Translate this page into:
Constituents of essential oil of Origanum minutiflorum and its in vitro antioxidant, scolicidal and anticancer activities
⁎Corresponding author. azema1@yahoo.com (Abdel-Azeem S. Abdel-Baki)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objective
Origanum minutiflorum essential oil was evaluated for its chemical components, in vitro antioxidant, scolicidal and anticancer activities.
Methods
Oil was extracted and its components were identified with spectrometry and flame ionisation gas chromatography. The oil antioxidant activity was evaluated by DPPH radical scavenging and β-Carotene/linoleic acid bleaching assay. Scolicidal effect of the oil against protoscolices of hydatid cysts was evaluated at different concentrations and exposure times. Also, different oil concentrations were testififed for its anticancer activities on breast cancer cell line (MCF-7), human lung cancer cell line (A-549) and human hepatocellular carcinoma cell line (HepG2)
Results
Analyses of the O. minutiflorum oil identified 64 components with carvacrol being the predominant compound. The oil showed a weak free radical scavenging capacity (IC50 at 310 ± 4.2 µg/mL) whilst it was moderately active in term of the suppression of linoleic acid oxidation (64.6%). All the tested oil concentrations, however, revealed a significant scolicidal activity on the protoscoleces of hydatid cysts. The anticancer activity of O. minutiflorum oil was found more in HepG2 cells followed by A-549 and MCF-7 cells and this was evidenced by the LC50 values.
Conclusions
The present work strongly suggests that O. minutiflorum could serve as a potential source of scolicidal and anticancer agents.
Keywords
Origanum
Antiparasite
Anticancer
Antioxidant
Carvacrol
1 Introduction
There is currently considerable debate regarding the relative inefficacy of chemically-derived drugs against drug resistant pathogens and newly emerging infections, as well as their safety aspects (Wangchuk 2018). In this context, there is increasingly pressure on drug manufacturers from both consumers and the authorities to substitute the synthetic chemical drugs with alternative natural compounds, that are believed to be likely to be less toxic and more effective. Plants contain a broad spectrum of natural compounds that could be sources of novel pharmacologically-active ingredients, and, indeed, plants have a long history as important sources of remedial agents (Veeresham 2012).
For a long time, Origanum minutiflorum (Labiatae) has been widely used as flavour enhancer in foods, moreover in traditional medication as a remedy for indigestion, loss of appetence and coughs (Göze et al. 2010). Its rich essential oil has also been shown in various studies to exhibit significant antibacterial, antiviral, antifungal, larvicidal and acaricidal activities (Cetin et al. 2009; Göze et al. 2010; Kılıçgün and Korkmaz 2014).
Despite these intensive studies, there remains a scarcity of knowledge on the scolicidal and anticancer activities of the constituents of the O. minutiflorum essential oil. The main aims of the present study therefore, were to determine: (i) the total active constituents of the oil; (ii) the in vitro antioxidant capacity; (iii) the in vitro scolicidal activity of O. minutiflorum essential oil on the protoscoleces of hydatid cysts; (iv) the in vitro anticancer activities on breast cancer cell line (MCF-7), human lung cancer cell line (A-549) and human hepatocellular carcinoma cell line (HepG2).
2 Materials and methods
2.1 Plant material
Origanum minutiflorum plants were collected from Devebeli Pass, Sütçüler-ISPARTA (37° 22́ N and 31° 07́ E, elevation 1340 m). Identification was approved by a plant taxonomist and a voucher specimen was deposited at the Herbarium of the Department of Biology, Cumhuriyet University (CUFH), Turkey. The O. minutiflorum leaves were dried and then ground to pass through a 2 mm mesh.
2.2 Extraction of essential oil
The dried ground leaves (100 g) were placed in a Clevenger-type distillation device with 2 L of double distilled water and then hydro-distilled for 3 h. The extracted essential oil (EO) was dried over anhydrous sodium sulphate, filtered and kept at 4 °C until use.
