Translate this page into:
Complement protein C1q binds soluble antigens of Leishmania major (SLA) via the globular head region, activates the classical pathway, and modulates macrophage immune response
⁎Corresponding author. salkahtani@ksu.edu.sa (Saad Alkahtani)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Leishmania species cause a range of moderate to severe diseases affecting cutaneous to visceral regions, thus having a great impact on morbidity and mortality. Leishmania is initially dealt with by human innate immune system immediately after its entry into the body, primarily by the complement system, one of the most potent humoral immune mechanisms that link innate and adaptive immunity. Complement can be activated via all its three pathways on the surface of Leishmania to varying degrees; however, it is considered that the classical pathway is the first to respond due to a lag phase preceding the alternative pathway activation. The present study illustrated that the recognition molecule, C1q bound to soluble leishmania antigens (SLA) derived from L. major via its domains and activates the classical pathway. This was confirmed in complement consumption assay where SLA was able to induce only ∼22% haemolysis of sensitized cells. Moreover, this study showed that recombinant individual head regions of C1q chains bind differentially to SLA while interaction studies with substitution mutants revealed that C1q-SLA binding relied on charge-charge interaction. When macrophage-like cell line, THP-1, was challenged with SLA in the presence of human C1q, it downregulated a number of cytokine and chemokine response considerably; the effects were mostly suppressive of Th1 immune response, suggesting that the requirements for potent adjuvants in SLA-mediated vaccine strategies.
Keywords
Complement
Leishmania
Macrophage
Cytokine
- SLA
-
Soluble Leishmania Antigens
- C1q
-
Complement Component 1q
- THP1
-
Human Monocytic Leukemia Cell Line
- Ig
-
Immunoglobulin
- MBL
-
Recognising mannan-binding C-type lectin
- MAC
-
Membrane Attack Complex
- LPG
-
Lipophosphoglycan
Abbreviations
1 Introduction
With over 12 million infected patients and between 0.7 and 1 million cases every year (Kedzierski, 2010; Alvar et al., 2012), Leishmania remains a formidable single-cell parasite that exploits sand flies for its transmission to human host (Sacks and Noben-Trauth, 2002; Kaye and Scott, 2011). Of its two major stages, Leishmania have two major stages: flagellar and non-flagellar; motile promastigotes are resident in the sand fly gut, while non-flagellar amastigotes are intracellular residents in human macrophages (Pearson and Sousa, 1996). Few species of Leishmania including L. major cause cutaneous leishmaniasis (Reithinger et al., 2007; David and Craft, 2009); in most case, the lesion heals on its own, and sometimes, becomes ulcerated. Other species such as L. donovani and L. brasilliensis have more severe prognosis and mortality rate (Murray et al., 2005; Chappuis et al., 2007; David and Craft, 2009; Kaye and Scott, 2011).
Like most instances, the host’s innate immune mechanisms play an essential function in clearing the pathogen until leishmania finds a way to manipulate innate as well as adaptive immune systems. Of several key innate immune components, the complement system is known to target leishmania parasite and lyse them. Complement system include more than 50 soluble and surface-bound proteins (regulators and receptors) in the blood that can detect a wide variety of pathogens and eliminate them from the body (Merle et al., 2015). The complement system operates by three pathways: classical, lectin and alternative pathways, depending on the first target recognition subcomponent of the pathway. The classical pathway is activated by C1q molecule binding to IgG or IgM opsonised pathogens although C1q can sometimes directly bind to pathogen ligands without involving immunoglobulins (Kishore et al., 2004), whereas lectin pathway is triggered by carbohydrate pattern recognising mannan-binding C-type lectin (MBL) that recognises sugar patterns on the pathogen surface (Merle et al., 2015). The alternative pathway is activated by auto-activation of C3 on the surface of pathogens. All three pathways assemble on C3 convertase enzyme that cleaves C3 into two parts (C3b, C3a) which enhances phagocytic clearance of the pathogens. C3b deposition can be followed by generation of C5 convertase, leading to creation of a lytic membrane attack complex (MAC) on the pathogen surface (Kouser et al., 2013). Leishmania parasites can activate complement via C3b deposition and MAC formation, leading to parasite lysis. However, there are escape mechanisms in place whereby Leishmania virulence factor lipophosphoglycan (LPG) and gp63 inhibit MAC formation; gp63 inactivates C3b (iC3b), and hence subsequent MAC complex formation. However, this does not prevent C3b-driven phagocytic uptake of amastigotes via macrophages. A CH50 value of 120 using normal human serum has cytotoxic effect on L. major (Hoover et al., 1984, 1985) that can happen within 5 min of C3b deposition (Dominguez et al., 2002). All three pathways play varying level in the clearance of leishmania, alternative pathway being the most important although it is preceded by activating the classical pathway (Hoover and Nacy, 1984b; Mosser and Edelson, 1985; Green et al., 1994; Dominguez et al., 2002).
In this study, we have examined if C1q can bind to soluble leishmania antigens (SLA) derived from Leishmania major and activate the classical pathway via its domains. C1q has a N-terminal collagen region and the ligand binding globular domain that is composed of C-terminal ends of A, B and C chains organised as a heterotrimer. Thus, we used individual head regions of human C1q and their substitution mutants for their interaction with SLA. In addition, we also examined the immune response of a macrophage-like cell line, THP-1, when challenged with SLA in the presence of human C1q.
2 Materials and methods
2.1 Preparation of soluble Leishmania antigens
The strain of L. major (MRHO/IR/75/ER) was used to prepare the SLA. The preparation of SLA was done as described by (Scott et al., 1987). In brief, the parasites were collected at stationary phase and treated with 20 mM HEPES, pH 7.5 with 5% w/v sucrose buffer. Then, promastigotes was treated with 1.2 × 109/ml of protease inhibitor cocktail (Sigma) and incubated over ice for 20 min. Promastigotes were lysed via freeze–thaw cycle and then sonicated (10 pulses, 30 sec each, every 2 min). The lysate was centrifuged at high speed centrifugation and the supernatant was dialysed extensively against the HEPES buffer and filter-sterilised. The BCA protein assay kit (Thermo Scientific, USA) was used to measure protein concentration of the SLA.
2.2 Purification of human C1q
C1q was essentially purified using a modified protocol as described earlier (Pondman et al., 2015). 50 ml of freshly frozen human plasma, adjusted to 5 mM EDTA, pH 7.4, was centrifuged for 30 min. at 12000 × g in order to eliminate debris, aggregates, and insoluble lipids. The supernatant was passed three times through a 5-ml column of IgG-Sepharose previously equilibrated with cold PBS-EDTA and extensively washed with the same buffer. The protein was stripped off the affinity column using elution buffer (1 M NaCl, 5 mM EDTA, 50 mM CAPS) at pH 11.2. The eluted fractions were neutralised using 0.5 M NaH2PO4. The contamination of IgG was removed by treated with a Hi-Trap protein G column. The flow was rich in C1q that was dialysed against HEPES buffer. The purity of C1q protein was assessed by 12% v/v SDS-PAGE. Before using the C1q in haemolytic assays, the haemolytic activity of C1q was checked through complement reconstitution assays.
