopic (EIS) measurements
The corrosion inhibitory effect of ligand and their metal complexes for soft-cast steel in 1 M hydrochloric acid was carried out by electrochemical impedance spectroscopic method. This technique explains about the inhibition mechanism of inhibitors with the surface investigation of the electrode surface. The Nyquist’s plots for the present study are shown in Fig. 6. The corrosion parameters obtained from EIS study such as polarization resistance (Rp), double layer capacitance (Cdl) were tabulated in Table 2, The Fig. 7 is an equivalent circuit is use to fit EIS data. The inhibition efficiency (ηz) for soft-cast steel by inhibitors were computed by the following expression (Ferreira et al., 2004),
![Impedance spectra of (i) HL, (ii)[Co(L)2(H2O)2], (iii)[Ni(L)2(H2O)2], (iv)[Fe(L)2(H2O)2] for corrosion of soft cast steel in 1 M HCl.](/content/185/2020/32/1/img/10.1016_j.jksus.2019.04.007-fig6.png)
Impedance spectra of (i) HL, (ii)[Co(L)2(H2O)2], (iii)[Ni(L)2(H2O)2], (iv)[Fe(L)2(H2O)2] for corrosion of soft cast steel in 1 M HCl.
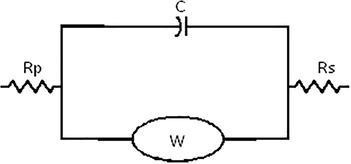
Equivalent circuit to fitting EIS data.
EIS spectra consist of semicircles, which are decapitated as the value of Rp, which are increased with rise in the inhibitor concentrations. The reported Cdl values are decreased with increase in the elevated inhibitor concentration in 1 M hydrochloric acid. This is attributed due to the increasing thickness of the electric double layer shows that the inhibitor strongly adsorbed on the surface of the soft-cast steel from the solution. The investigated results shows that the metal complexes are shows good corrosion inhibition efficiency because of the strong adsorption of inhibitors on soft-cast steel surfaces. There is a strong adsorption occurs by Ni complex, which shows maximum inhibition efficiency while compared to ligand and Co & Fe metal complexes. The order of inhibition efficiency was reported by this method is ([Ni (L)2(H2O)2] > [Fe(L)2(H2O)2] > [Co (L)2(H2O)2]) for corrosion of soft cast steel in 1 M HCl. Ni metal complex of ligand shows excellent inhibition efficiency due to the strong adsorption or increasing the thickness of the double layer (Bentiss et al., 2009)
EIS measurements for the corrosion study for soft-cast steel by the selected inhibitors revealed good agreement by the outcome found from Tafel polarisation and weight loss method. Hence, selected metal complexes of ligand especially Ni metal complex for this study act as corrosion inhibitors for soft-cast steel in 1 M hydrochloric acid solution.
3.3 Surface morphology
3.3.1 SEM measurements
The surface morphology of investigated soft-cast steel without and with the inhibitors in 1 M hydrochloric acid solution was studied by SEM measurements. The SEM graphs for corrosion of soft-cast steel in 1 M hydrochloric acid has a large pits and cracks because of attack of corrosion is as shown in Fig. 8i. But in the presence of inhibtors with 25 mg/L concentration shows uniform passive protective layer produced on the surface (Bammou et al., 2014) because of the inhibtors molecules adsorbed on the surface of soft-cast steel from the solution as shown in Fig. 8ii–v. However the SEM of steel surface in presence of Ni metal complex give moderately good protective layer due to the strong and uniform adsorption, which exhibit the excellent inhibitive action rather than ligand and other metal complexes. This investigation indicates that the steel surface damaged in aggressive corrosive media (i.e. 1 M Hydrochloric acid), but in the presence of inhibitors surfaces are protected by the attack of corrosion due to the founding of a shielding barrier on surfaces of the metal.![SEM micrographs of (i) absence of inhibitor and in presence of (ii) HL, (iii) [Co (L)2(H2O)2], (iv) [Ni(L)2(H2O)2], (v) [Fe(L)2(H2O)2] in 1 M hydrochloric solution.](/content/185/2020/32/1/img/10.1016_j.jksus.2019.04.007-fig8.png)
SEM micrographs of (i) absence of inhibitor and in presence of (ii) HL, (iii) [Co (L)2(H2O)2], (iv) [Ni(L)2(H2O)2], (v) [Fe(L)2(H2O)2] in 1 M hydrochloric solution.