2.3 Characterization of essential oil (GC–MS and GC-FID Analyses)
The identification of the EO chemical profile was carried out according to Busatta et al. (2017). This was done using gas chromatography with a mass detector (GC–MS), model Shimadzu GC 17A, ion source temp: 200 °C, interface temp: 250 °C, solvent cut time: 4 min. Chromatographic patterns as cis-sabinene hydrate, α-terpinene, γ-terpinene (Fluka), α-terpineol, camphene, α-pinene, carvacrol, β-pinene, β-myrcene, p-cymene, limonene, eucalyptol, terpinolene and linalool (Sigma-Aldrich) were utilized for the chemical characterization of the essential oils.
Gas chromatography (GC) in a Shimadzu GC-210 with a data processor, using biphenyl as an internal standard for The quantification of essential oils. The analyses were performed in capillary column RESTEK, Rxi-5 Sil MS 30 Meter 0,25 mm ID 0,25 µm df, detector FID, following the temperature programme: 40–180 °C (3 °C min−1), 180–240 °C (20 °C min−1), 240 °C (20 min), temperature of injector 250 °C, injection mode split, carrier gas He, injection volume of 0.4 μL (sample diluted in n-hexane 1:10).
2.4 Antioxidant activity
2.4.1 2,2-Diphenyl-1-picrylhydrazyl DPPH radical scavenging assay
The assay was performed according to Demir et al. (2015). Briefly, the stock solutions (10–20 mg mL−1) were prepared in methanol. Aliquots of 50 µL of the extract solution was added to 5 mL of a newly prepared 0.004% (w/v) DPPH radical methanol solution and left in the dark for 30 min at room temperature allowing for any reaction to occur. Absorbance values of these solutions were measured using spectrometer (Unicam UV2-100) at 517 nm. The anti-free radical activity was determined in percent (I%) as follows:
where, Ablank is the control reaction absorbance while Asample is the tested compound absorbance. The concentration of the extract exhibiting 50% inhibition (IC50) was calculated from the graph-plotted for the percent of inhibition against the concentration of the extract. The assay was performed in triplicate with butylated hydroxytoluene (BHT) serving as a positive control.
2.4.2 β-Carotene/linoleic acid bleaching assay
In this assay, a mixture of 0.5 mg β-carotene in 1 mL chloroform (HPLC grade), 25 µL linoleic acid and 200 mg Tween 20 was prepared. Chloroform was then totally evaporated in vacuo and thereafter the residue was diluted with 100 mL of oxygenated distilled water to form a clear yellowish emulsion. This must be used soon as possible. The extract and BHT (positive control) were individually dissolved in ethanol (2 mg ml−1) and 350 µL in a test tube, followed by a 2.5 mL of the β-carotene and linoleic acid mixture, and mixed thoroughly. The test tubes were kept for 24 h at room temperature together with a negative control (blank). The values of the absorbance were estimated at 490 nm on an ultraviolet and visible (UV–vis) spectrometer (Burits and Bucar 2000). The Relative Antioxidant Activity (RAA %) was estimated as following:
All assays were performed in triplicate and the percentages of inhibition were given as means ± SD.