2.3 Purification and expression of the human C1q
The recombinant head regions of human C1q, ghA, ghB, and ghC modules were expressed in E. coli BL21 (Kishore et al., 2003; Kojouharova et al., 2004). Plasmids pKBM-B, pKBM-A and pKBM-C containing complementary DNA (cDNA) for ghA, ghB and ghC regions were transformed separately into competent E. coli cells. Cells were grown in Luria-Bertani medium containing (50 μg/mL) antibiotic at 37 °C with shaking, and subsequently induced with 1.5 mM isopropylthio-β-galactoside (IPTG) at OD600 of 0.75 for 2 h. The culture was then centrifuged at 4,500 × g for 15 min, the medium discarded. The pellet was suspended in 20 ml of lysis buffer. After incubation for 2 h at 4 °C cell lysate was sonicated 10–15 times, 30 sec each. The sonicate was then centrifuged (15,000 × g for 30 min), and the collected supernatant was diluted 5-fold using column buffer 1 (100 mM NaCl, 0.2% v/v Tween 20, 5 mM EDTA, 20 mM Tris–HCl, pH 8.0 and 5% v/v glycerol) and loaded on a 10-ml amylose resin column re-equilibrated with column buffer 1(New England Biolabs). After extensively washing the amylose resin column with buffer 2 (20 mM Tris–HCl, pH 8.0, 100 mM NaCl, 5 mM EDTA, and 5% v/v glycerol), the bound protein was eluted in 1 ml fractions with appropriate volumes of buffer 2 containing 10 mM maltose. The protein concentration was determined via Nanodrop and purity was examined via SDS-PAGE.
2.4 Expression and purification of substitution mutants of human C1q
The ghA, ghB and ghC derived substitution mutants (ArgA162Ala/Glu, ArgB114Gln/Glu, HisB117Asp, ArgB129Ala/Glu, LysB136Glu, ArgB163Ala/Glu, TyrB175Leu, HisC101Ala, ArgC156Glu, LysC170Glu) were expressed as described above (Kishore et al., 2003; Kojouharova et al., 2004). Similar to the wild-type proteins, the substitution mutants were purified using an amylose resin column under gravity. These mutants were investigated based on previous data showing their essential role in C1q binding to wide range of ligands such as IgG, IgM, pentraxin 3 and C-reactive protein (Roumenina et al, 2006; Zlatarova et al., 2006). Contaminating Lipopolysaccharide (endotoxin) was eliminated using Polymyxin B column (Sigma). Limulus amebocyte lysate assay (BioWhittaker) was used to measure the level of contaminating and found to be less than ∼4 pg per μg of the recombinant protein.
2.5 Complement assay for the classical pathway
The modulation of classical pathway activation by SLA was examined by a complement consumption assay (Kishore et al., 1998). Different concentrations with an equal volume of normal human serum (NHS) were incubated with occasional shaking at 37 °C for 1 h. 1:1 diluted in dextrose gelatin veronal buffer composed of (DGVB++; 2.5 mM sodium barbital, 71 mM NaCl, 0.15 mM CaCl2, 0.5 mM MgCl2, 2.5% w/v glucose, 0.1% w/v gelatin, pH 7.4). The supernatants were tested for their complement activity by measuring their ability to lyse antibody-sensitised sheep erythrocytes (EA). 100 µl of EA cells (1x108 cells/ml in DGVB++) were added to serial dilutions (100 µl) of each supernatant in DGVB++ and incubated for 1 h at 37 °C. Supernatant was centrifuged at 5000 rpm for 5 min. The released haemoglobin from 100 µl of the supernatant was measured at 405 nm. Total haemolysis was measured by lysing EA with water. Diluted Serum was used without SLA as positive control. In addition, non-sensitised sheep erythrocytes (E) were used as a control for EA lysis at the same dilution of NHS.
2.6 Direct binding ELISA to examine interactions of C1q and recombinant wild type and mutant globular domain proteins with SLA
SLA was diluted in carbonate-bicarbonate buffer (Sigma-Aldrich) in the required dilutions (2.5 µg, 1.25 µg, 0.625 µg and 0.312 µg per well in 100 µl volume) and incubated 12 h at 4 °C. 100 µl of bovine serum albumin (BSA) was used to block microtitre wells at 37 °C for 4 h. The wells were then washed multiple times with PBS containing 0.05% v/v Tween 20 by filling up the wells and discarding the washing buffer. C1q and recombinant globular heads (ghA, ghB, ghC) were concentration-adjusted per well in binding buffer (20 mM Tris pH 8.0, 100 mM NaCl, 10 mM CaCl2) and incubated for one hour at 37 °C and another one hour at 4 °C. Washing buffer PBS 0.05%Tween 20 was added to the wells and the primary antibody was added to the wells. C1q was probed with anti-human C1q monoclonal antibody (1:5000) while monoclonal anti-MBP antibody (1:7000) (Sigma-Aldrich) was used to probe ghA, ghB, ghC and their respective substitution mutants; the incubation lasted for one hour at 37 °C. After another round of extensive washing, 100 µl of secondary antibody rabbit anti-mouse IgG-horseradish peroxidise conjugate (1:5000) in PBS was added to each microtitre well for one hour at 37 °C. The colour was developed using o-Phenylenediamine dihydrochloride and measured at A450 using the BioRad spectrophotometer.
2.7 Secreted cytokine, chemokine and growth factor multiplex assay
Different type of cytokines concentrations such as; (IL-6, IL-8, IL-10, IL12p40, IL12p70, IL-23, IL-27, IL-1α and IL-1β) and chemokines/growth factors concentrations such as; (MIG, I-TAC, MCP-1, G-CSF and M-CSF) were measured by MagPix Milliplex kit (EMD Millipore). THP-1 cells (1 × 1010 per well) were incubated with SLA (5 µg) with and without purified C1q (5 µg) for 24 h and 48 h, cells were spun down at 300 × g, and supernatants were collected for measuring a wide range of secreted pro-inflammatory and anti-inflammatory cytokines, chemokines, growth factors, and soluble ligands and receptors. The assay was done by adding 25 µl each of assay buffer and the supernatant from the THP-1 cell culture in a 96-well microtiter plate. A mixture of magnetic beads representing individual analytes mixed with dilution buffer was added to each well and incubated overnight at 4 °C. Next morning, the wells were washed with assay buffer and then probed with 25 µl of detection antibodies (1 h at room temperature). This was followed by addition of 25 µl of Streptavidin-Phycoerythrin conjugate (since target primary antibodies were biotinylated) to each well and incubated for 30 min at RT. Then, 150 µl of sheath fluid was added to each well. The Luminex Magpix instrument was used to read the plate wells.