3.4 Theoretical study
3.4.1 Quantum parameters
The quantum chemical parameters were studied by quantum method via ZINDO method. The computed quantum parameters such as energy of HOMO (Fig. 9), energy of LUMO (Fig. 10) and dipole moment (μ) are discussed and reported in Table 3. The 4s, 3p and 3d orbitals mixing in the valence state of Cobalt in HOMO and LUMO is predicted to be less polarized in comparison to Fe and Ni metals due to high electron affinity in Cobalt metal (Table3).![HOMO energy state of (A) HL, (B) [Co (L)2(H2O)2], (C) [Ni(L)2(H2O)2], (D) [Fe(L)2(H2O)2]](/content/185/2020/32/1/img/10.1016_j.jksus.2019.04.007-fig9.png)
HOMO energy state of (A) HL, (B) [Co (L)2(H2O)2], (C) [Ni(L)2(H2O)2], (D) [Fe(L)2(H2O)2]
![HOMO energy state of (A) HL, (B) [Co (L)2(H2O)2], (C) [Ni(L)2(H2O)2], (D) [Fe(L)2(H2O)2]](/content/185/2020/32/1/img/10.1016_j.jksus.2019.04.007-fig10.png)
HOMO energy state of (A) HL, (B) [Co (L)2(H2O)2], (C) [Ni(L)2(H2O)2], (D) [Fe(L)2(H2O)2]
![LUMO energy state of (A) HL, (B) [Co (L)2(H2O)2], (C) [Ni(L)2(H2O)2], (D) [Fe(L)2(H2O)2].](/content/185/2020/32/1/img/10.1016_j.jksus.2019.04.007-fig11.png)
LUMO energy state of (A) HL, (B) [Co (L)2(H2O)2], (C) [Ni(L)2(H2O)2], (D) [Fe(L)2(H2O)2].
![LUMO energy state of (A) HL, (B) [Co (L)2(H2O)2], (C) [Ni(L)2(H2O)2], (D) [Fe(L)2(H2O)2].](/content/185/2020/32/1/img/10.1016_j.jksus.2019.04.007-fig12.png)
LUMO energy state of (A) HL, (B) [Co (L)2(H2O)2], (C) [Ni(L)2(H2O)2], (D) [Fe(L)2(H2O)2].
Properties compounds
Dipole moment (Debyes)
EHOMOeV
ELUMOeV
ΔE
L
7.465
−5.070
3.895
8.965
[Ni(L)2(H2O)2]
24.537
−3.101
3.538
6.639
[Co (L)2(H2O)2]
13.468
−4.766
4.117
8.883
[Fe(L)2(H2O)2]
21.175
−5.472
3.318
8.790
The molecular orbital energies (i.e, EHOMO & ELUMO) play a major role to predicting the chemical reactivity of species. The energy of EHOMO is the electron contributing capacity of the molecule. Therefore, increasing values of energy EHOMO indicates a greater affinity for the contribution of electrons to a suitable acceptor molecule with lesser energy and vacant molecular orbital. The results obtained from Table 3, the lowermost value of ΔE (6.639 eV) was found for Ni complexes. It can be confirmed that Ni Complex has more tendency to be adsorbed on the surface of soft-cast steel than other metal complexes and ligand (Gopalji Shukla et al., 2011). And also dipole moment (μ) of the molecule is also an important parameter to decide the inhibition capacity of the molecule for steel in corrosive media. The inhibition efficiency for soft-cast steel by the inhibitor increases with the increasing value of dipole moment of the molecule (Yadav et al., 2013; Stoyanova et al., 2002; Benali et al., 2007). The dipole moment (μ) of the Ni complex is 24.53, indicates that Ni behaves as a strong inhibitor for soft-cast steel than other complexes.