2.5 Scolicidal activity
2.5.1 Collection of protoscoleces from hydatid cysts and the viability test
Hydatid cysts were carefully excised without any spillage from the naturally infected livers of slaughtered sheep. The protoscoleces and hydatid fluid were aspirated and transferred into glass cylinders and left for 20 min so as to permit the protoscoleces to settle down. The supernatant fluid was then evacuated while the retained protoscoleces were washed three times in saline solution. The viability of the protoscoleces was affirmed by 0.1% eosin staining. After exposure to the stain for five minutes, protoscoleces that did not take the dye were considered viable while the stained protoscoleces were considered dead (Fig. 1) (Haghani et al., 2014). In order to calculate the percentage of viability, at least 450 protoscoleces were counted and then the number of viable protoscoleces was divided by the total number of protoscoleces. Finally, samples with viability of more than 95% were considered suitable for further studies.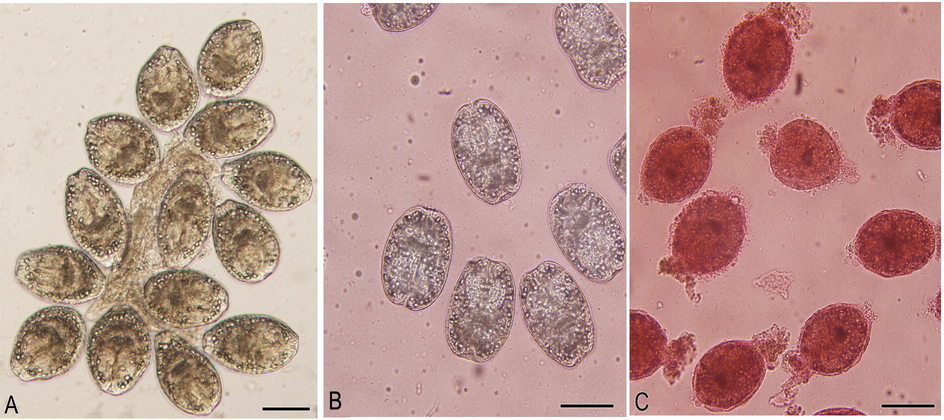
Live non stained protoscolices (A), Live protoscolices after staining with 0.1% eosin (B), Dead protoscolices after treatment with O. minutiflorum EO and staining with 0.1% eosin. Scale-bar = 100 µm.
2.5.2 Determination of in vitro activity
In the present investigation, three concentrations (0.025, 0.05 and 0.1%) of the essential oil were tested on the protoscoleces. 2 mL of each concentration was placed in three test tubes to which about 5 × 103 protoscolices were added and gently mixed. These three tubes for each concentration (i.e. nine in total) were then incubated at 37 °C for 1, 3 and 5 min, respectively. After incubation, the supernatant was carefully disposed to avoid disturbing the remaining settled protoscoleces. Then, 1 mL of 0.1% eosin stain was mixed gently with settled protoscoleces. Five minutes later, the viability of these protoscoleces were microscopically estimated. At least 5 × 103 protoscoleces in 2 mL distilled water with no treatment with the oil was considered as a control group.
2.6 Cytotoxic activity
2.6.1 Cell culture
Human breast cancer cell line (MCF-7), human lung cancer cell line (A-549) and human hepatocellular carcinoma cell line (HepG2) were cultured in DMEM culture medium supplemented with 10% foetal bovine serum (FBS), 0.2% sodium bicarbonate and antibiotic/antimycotic solution. The cells were grown at 37 °C in a CO2 incubator (5% CO2–95% atmosphere) under high humidity (Siddiqui et al., 2008). The viability of the all cell lines were estimated using trypan blue dye exclusion assay and the batches of cells with more than 98% cell viability were utilized in the current work.
2.6.2 Cytotoxicity assessment by MTT assay
The cytotoxicity assessment was performed according to the protocol of Siddiqui et al. (2008). In brief, MCF-7, A-549 and HepG2 cells were plated in 96 well culture plates and were allowed to adhere for 24 h in a CO2 incubator at 37 °C. Then, the cells were subjected to various concentrations (0.0156 – 1%) of O. minutiflorum oil for 24 h. After the exposure, 10 μL of MTT (5 mg/mL of stock) was added in each well and plates were incubated for a further 4 h in a CO2 incubator. Then, the supernatant was discarded and 200 µL of DMSO was added in each well and mixed gently. Plates were read at 550 nm wavelength. Control sets were also kept under the same conditions. The cytotoxic activity of O. minutiflorum oil against the three cancer cell lines was compared by estimating the LC50 values.
2.7 Statistical analysis
Statistical analysis was accomplished by one-way analysis of variance (ANOVA). All analyses were carried out using a statistical package program (Sigma Plot version 11.0). Results were considered statistically significant at P ≤ 0.001.