2.8 Statistical analysis
For the classical pathway assays, the readings were plotted as percentage of lysis using the following equation
; Where Cx = OD415 of sample, B = spontaneous lysis, L = maximum lysis. Graphs were generated using GraphPad Prism 6.0; and the statistical analysis was done by using unpaired one-way ANOVA test. Error bars represents SD or SEM (n = 3). The significant values were measured based on p < 0.05. A 2-sided unpaired t-test was used to compare the mean of the cytokine targets between SLA +/- C1q for any significant differences. P values were computed, and graphs were compiled and analysed.
3 Results
3.1 Soluble Leishmania antigens activate the classical pathway
In order to assess if SLA can activate the classical pathway, we prepared haemolysin-sensitised sheep erythrocytes and assessed only classical pathway activation via determination of CH50 value (Fig. 1A). SLA (5 µg) was coated on microtiter wells and serially double-diluted. NHS was added to the wells for complement consumption for 1 h. The values derived were then plotted on the graph as percentage lysis. EA cells exposed to 1:10 diluted serum incubated with SLA exhibited only ∼22% complement activity, suggesting ∼77% of the total complement activity of NHS, when compared to MBP (Fig. 1B). NHS, untreated with SLA, showed ∼82% lysis at the same dilution and the CH50 value was attained earlier at ∼1:60 dilution of NHS. However, NHS consumed by SLA did not attain a CH50 value, suggesting high level of complement consumption.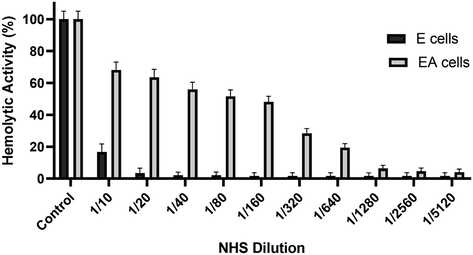
Haemolytic assay of normal human serum antibodies. 100 µl of human serum was treated with 200 µl of washed sheep red blood cells overnight followed by centrifugation and collection of supernatants. The supernatant serum was incubated at different dilutions (as shown in the graph) in DGVB2+ and incubated with 100 µl of sensitised and non-sensitised erythrocytes (EA and E cells respectively) for one hour at 37 °C. After centrifugation, the supernatants were collected and the absorbance was read at OD415. EA and E with water was used as 100% lysis and the graph was plotted as a percentage of lysis.
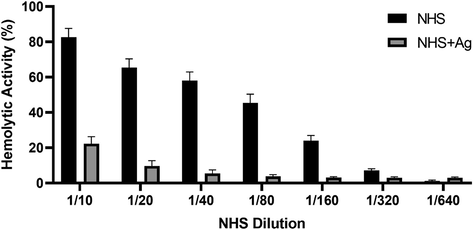
Classical pathway haemolytic assay via complement consumption by soluble leishmania antigens (SLA). 5 µg of SLA was coated in microtitre wells using carbonate-bicarbonate buffer overnight. After blocking with 2% w/v BSA in DGVB2+ buffer, serially double-diluted normal human serum (NHS) were added to the wells. Recombinant E. coli Maltose-binding protein (MBP) was used as a negative control. Following 1 h incubation at 37 °C, haemolysin-sensitised EA cells (100 μl) were added to each well and incubated for another 1 h. After centrifugation, the supernatants were collected and the absorbance was read at OD415. NHS diluted serially was used as a negative control of complement consumption. EA and E with water was used as 100% lysis and the graph was plotted as a percentage of lysis. The experiment was carried out in duplicates. SLA (NHS + Ag) consumed nearly 80% of the total complement in the serum. Results are expressed as percentage lysis with reference to a standard curve established using CH50 value from Fig. 1A (calculation formula under the Methods section). The data were expressed as mean of three independent experiments (n = 3) done in triplicates ± SEM. Statistical significance was established using the unpaired one-way ANOVA test (p < 0.01).
3.2 Full length human C1q binds SLA via its globular domain
Different concentrations of SLA were coated on microtitre wells and allowed to bind C1q, ghA, ghB, and ghC. Recombinant forms of head regions of C1q A, B and C chains (ghA, ghB and ghC, respectively) were expressed and purified (Fig. 2A) to ascertain if C1q binds to SLA via its globular domain or triple-helical collagen domain. C1q and ghB bound SLA dose-dependently (Fig. 3A) and more specifically when compared to ghA, ghC and MBP (Fig. 3B). This suggested that SLA interacted to C1q via the most apically oriented module of the globular domain i.e. ghB. Next, we examined if single amino acid mutations made in the ghA, ghB and ghC modules impacted upon C1q-SLA interaction.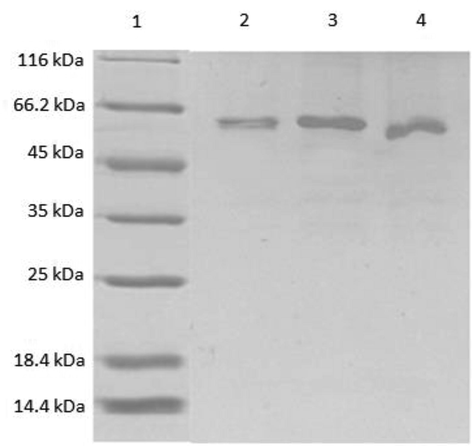
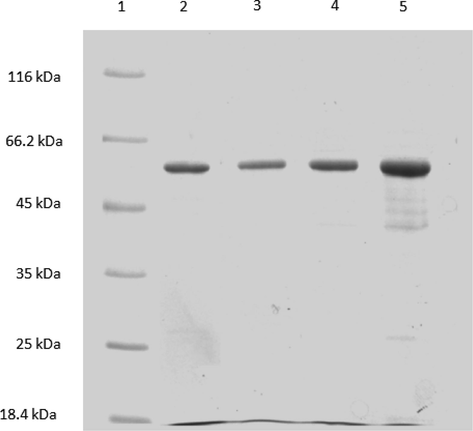
SDS-PAGE analysis of purified MBP-ghA, MBP-ghB, MBP-ghC after elution from amylose resin. All three proteins were run simultaneously on a 12% acrylamide SDS-PAGE gel. The three proteins ran as a single thick band at ∼60 kDa. Lane 1: Standard molecular weight markers; Lane 2: MBP-ghA; Lane 3: MBP-ghB; Lane 4: MBP-ghC.