4 Conclusion
-
The novel transition metal complexes of Antipyrine ligand show good inhibition efficiencies for corrosion of soft-cast steel in 1 M hydrochloric acid solution. The order of inhibiting effect of the inhibitors were reported as [Ni(L)2(H2O)2] > [Fe(L)2(H2O)2] > [Co (L)2(H2O)2].
-
The slight deviation in Ecorr values with regard to the blank indicates that all the inhibitors are mixed type.
-
In the EIS measurements, increased Rp value and decreased Cdl values with the inhibitors representing the adsorption of the inhibitor on mild steel surfaces.
-
Quantum results strengthen the experimental results of polarisation and EIS measures. This shows that the Ni metal complex shows maximum inhibition efficiency of around 90% than that of the ligand and metal complexes.
References
- Corrosion inhibition of steel in sulfuric acidic solution by the Chenopodium Ambrosioides Extracts. J. Assoc. Arab Univ. Basic Appl. Sci.. 2014;16:83-90.
- [CrossRef] [Google Scholar]
- Electrochemical and theoretical investigation on the corrosion inhibition of mild steel by thiosalicylaldehyde derivatives in hydrochloric acid solution. Corros. Sci.. 2008;51:2172-2181.
- [Google Scholar]
- The inhibitive effect of some bis-N, sbidentate schiff bases on corrosion behaviour of 304 stainless steel in hydrochloric acid solution. Corros. Sci.. 2009;51:1073-1082.
- [Google Scholar]
- Enhanced corrosion resistance of carbon steel in normal sulfuric acid medium by some macrocyclic polyether compounds containing a 1,3,4-thiadiazole moiety: AC impedance and computational studies. Corros. Sci.. 2009;51:2165-2173.
- [Google Scholar]
- Evaluation of the inhibitor effect of 1-ascorbic acid on the corrosion of mild steel. Mat. Chem. Phys.. 2004;83:129-134.
- [Google Scholar]
- Inhibitive effect of argemone mexicana plant extract on acid corrosion of mild steel. Ind. Eng. Chem. Res.. 2011;50:11954-11959.
- [Google Scholar]
- Novel thiophene symmetrical schiff base compounds as corrosion inhibitor for mild steel in acidic media. Corros. Sci.. 2011;53:1484-1488.
- [Google Scholar]
- Cobalt (III) complexes of macrocyclic-bidentate type as a new group of corrosion inhibitors for iron in perchloric acid. Corros. Sci.. 2006;48:3014-3034.
- [Google Scholar]
- Electrochemical behaviour of some transition metal acetylacetonate complexes as corrosion inhibitors for mild steel. Corros. Sci.. 2009;53:409-414.
- [Google Scholar]
- J. Bio. Tribo. Corros.. 2015;1:19.
- Organic Inhibitors. Houston: NACE; 1997.
- Ketosulfone drug as a green corrosion inhibitor for mild steel in acidic medium. Indus. Eng. Chem. Res.. 2014;53:8436-8444.
- [Google Scholar]
- The influence of metal complexes of tetramethyldithio-oxamide on the rate of acid corrosion of steel. Corros. Sci.. 1996;38:301-306.
- [Google Scholar]
- Investigation of adsorption and inhibitive effect of 2-mercaptothiazoline on corrosion of mild steel in hydrochloric acid media. Electrochim. Acta. 2008;53:5941-5952.
- [Google Scholar]
- Correlation between the molecular structure and the corrosion inhibiting effect of some pyrophthalone compounds. Chem. Phys.. 2002;279:1-6.
- [Google Scholar]
- Studied corrosion inhibition performance of three benzmidazole derivatives for mild steel in Hydrochloric acid. Ind. Eng. Chem. Res.. 2013;52:11954-16318.
- [Google Scholar]