3 Results
3.1 Yield and chemical composition of the essential oil
Hydrodistillation of ground leaves provided a pale yellow-coloured essential oil with a 3.1% (v/w) yield. The oil’s odour was typical of carvacrol. GC–MS and GC-FID analyses also confirmed this observation, identifying 64 components (representing ca. 98.9% of the total identified components) with carvacrol (64.29%) being the predominant compound, followed by p-cymene (9.56%). The main oil constituents are listed in Table 1, except for 43 compounds each representing less than 0.1% of the total, which were considered as. Abbreviations: RT, Retention time, RI, Retention Index. *Components represented less than 0.1% are collectively referred as minors (43 in total). **Two peaks were not identified and named as unknown.
Peak No
RT
RI
Components
%
1
9.042
834.00
α-Pinene
1.33
2
9.629
846.00
Camphene
1.03
3
10.681
874.00
(+) β-Pinene
0.26
4
10.901
879.00
1-Octen-3-ol
0.51
5
11.227
888.00
β-Myrcene
1.66
6
11.561
897.00
3-Octanol
0.18
7
11.815
904.00
δ-3-Carene
0.10
8
11.915
906.00
α-Phellandrene
0.99
9
12.249
915.00
α-Terpinene
1.47
11
12.590
923.00
p-cymene
9.56
12
12.739
927.00
Bornylene
0.65
13
12.847
930.00
Eucalyptol
0.50
14
13.898
957.00
γ-Terpinene
3.94
15
14.366
969.00
cis-sabinene hydrate
1.03
16
14.932
983.00
α-Terpinolene
0.23
17
15.555
999.00
Linalool
0.74
18
18.315
1071.00
Borneol
2.31
19
18.637
1079.00
Terpinen-4-ol
1.14
20
19.446
1100.00
α-terpineol
0.50
21
20.801
1137.00
Carvacrol methyl ether
0.10
22
23.454
1210.00
Carvacrol
64.29
23
27.227
1320.00
Trans-β-Caryophyllene
2.39
24
28.387
1355.00
α-Humulene
0.10
25
31.558
1455.00
4-Ethylguaiacol
0.55
26
32.259
1477.00
(+) spathulenol
0.27
27
32.419
1483.00
(−)-Caryophyllene oxide
0.42
28
41.879
1819.00
α-Chamigrene
0.21
29
46.892
2022.00
Nerolidol
0.22
30
48.712
2100.00
Heneicosane
0.17
31
50.948
2200.00
Docosane
0.23
Minors *
1.81
Unknown **
1.11
Total
100.00
Monoterpene hydrocarbons 20.57%
Oxygenated Monoterpenes 70.51%
Hydrocarbon sesquiterpenes 2.70
Oxygenated sesquiterpenes 1.46%
Others 1.85%
3.2 Antioxidant activity
The radical scavenging capacity of O. minutiflorum oil as evaluated spectrophotometrically by determining its capability to donate hydrogen to stabilize the radical, DPPH. BHT was employed as a positive control. It was found that the DPPH radical scavenging activities of the oil was insignificant, with an IC50 at 310 ± 4.2 µg/mL when compared to that of BHT, which have IC50 at 19.8 ± 0.5 µg/mL.
In the β-carotene/linoleic acid assay, the EO was found to inhibit the oxidation of linoleic acid moderately with an RAA of 64.6%. This compares, however, with an RAA for the positive control, BHT, of 100%.
3.3 In vitro scolicidal activity
The scolicidal activity of O. minutiflorum oil is summarized in Table 2. The scolicidal effect of O. minutiflorum oil at a concentration of 0.025% was 32.4%, 50.3% and 91.1% after 1, 3 and 5 min, respectively. The effect at a concentration of 0.05% was 42.03%, 75.4% and 100% after 1, 3 and 5 min respectively. At 0.1% concentration of the oil exhibited 100% death after 3 and 5 min of exposure time. Overall, the scolicidal activity of the oil was evident at these concentrations.