3.3 SLA-C1q binding involves charge-charge interaction
The nature of interaction between SLA, and recombinant ghB and its single residue mutants (ghB-R114Q, ghB-R129A, ghB-R163E, ghB-R114E, ghB-R129E, ghB-H117D, ghB-T175L, ghB-L163G) were examined using solid-phase binding assays (Fig. 2B, C, D). At 10 µg concentration of SLA, the substitution of ghB mutant Arg129 to alanine and glutamate resulted in 2% and 13% reduction in binding, respectively. Arg114 substitution of ghB led to 39% decrease of binding when substituted with both glutamic acid and glutamine. A similar decrease of binding was observed in the Arg163Glu substitution (31%). However, substitution mutants His117 to aspartic acid, Thr175 to leucine and Leu136 to glycine had more significant effects on SLA-ghB binding i.e., 49%, 61% and 46%, respectively. At 5 µg concentration of SLA, the substitutional ghB mutant Arg129 to alanine resulted in a reduction of 34%. However, at this particular concentration, Arg129 substitution to a negatively charged glutamic acid leads to an increase of 6% compared to wild type ghB. Arg114 substitution of ghB showed a minor reduction of binding i.e. 13% for glutamine substitution and 24% for glutamic acid substitution. However, substituting Arg163 with glutamic acid exhibited a decreased binding of 62% to SLA. His117 to aspartic acid, Thr175 to leucine and Leu136 to glycine had more significant effects on SLA-ghB binding at 5 µg i.e., 52%, 60% and 46%, respectively. On an average, Arg substitutions resulted in less reduced binding i.e. 29% reduced binding in R114Q, 33% reduced binding in R114E, 25% less binding in R129A and 13% reduced binding in R129E to SLA compared to His and Leu substitutions leading to 50% less binding and Thr substitution leading to 61% less binding. However, one arginine substitution (R163E) showed a reduced binding of 48% to SLA (Table 1).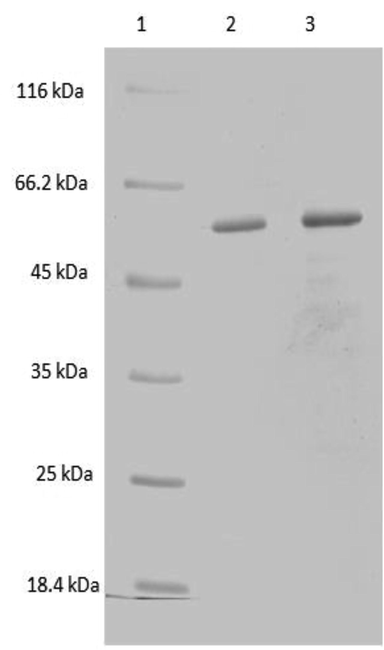
SDS-PAGE analysis of purified ghA mutants. The ghA mutants expressed as MBP-fusion proteins were purified over amylose resin matrix, eluted with 10 mM maltose, and run on a 12% vol/vol acrylamide gel. Purified ghA mutants: lane 1: molecular marker; lane 2: ghA-R162A; lane 3: ghA-R162E.
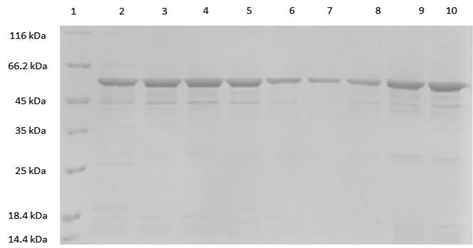
SDS-PAGE analysis of purified ghB mutants. The ghB mutants expressed as MBP-fusion proteins were purified on amylose resin column and eluted with 10 mM maltose. The purified recombinant proteins were run on a 12% vol/vol acrylamide gel, as described under Methods section. Lane 1: Molecular marker; Lane 2: ghB-R163A; Lane 3: ghB-R163E; Lane 4: ghB-R114Q; Lane 5: ghB-R114E; Lane 6: ghB-R129E; Lane 7: ghB-R129A; Lane 8: ghB-T175L; Lane 9: ghB-L136G; Lane 10: ghB-H117D.
SDS-PAGE analysis of purified ghC mutants. The ghC mutants expressed as MBP-fusion proteins were purified using an amylose resin matrix and eluted with 10 mM; each purified mutant protein was separately run on a 12% vol/vol acrylamide gel. Lane 1: Molecular marker; Lane 2: ghC-H101A; Lane 3: ghC-R156E; Lane 4: ghC-L170E; Lane 5: ghC-R156Q.
Substitution mutants
% reduction in binding to SLA compared to wild type used as 100%
% direct binding to SLA compared to wild type used as 100%
ghB-R114Q
39%
61%
ghB-R129A
2%
98%
ghB-R163E
62%
28%
ghB-R114E
39%
61%
ghB-R129E
13%
87%
ghB-H117D
49%
51%
ghB-T175L
61%
39%
ghB-L163G
46%
54%
3.4 C1q modulates SLA-induced immune response by macrophages
To further understand the immune response of THP-1 cells, when challenged with SLA, with and without C1q, we cultured THP-1 cells in serum for 24 h and 48 h, collected their supernatants, and subjected them to analyte measurement of cytokines (Fig. 4), chemokines (Fig. 5), growth factors (Fig. 6) and soluble ligands (Fig. 7) using Multiplex array. SLA on its own induced in THP-1 cells secretion of IL-1α, IL-6, IL-1 α, IL-12, IL-17A, and IL-15 (Fig. 4). Similarly, chemokines and growth factors and soluble ligands were induced in THP-1 cells and secreted (Figs. 5–7). These data suggested a pro-inflammatory effect of SLA on THP-1 cells. Using 48 h as a reference point, we found that C1q seemed to upregulate IL-1 α, while it downregulated in THP-1 cells IL-6, IL-1 α, IL-12, IL-17A, and IL-15; the suppressive effects of C1q on IL-1 α, IL-12 and IL-17A quite considerable (Fig. 4). On the chemokine side, MIP-1a, was upregulated by C1q + SLA combination, while IL-8, IP-10, GRO and Eotaxin were downregulated; the suppression of IP-10 in the presence of C1q was very dramatic (Fig. 5). Interestingly, almost all growth factors (FGF, VEGF, EGF, GM-CSF and G-CSF) were downregulated by the presence of C1q (Fig. 6). With respect to other soluble ligands, such as IFN-g, IFN-a, Flt-3, IL-1RA and sCD40L, C1q was able to suppress their levels triggered by SLA consistently (Fig. 7). These results suggest that C1q can considerably influence pro-inflammatory soluble factors secreted by THP-1 cells, with the exception of IL-1 α (Fig. 4) and MIP-1 α (Fig. 5). Of note is the suppression of IL-12 (Fig. 4) that appears to suggest that C1q may be altering negatively to Th1 response that is required for parasite clearance as well as vaccine outcome using SLA.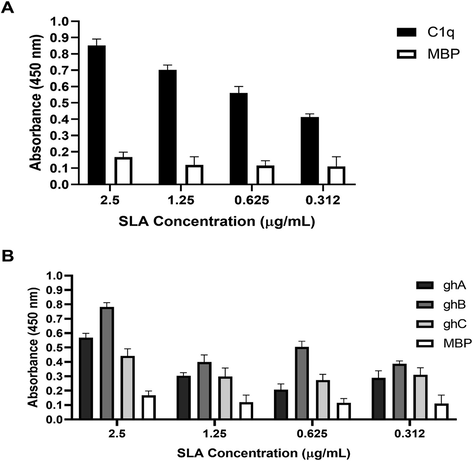
Binding of human C1q (A), ghA, ghB and ghC (B) to solid phase SLA. Various concentrations of SLA were coated on microtitre wells and a fixed concentration (5 µg) of C1q, ghA, ghB and ghC were added. The interactions were detected by probing with a primary antibody, rabbit anti-human C1q antibody for C1q and rabbit anti-MBP antibody for globular head regions, followed by secondary antibody IgG conjugated with HRP. The colour was developed using o-Phenylenediamine dihydrochloride and A415 values were measured. All experiments were done in triplicates with MBP used as a negative control and C1q used as a positive control. The data were expressed as mean of three independent experiments (n = 3) done in triplicates ± SEM. Statistical significance was established using the unpaired one-way ANOVA test (p < 0.05).