Concentrations
Experiment
% of mortality rates after exposure
1 min
3 min
5 min
0.025%
1
30.6
50
90.2
2
32.2
48.9
93.4
3
34.3
52.1
89.7
Average
32.4
50.3
91.1
0.05%
1
40.6
77.3
100
2
44.2
75.3
100
3
41.3
73.6
100
Average
42.03
75.4
100
0.1%
1
72
100
100
2
76.6
100
100
3
74.8
100
100
Average
74.5
100
100
Control
1
3.1
3.6
5.5
2
3.3
4.2
5.3
3
3.0
3.8
4.8
Average
3.1
3.9
5.2
3.4 Cytotoxicity assessment
Highlights of the results obtained by mtt assay in mcf-7, a-549 and hepg2 cells are summarized in figures (2–4). the results exhibited a concentration dependent decrease in the cell viability of mcf-7, a-549 and hepg2 cells following 24 h exposure to o. minutiflorum oil. the percentage of cell viability, even at lower concentrations i.e. 0.0156, 0.03125 and 0.0625% of o. minutiflorum oil was recorded as 93%, 14%, and 14%, respectively in mcf-7 cells (Fig. 2). in a-549 cells the cell viability was recorded as 83%, 6% and 6% with o. minutiflorum oil concentrations of 0.0156, 0.03125 and 0.0625%, respectively (Fig. 3). in hepg2 cells, meanwhile, cell viability was 78%, 12% and 5% at concentrations of 0.0156, 0.03125 and 0.0625%, respectively (Fig. 4). the lc50 were 0.028457%, 0.028682%, 0.0349235% for hepg2, a-549 and mcf-7 cells respectively. overall, therefore, the cytotoxic effect of o. minutiflorum oil was strongest in hepg2 cells followed by a-549 and mcf-7 cells of the results obtained by MTT assay in MCF-7, A-549 and HepG2 cells are summarized in figures (2–4). The results exhibited a concentration dependent decrease in the cell viability of MCF-7, A-549 and HepG2 cells following 24 h exposure to O. minutiflorum oil. The percentage of cell viability, even at lower concentrations i.e. 0.0156, 0.03125 and 0.0625% of O. minutiflorum oil was recorded as 93%, 14%, and 14%, respectively in MCF-7 cells (Fig. 2). In A-549 cells the cell viability was recorded as 83%, 6% and 6% with O. minutiflorum oil concentrations of 0.0156, 0.03125 and 0.0625%, respectively (Fig. 3). In HepG2 cells, meanwhile, cell viability was 78%, 12% and 5% at concentrations of 0.0156, 0.03125 and 0.0625%, respectively (Fig. 4). The LC50 were 0.028457%, 0.028682%, 0.0349235% for HepG2, A-549 and MCF-7 cells respectively. Overall, therefore, the cytotoxic effect of O. minutiflorum oil was strongest in HepG2 cells followed by A-549 and MCF-7 cells.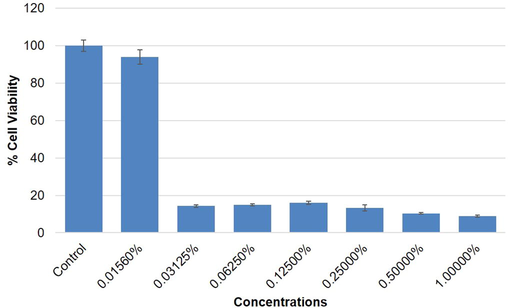
Cytotoxic activity of O. minutiflorum EO in MCF-7 cells by MTT assay. Cells were exposed to different concentrations of EO for 24 h. All values are presented as mean ± SD.
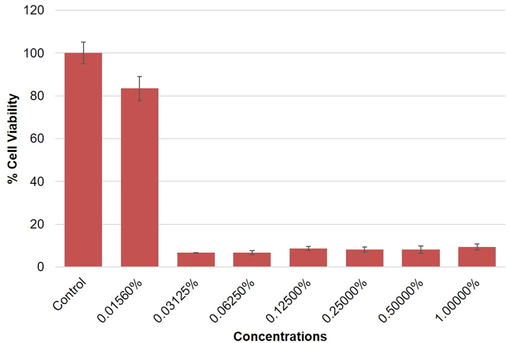
Cytotoxic activity of O. minutiflorum EO in A-549 cells. Cells were exposed to different concentrations of EO for 24 h. All values are presented as mean ± SD.
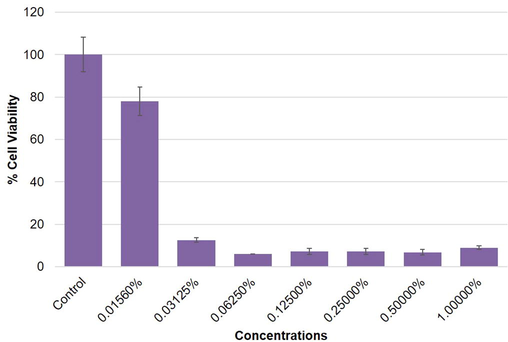
Cytotoxic activity of O. minutiflorum EO in HepG2 cells. Cells were exposed to different concentrations of EO for 24 h. All values are presented as mean ± SD.
4 Discussion
The constituents of O. minutiflorum EO have been studied by many researchers and as mentioned here, carvacrol was found to be the predominant component (64.29%). In the literature, however, the carvacrol content varies greatly between different studies. For instance, studies by Demirel and Erdogan (2017), Toker et al. (2017) and Albayrak and Aksoy (2019) reported carvacrol content of 52.04%, 74.63% and 90.87%, respectively. After carvacrol, p-cymene was the second major component in our study. This finding is also consistent with the reports mentioned above.
It was found that both the yield and composition of EOs are dramatically affected by the time of the plant collection and climatic factors, the oregano populations selected, the duration of hydrodistillation and the plant parts employed also have significant effect (Toker et al. 2017).
It is a generally accepted pattern that polar extracts obtained from aromatic plants show much higher antioxidant activity than those of EOs. This phenomenon is attributed to the presence of phenolics in the polar extracts. Although polar extracts of mountain oregano have not been included in our findings, our preliminary experiments support this phenomenon. Here, the free radical scavenging capacity of EO is weak (310 µg/mL) whilst it exerts moderate action on inhibition the linoleic acid oxidation. The non-polar nature of EOs may explain the poor efficiency in stabilizing the hydrophilic DPPH radical (Sahin et al. 2004). On the other hand, the moderate effect in the β-carotene/linoleic acid assay might be due to that the oil contains carvacrol and the other monoterpene hydrocarbons (Albayrak and Aksoy 2019).
The application of scolicidal agents is obligatory for the interventional treatment of hydatid cysts and to avoid dissemination of the protoscoleces during surgery (Abdel-Baki et al. 2016). The currently available scolicidal agents have some unacceptable adverse effects, however, and this has forced researchers to look for alternative safer scolicidal agents. Recently, intensive efforts have been devoted to explore herbal extracts as a possible source for new anti-scolicidal components with high activity and low toxicity (Almalki et al. 2017). The essential oils of many Origanum species are established to contain highly bioactive ingredients that exhibit antimicrobial (Vardar-Ünlü et al., 2006; Göze et al. 2016), antifungal (Albayra and Aksoy, 2019), acaricidal and insecticidal (Cetin et al., 2009) activities. Building on this concept, here we assessed the in vitro scolicidal activity of O. minutiflorum essential oil on the protoscoleces of hydatidosis at a various concentrations and exposure times. All the tested oil concentrations showed a significant level of scolicidal activity. Many herbal extracts and their volatile components have been proven to have scolicidal activity, including Allium sativum, Zataria multiflora, Mentha pulegium, Nigella sativa, Salvadora persica and Curcuma longa and Zingiber officinale (see Moazeni et al. 2014, Abdel-Baki et al. 2016, Almalki et al. 2017). Pensel et al. (2014) established that the Origanum vulgare essential oil have anti-parasitic activities against protoscoleces and cysts of Echinococcus granulosus. The intrinsic activities of the essential oils are probably going to be associated with the proportion in which the constituents are present and also the interactions between them (Göze et al., 2016). It is possible, therefore, that the scolicidal activity of O. minutiflorum essential oil is due to its high carvacrol content (64.29%). This is suggested, for example, by the work of Fabbri et al. (2016) who showed that carvacrol applied at a dose of 10 μg/mL led to maximum protoscolicidal effect. Although p-cymene is another major component (9.56%) identified in O. minutiflorum, it has been shown to have no anti-microbial activity when used alone (Juliano et al. 2000). That said, the use of p-cymene in combination with other phenolic compounds, such as carvacrol, appeared to increase scolicidal activity (Moazeni et al. 2014).