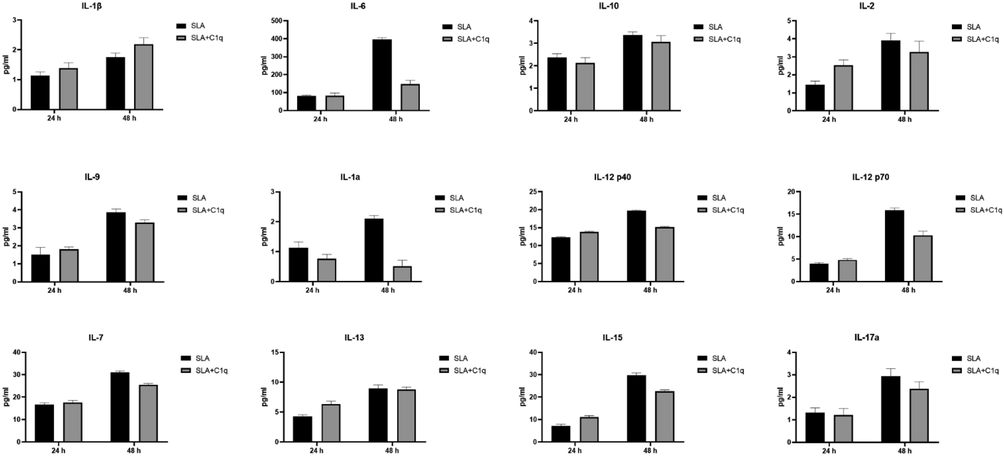
Multiplex analysis of cytokine analytes at 24 and 48 h using supernatant from the SLA challenged THP-1 cells with (SLA + C1q) and without C1q (SLA). Error bars represent ± standard deviation. A 2-side unpaired t-test (with Welch’s correction) was performed on the data to determine significant differences in protein levels between each group of treated and untreated supernatant. All these comparisons were significant (p < 0.05). IL, interleukin.
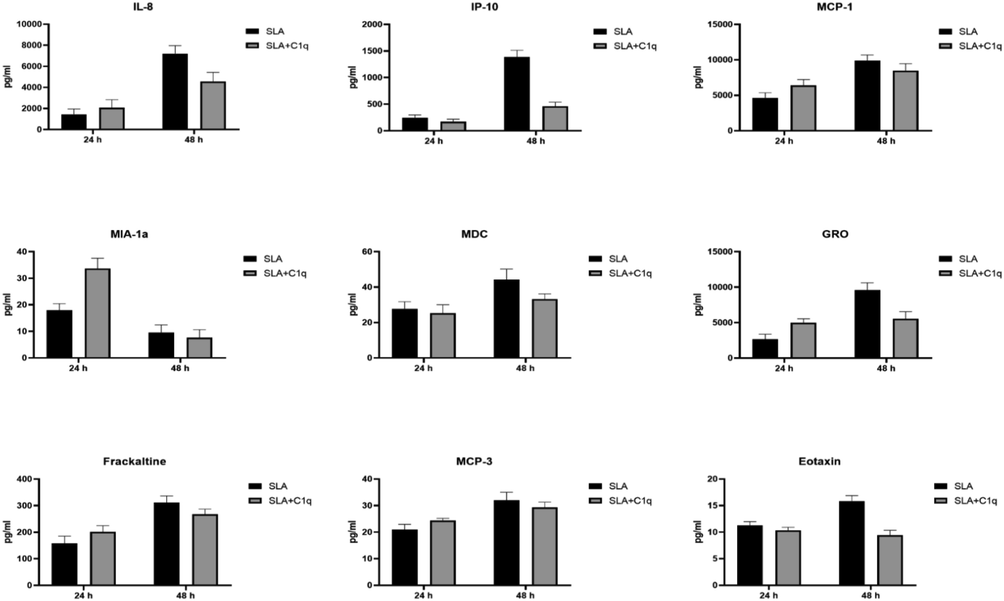
Multiplex analysis of chemokine analytes at 24 and 48 h using supernatant from the SLA challenged THP-1 cells with and without C1q. Error bars represent ± standard deviation. A 2-side unpaired t-test (with Welch’s correction) was performed on the data to determine significant differences in protein levels between each group of treated and untreated supernatant. All these comparisons were significant (p < 0.05). IL, interleukin; IP, interferon-gamma induced protein; MCP, monocyte chemoattractant protein; MDC, macrophage derived chemokine; GRO, growth-related oncogene.
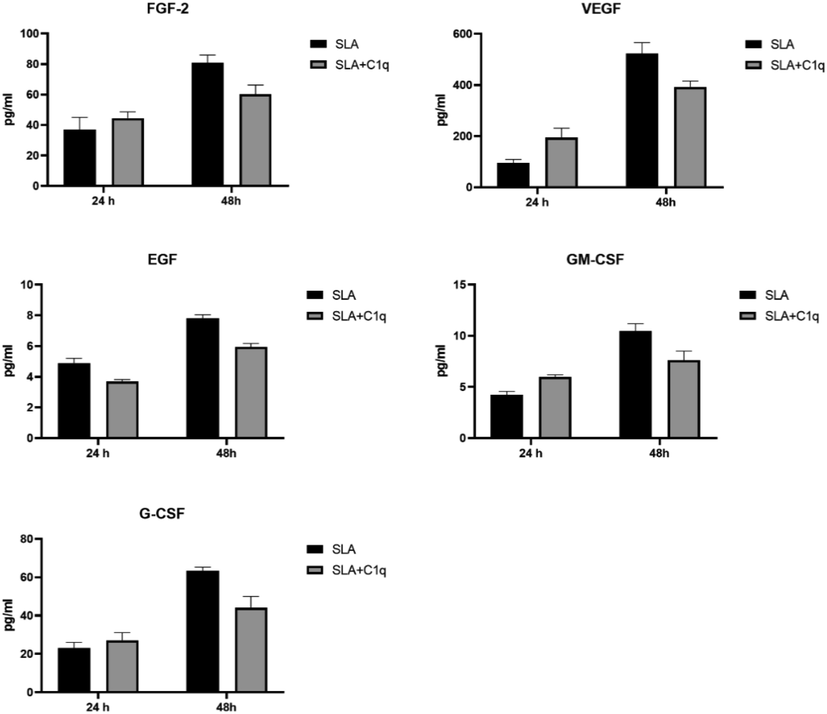
Multiplex analysis of growth factor analytes at 24 and 48 h using supernatant from the SLA challenged THP-1 cells with and without C1q. Error bars represent ± standard deviation. A 2-side unpaired t-test (with Welch’s correction) was performed on the data to determine significant differences in protein levels between each group of treated and untreated supernatant. All these comparisons were significant (p < 0.05). FGF, fibroblast growth factor; VEGF, vascular endothelial growth factor; EGF, epidermal growth factor; GM-CSF, granulocyte-monocyte-colony stimulating factor; G-CSF, granulocyte-colony stimulating factor.