İn the present study, the in vitro cytotoxic effect of O. minutiflorum oil against HepG2, A-549 and MCF-7 cells was evaluated. The results showed that O. minutiflorum oil was highly cytotoxic in HepG2 cells, followed by A-549 and MCF-7 cells. Bostancıoğlu et al. (2012) proved that Origanum onites oil, also with a high carvacrol content, significantly suppressed the cell viability and triggered apoptosis in rat adipose tissue endothelial cells (RATECs) and 5RP7 (c-H-ras transformed rat embryonic fibroblasts) cells in addition to obstructing the in vitro tube formation and migration of RATECs. Similarly, it was found that carvacrol has the ability to induce apoptosis in human glioblastoma cells and reduce hyperalgesia in mice with tumours, suggesting that Origanum EO can be useful choice for cancer pain management (Liang and Lu, 2012; Guimarães et al. 2015 respectively). Recently, Han and Parker (2017) found that Origanum vulgare, again with a high carvacrol content, markedly amended the global gene expression and modulated signalling pathways which are critical in cancer signalling processes. When combined with this existing literature, the present work strongly implies that O. minutiflorum, with carvacrol as the main active compound, might act as a possible source of an anticancer agent.
5 Conclusion
To date, no information has been available about the in vitro scolicidal activity of O. minutiflorum EO on the protoscoleces of hydatid cysts as well as its anticancer activities on MCF-7, A-549 and HepG2 cell lines. The findings presented here offer important new data in these respects. Further studies are now needed to identify the nature of the activity created by O. minutiflorum EO and to determine if it would be a viable in vivo remedy to eradicate scolicides as well as cancer cells.
Acknowledgements
This work was supported by Researcher supporting Project (RSP-2019/3), King Saud University and Konya Food and Agriculture University’s Scientific Research Projects Units (Project No. KGTU- 374 BAP 2018-0022).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. The authors declare that they have no conflicts of interest.
References
- In vitro scolicidal effects of Salvadora persica root extract against protoscolices of Echinococcus granulosus. Korean J. Parasitol.. 2016;54:61-66.
- [CrossRef] [Google Scholar]
- Phenolic contents and biological activity of endemic Origanum minutiflorum grown in Turkey. Indian J. Pharm. Edu. Res.. 2019;53:160-170.
- [CrossRef] [Google Scholar]
- In vitro effectiveness of Curcuma longa and Zingiber officinale extracts on Echinococcus protoscoleces. Saudi J. Biol. Sci.. 2017;24:90-94.
- [CrossRef] [Google Scholar]
- Assessment of anti-angiogenic and anti-tumoral potentials of Origanum onites L. essential oil. Food Chem. Toxicol.. 2012;50:2002-2008.
- [CrossRef] [Google Scholar]
- Antioxidant activity of Nigella sativa essential oil. Phytother. Res.. 2000;14:323-328.
- [CrossRef] [Google Scholar]
- Chemical profiles of essential oils of marjoram (Origanum majorana) and oregano (Origanum vulgare) obtained by hydrodistillation and supercritical CO2. J. Essent. Oil Res.. 2017;29:367-374.
- [CrossRef] [Google Scholar]
- Acaricidal effects of the essential oil of Origanum minutiflorum (Lamiaceae) against Rhipicephalus turanicus (Acari: Ixodidae) Vet. Parasitol.. 2009;160:359-361.
- [CrossRef] [Google Scholar]
- Antioxidant activity of Alhagi camelorum phenolics extracted by automated and standard extraction techniques. Sep. Sci. Technol.. 2015;50:529-535.