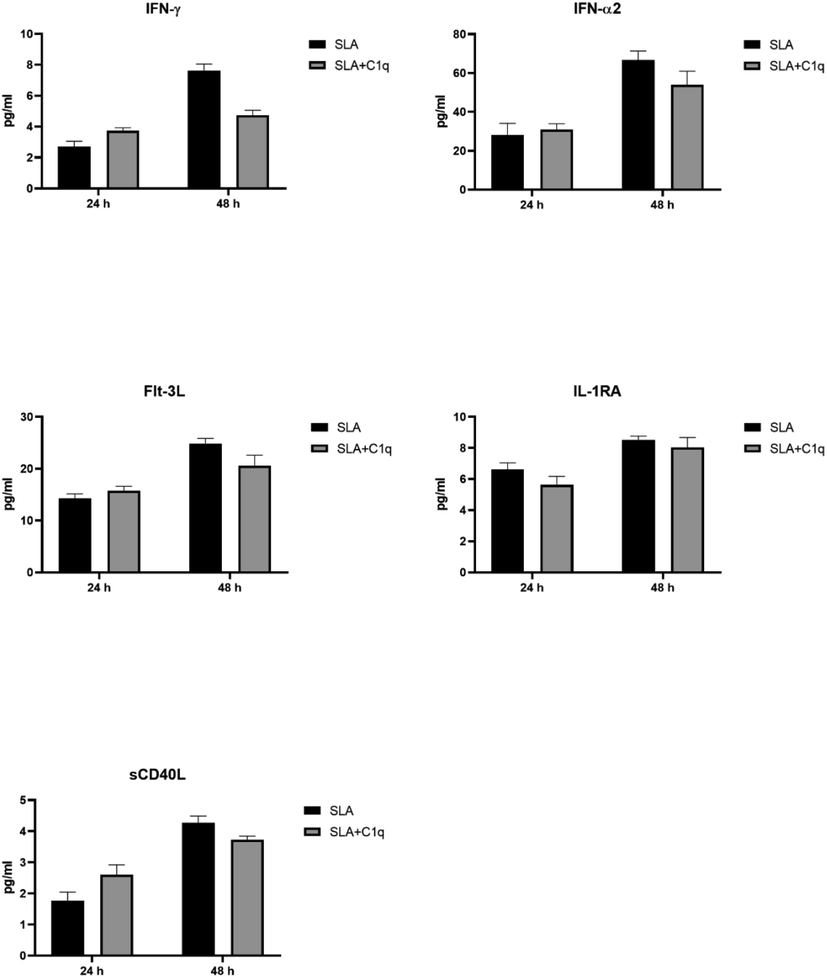
Multiplex analysis of soluble immune ligand analytes at 24 and 48 h using supernatant from the SLA challenged THP-1 cells with and without C1q. Error bars represent ± standard deviation. A 2-side unpaired t-test (with Welch’s correction) was performed on the data to determine significant differences in protein levels between each group of treated and untreated supernatant. All these comparisons were significant (p < 0.05). IFN, interferon; Flt-3L, Fms-related tyrosine kinase 3 ligand.
4 Discussion
Leishmania is an intracellular parasite, and thus prior to macrophage entry, it has to deal with the most important humoral factor i.e. complement system, which can lyse Leishmania very quickly and efficiently (Hoover et al., 1984a, 1985), including promastigotes (Dominguez et al., 2002). The complement alternative pathway is a major player when it comes to complement-mediated lysis of Leishmania (Hoover et al., 1984a; Mosser et al., 1984; Hoover et al., 1985; Mosser and Edelson, 1985; Mosser et al., 1986; Puentes et al., 1989); however, classical and lectin pathways have their own share of contribution in defence against Leishmania (Pearson and Steigbigel, 1980; Navin et al., 1989; Dominguez et al., 2002; Green et al., 1994). This is important since C1q can act in an IgG-dependent manner as well as by directly binding to target ligands (Kishore et al., 2004). Not surprisingly, Leishmania can escape from complement attack by virtue of using its lipophosphoglycan (LPG) and gp63; their deficiency can render L. major parasites susceptible to MAC mediated killing (Joshi et al., 2002; Spath et al., 2003; Yao et al., 2013). LPG allows complement activation to proceed but release MAC as soluble product (Puentes et al., 1990), whereas gp63 inactivates C3b yielding the inactivated fragment iC3b (Ramer-Tait et al., 2012; Brittingham et al., 1995). However, the ability to deposit C3b by LPG and gp63, and not continuing beyond, seems essentially designed to exploit complement receptors present on macrophages to acquire intracellular habitat.
In the current study, we used soluble leishmania antigens (SLA) derived from Leishmania major for its ability to activate the classical pathway (Scott et al., 1987). We used SLA for this study since it is a part of vaccination strategy (with a range of adjuvants) against Leishmania. Thus, it was imperative to understand if SLA activated the complement system, especially the classical pathway, whose first recognition subcomponent C1q can modulate a variety of immune cells including macrophages. We found that SLA was quite efficient in activating the classical pathway to a remarkable level so that the NHS was not able to reach a CH50 value (Fig. 1). We then examined if purified C1q bound to immobilised SLA, which it did (Fig. 3). This prompted us to speculate if the globular domain of C1q was the SLA-binding region. Thus, generated recombinant forms of ghA, ghB and ghC (Fig. 2A), have been shown to interact with a range of C1q ligands differentially (Kishore et al., 1998, 2003; Roumenina et al., 2004) via its globular domain and activates the classical pathway. The recombinant individual regions of C1q A, B and C chains i.e. ghA, ghB and ghC, respectively, bound differentially to SLA, with ghB being the best binder in a dose-dependent manner (Fig. 3A, B). Since ghB as the apical orientation within the globular domain of C1q structure, it was interesting, but not surprising, as reported earlier by Kishore et al. (2003), that C1q relied on this segment for interaction with a complex mixture of target ligands. In order to identify the importance of residues that were involved in this interaction, we generated a range of substitution mutations of the recombinant globular head fragments and characterised their binding to SLA. These substitution mutants have previously been examined for their interaction with IgG, IgM, and other C1q ligands (Kojouharova et al., 2004; Roumenina et al, 2006; Zlatarova et al., 2006). Studies with substitution mutants revealed that C1q-SLA binding relied heavily on charge-charge interaction (Table 1).