- [CrossRef] [Google Scholar]
- Insecticidal effects of essential oils from Labiatae and Lauraceae families against cowpea weevil, Callosobruchus maculatus (F.) (Coleoptera: Bruchidae) in stored pea seeds. Entomol. Appl. Sci. Lett.. 2017;4:13-19.
- [Google Scholar]
- In vitro and in vivo efficacy of carvacrol against Echinococcus granulosus. Acta Trop.. 2016;164:272-279.
- [CrossRef] [Google Scholar]
- In vitro antimicrobial, antioxidant, and antispasmodic activities and the composition of the essential oil of Origanum acutidens (Hand -Mazz.) Ietswaart. J. Med. Food. 2010;13:705-709.
- [CrossRef] [Google Scholar]
- In vitro antimicrobial and antioxidant activities and chemical composition of essential oils of the leaf and flower of Origanum minutiflorum O. Schwarz et. P. H. Davis. Cumhuriyet Üniv. Sağ. Bil Enst. Derg.. 2016;2:17-23.
- [Google Scholar]
- Encapsulation of carvacrol, a monoterpene present in the essential oil of oregano, with β-cyclodextrin, improves the pharmacological response on cancer pain experimental protocols. Chem. Biol. Interact.. 2015;227:69-76.
- [CrossRef] [Google Scholar]
- Low scolicidal effect of Ocimum bacilicum and Allium cepa on protoccoleces of hydatid cyst: an in vitro study. Comp. Clin. Pathol.. 2014;23:847-853.
- [CrossRef] [Google Scholar]
- Anti-inflammatory, tissue remodeling, immunomodulatory, and anticancer activities of oregano (Origanum vulgare) essential oil in a human skin disease model. Biochim. Open. 2017;4:73-77.
- [CrossRef] [Google Scholar]
- Composition and in vitro antimicrobial activity of the essential oil of Thymus herba-barona Loisel growing wild in Sardinia. J. Essent. Oil Res.. 2000;12:516-522.
- [CrossRef] [Google Scholar]
- Hepatoprotective and antidiabetic activity of Origanum minutiflorum grown wild in Turkey. Bothalıa J. 2014;44:3.
- [Google Scholar]
- Carvacrol-induced [Ca2+]i rise and apoptosis in human glioblastoma cells. Life Sci.. 2012;90:703-711.
- [CrossRef] [Google Scholar]
- Moazeni M, Larki S, Oryan A, Saharkhiz MJ (2014) Preventive and therapeutic effects of Zataria multiflora methanolic extract on hydatid cyst: an in vivo study. Vet. Parasitol. 205: 107–112. https://doi.org/ 10.1016/j.vetpar.2014.07.006
- Efficacy of essential oils of Thymus vulgaris and Origanum vulgare on Echinococcus granulosus. Interdiscip Perspect. Infect. Dis.. 2014;2014:693289
- [CrossRef] [Google Scholar]
- Biological activities of the essential oils and methanol extract of Origanum vulgare ssp. vulgare in the Eastern Anatolia region of Turkey. Food Control. 2004;15:549e557.
- [CrossRef] [Google Scholar]
- Influence of cytotoxic doses of 4-hydroxynonenal on selected neurotransmitter receptors in PC-12 cells. Toxicol. In Vitro. 2008;22:1681-1688.
- [CrossRef] [Google Scholar]
- Effects of distillation times on essential oil compositions of Origanum minutiflorum O. Schwarz Et. and P.H Davis. J. Essent Oil Res. 2017;29:30-335.
- [CrossRef] [Google Scholar]
- Chemical composition and in vitro antimicrobial activity of the essential oil of Origanum minutiflorum O Schwarz & PH Davis. J. Sci. Food Agricult.. 2006;87:255-259.
- [CrossRef] [Google Scholar]
- Natural products derived from plants as a source of drugs. J. Adv. Pharm. Technol. Res.. 2012;3:200-201.
- [CrossRef] [Google Scholar]
- Therapeutic applications of natural products in herbal medicines, biodiscovery programs, and biomedicine. J. Biol. Active Prod. Nat.. 2018;8:1-20.
- [CrossRef] [Google Scholar]