Having established a clear involvement of C1q with SLA handling, we next checked if C1q can affect immune response of a macrophage-like cell line, THP-1, when challenged with SLA. We cultured THP-1 cells in serum-free medium for 24 h and 48 h, collected their supernatants, and subjected them to multiplex array analysis for secreted cytokines (Fig. 4), chemokines (Fig. 5), growth factors (Fig. 6) and soluble ligands (Fig. 7). Using 48 h as a reference, we found that C1q upregulated IL-1 α, while it downregulated IL-6, IL-1 α, IL-12, IL-17A, and IL-15; the suppressive effects of C1q on IL-1 α, IL-12 and IL-17A quite considerable (Fig. 4). On the chemokine side, MIP-1a was upregulated by C1q, while IL-8, IP-10, GRO and Eotaxin were downregulated; the suppression of IP-10 in the presence of C1q was very dramatic (Fig. 5). Interestingly, almost all growth factors (FGF, VEGF, EGF, GM-CSF and G-CSF) were downregulated by the presence of C1q (Fig. 6). IFN-γ, IFN- α, Flt-3, IL-1RA and sCD40L levels were suppressed by C1q (Fig. 7). Thus, C1q considerably downregulated pro-inflammatory soluble factors secreted by THP-1 cells, with the exception of IL-1 α (Fig. 4) and MIP-1 α (Fig. 5). C1q also suppressed IL-12 secretion in the presence of SLA (Fig. 4), hinting that it may be suppressing Th1 response intended to be upregulated by SLA since IL-12 is an obligate differentiation factor for Th1 cells secreting IFN- γ, required for parasite clearance as well as good vaccination outcome using SLA (Eskandari et al., 2014). SLA has an inherent tendency to develop Th2 responses (Watanabe et al., 2004). In short, C1q seemed to have a suppressive effect of SLA-induced pro-inflammatory cytokines and chemokines. Whether this suppressive effect of C1q would benefit host or the parasite, remains to be ascertained.
Myd88 gene knock-out (−/−) mice has hematopoietic system defects, as well as signalling and apoptotic defects. They have increased susceptibility to bacterial and viral infection due to immune system abnormalities. Treatment of MyD88−/− mice with IL-12 protected them from L. major infection (Muraille et al., 2003). As a result of increased IL-12 production, Tlr2−/− mice became resistant to Leishmania infections (Vargas-Inchaustegui et al., 2009; Guerra et al., 2010). Glycosphingophospholipids and glycoproteins from L. donovani have been suggested as potential ligands for TLR4. These compounds have the ability to induce effector proteins such as IL-12 (Karmakar et al., 2012; Paul et al., 2012). NK cell activation led to increasing of IL-12 production and specific target cells (Liese et al., 2007; Schleicher et al., 2007)., suggesting triggering of Th1 immune response. Indeed, recognition of Leishmania LPG seems to enhance Leishmania growth by reducing IL-12 production. These results argue the function of TLR stimulation as a potential vaccine therapy. NOD-like receptors (NLRs), which are cytoplasmic Pathogen-Associated Molecular Pattern Recognising Receptors (PRRs), contribute to the constitution of inflammasomes (Martinon et al., 2002; Kanneganti, 2015), which bring about cleavage and activation of cytokines, such as IL-1 α, IL-1β and IL-18. It is interesting to note that C1q upregulated IL-1 α, while downregulating IL-1 αFig. 4). It is known that the infection restriction (in C57BL/6 mice) results from IL-12-dependent production of IFN-γ. In contrast, Balb/c mice succumb to infection because of preferential Th2-type cytokine induction. Therefore, the role of these cytokines in Leishmaniasis is debatable and dictated by the phenotype of the mice strain used. IL-1 α and IL-1 α β can be pathogenic under Th2 bias i.e. in Balb/c mice (Voronov et al., 2010) or protective under Th1 bias in infection models i.e. in C57BL/6 mice (Kautz-Neu et al., 2011). These discrepancies are of great importance in interpreting the alteration in their levels by C1q. In general, cytokines such as IFN- γ, IL-12, TNF- α, IL-2, IL-15 and IL-8 are considered to be protective in against Leishmania, while IL-10, TGF-β, IL-5, IL-6, IL-9, IL-27 and IL-33 are pathogenic (Dayakar et al., 2019).
In conclusion, we report here that SLA, which is used as a candidate vaccine material, seems to bind complement protein C1q via its globular domain, activate the complement classical pathway, and downregulate most of the pro-inflammatory cytokines and chemokines, with the exception of IL-1 β and MIP-1 α. It also seems to downregulate the protective Th1 response via suppression of IL-12 level by SLA-challenged THP-1 cells. These data are quite relevant in understanding weak or lack of Th1 response in mice immunised with SLA alone (Voronov et al., 2010; Kautz-Neu et al., 2011). Complement proteins, especially C1q, may be contributing to this, and hence the requirement for potent adjuvants.
Acknowledgments
This Project was funded by the National Plan for Science, Technology and Innovation (MAARIFAH), King Abdulaziz City for Science and Technology, Kingdom of Saudi Arabia, Award Number (12-MED2681-02).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Leishmaniasis worldwide and global estimates of its incidence. PloS One. 2012;7:e35671
- [Google Scholar]
- Role of the Leishmania surface protease gp63 in complement fixation, cell adhesion, and resistance to complement-mediated lysis. J. Immunol.. 1995;155:3102-3111.
- [Google Scholar]
- Visceral leishmaniasis: what are the needs for diagnosis, treatment and control? Nat. Rev. Microbiol.. 2007;5:873-882.
- [Google Scholar]
- Cytokines: key determinants of resistance or disease progression in visceral leishmaniasis: opportunities for novel diagnostics and immunotherapy. Front Immunol.. 2019;5(10):670.
- [Google Scholar]
- Complement interaction with trypanosomatid promastigotes in normal human serum. J. Exp. Med.. 2002;195:451-459.
- [Google Scholar]
- Immunoliposomes containing Soluble Leishmania Antigens (SLA) as a novel antigen delivery system in murine model of leishmaniasis. Exp. Parasitol.. 2014;146:78-86.
- [Google Scholar]
- Recognition of the major cell surface glycoconjugates of Leishmania parasites by the human serum mannan-binding protein. Mol. Biochem. Parasitol.. 1994;66:319-328.
- [Google Scholar]
- Histopathological analysis of initial cellular response in TLR-2 deficient mice experimentally infected by Leishmania (L.) amazonensis. Int. J. Exp. Pathol.. 2010;91:451-459.
- [Google Scholar]
- Complement-mediated serum cytotoxicity for Leishmania major amastigotes: killing by serum deficient in early components of the membrane attack complex. J. Immunol.. 1985;135:570-574.
- [Google Scholar]
- Killing of Leishmania tropica amastigotes by factors in normal human serum. J. Immunol.. 1984;132:893-897.
- [Google Scholar]
- Macrophage activation to kill Leishmania tropica: defective intracellular killing of amastigotes by macrophages elicited with sterile inflammatory agents. J. Immunol.. 1984;132:1487-1493.
- [Google Scholar]
- Targeted gene deletion in Leishmania major identifies leishmanolysin (GP63) as a virulence factor. Mol. Biochem. Parasitol.. 2002;120:33-40.
- [Google Scholar]
- TLR4 and NKT cell synergy in immunotherapy against visceral leishmaniasis. PLoS Pathog.. 2012;8:e1002646
- [Google Scholar]
- IL-1 signalling is dispensable for protective immunity in Leishmania-resistant mice. Exp. Dermatol.. 2011;20:76-78.
- [Google Scholar]
- Properdin and factor h: opposing players on the alternative complement pathway “see-saw”. Front Immunol.. 2013;23(4):93.
- [CrossRef] [Google Scholar]
- Leishmaniasis: complexity at the host-pathogen interface. Nat. Rev. Microbiol.. 2011;9:604-615.
- [Google Scholar]
- Functional characterization of a recombinant form of the C-terminal, globular head region of the B-chain of human serum complement protein, C1q. Biochem J.. 1998;333(Pt 1):27-32.
- [CrossRef] [Google Scholar]
- Modular organization of the carboxyl-terminal, globular head region of human C1q A, B, and C chains. J. Immunol.. 2003;171(2):812-820.
- [CrossRef] [Google Scholar]
- Mutational analyses of the recombinant globular regions of human C1q A, B, and C chains suggest an essential role for arginine and histidine residues in the C1q-IgG interaction. J. Immunol.. 2004;172(7):4351-4358.
- [CrossRef] [Google Scholar]
- Structural and functional anatomy of the globular domain of complement protein C1q. Immunol Lett.. 2004;95(2):113-128.
- [CrossRef] [Google Scholar]
- Liese J, Schleicher U, Bogdan C. TLR9 signaling is essential for the innate NK cell response in murine cutaneous leishmaniasis. European journal of immunology. 2007; 37:3424–3434. [PubMed] [Google Scholar].
- Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Molecular cell. 2002;10:417–426. [PubMed] [Google Scholar]
- Complement system part I - molecular mechanisms of activation and regulation. Front. Immunol.. 2015;2(6):262.
- [CrossRef] [Google Scholar]
- Leishmania species: mechanisms of complement activation by five strains of promastigotes. Exp. Parasitol.. 1986;62:394-404.
- [Google Scholar]
- The mouse macrophage receptor for C3bi (CR3) is a major mechanism in the phagocytosis of Leishmania promastigotes. J. Immunol.. 1985;135:2785-2789.
- [Google Scholar]
- Genetically resistant mice lacking MyD88-adapter protein display a high susceptibility to Leishmania major infection associated with a polarized Th2 response. J. Immunol.. 2003;170:4237-4241.
- [Google Scholar]
- Effect of immunoglobulin M from normal human serum on Leishmania donovani promastigote agglutination, complement-mediated killing, and phagocytosis by human monocytes. Infect. Immun.. 1989;57:1343-1346.
- [Google Scholar]
- TLR-mediated distinct IFN-gamma/IL-10 pattern induces protective immunity against murine visceral leishmaniasis. Eur. J. Immunol.. 2012;42:2087-2099.
- [Google Scholar]
- Clinical spectrum of Leishmaniasis. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America.. 1996;22:1-13.
- [Google Scholar]
- Mechanism of lethal effect of human serum upon Leishmania donovani. J. Immunol.. 1980;125:2195-2201.
- [Google Scholar]
- Innate immune humoral factors, C1q and factor H, with differential pattern recognition properties, alter macrophage response to carbon nanotubes. Nanomedicine. 2015;11(8):2109-2118.
- [Google Scholar]
- Serum resistance of metacyclic stage Leishmania major promastigotes is due to release of C5b–9. J. Immunol.. 1990;145:4311-4316.
- [Google Scholar]
- Binding and release of C3 from Leishmania donovani promastigotes during incubation in normal human serum. J. Immunol.. 1989;143:3743-3749.
- [Google Scholar]
- Differential surface deposition of complement proteins on logarithmic and stationary phase Leishmania chagasi promastigotes. J. Parasitol.. 2012;98:1109-1116.
- [Google Scholar]
- Interaction of C1q with IgG1, C-reactive protein and pentraxin 3: mutational studies using recombinant globular head modules of human C1q A, B, and C chains. Biochemistry. 2006;45(13):4093-4104.
- [Google Scholar]
- The immunology of susceptibility and resistance to Leishmania major in mice. Nat. Rev. Immunol.. 2002;2:845-858.
- [Google Scholar]
- NK cell activation in visceral leishmaniasis requires TLR9, myeloid DCs, and IL-12, but is independent of plasmacytoid DCs. J. Exp. Med.. 2007;204:893-906.
- [Google Scholar]
- Vaccination against cutaneous leishmaniasis in a murine model. II. Immunologic properties of protective and nonprotective subfractions of soluble promastigote extract. J Immunol.. 1987;139(9):3118-3125.
- [Google Scholar]
- The role(s) of lipophosphoglycan (LPG) in the establishment of Leishmania major infections in mammalian hosts. PNAS. 2003;100:9536-9541.
- [Google Scholar]
- Distinct roles for MyD88 and Toll-like receptor 2 during Leishmania braziliensis infection in mice. Infect. Immun.. 2009;77:2948-2956.
- [Google Scholar]
- IL-1-induced inflammation promotes development of leishmaniasis in susceptible BALB/c mice. Int. Immunol.. 2010;22:245-257.
- [Google Scholar]
- Innate immune response in Th1-and Th2-dominant mouse strains. Shock. 2004;22:460-466.
- [Google Scholar]
- Attenuation of Leishmania infantum chagasi metacyclic promastigotes by sterol depletion. Infect. Immun.. 2013;81:2507-2517.
- [Google Scholar]
- Existence of different but overlapping IgG- and IgM-binding sites on the globular domain of human C1q. Biochemistry. 2006;45(33):9979-9988.
- [Google Scholar]







