Translate this page into:
Comparison of short term and long term multidrug resistant tuberculosis treatment outcomes in tertiary care settings
⁎Corresponding author. saba.shamim@imbb.uol.edu.pk (Saba Shamim)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction
Tuberculosis is a contagious and communicable disease while drug resistant tuberculosis is difficult to treat and requires long duration. Various strategies are focused to treat the drug resistant tuberculosis focusing lesser side effects and shorter duration of treatment.
Objective
To compare treatment outcomes between short-term and long-term regimens among patients registered at a tertiary care hospital's Programmatic Management of Drug-Resistant TB (PMDT) site in Pakistan.
Methods
A comparative prospective cohort study was conducted at IMBB, The University of Lahore, in collaboration with the Health Research Institute (HRI) TB Research Centre at King Edward Medical University/Mayo Hospital, Lahore. The study included a sample size of 136 patients, with 68 patients in each treatment regimen (long-term and short-term). Adult pulmonary MDR TB patients seeking treatment from the facility were included based on predefined criteria. Data was collected using a pre-designed questionnaire after obtaining ethical approval and informed consent. The rates of patients' cure, treatment completed, treatment failure and death were calculated.
Results
A total of 131 patients were successfully followed up and included in the final analysis. Of these, 66 patients were in the long-term treatment group and 65 patients were in the short-term treatment group. The cure rate was 74.8 %, with 98 patients successfully cured, and 13.8 % of patients completed their treatment successfully. In the long-term treatment group, the cure rate was 75.6 %, with a treatment failure rate of 6.2 %. In the short-term treatment group, the cure rate was 73.8 %, with a treatment failure rate of 6.1 %. The death rate was 4.5 % in the short-term treatment group and 6.2 % in the long-term treatment group. However, these differences in treatment outcomes were statistically insignificant (p-values > 0.05). The newer drugs, bedaquiline and delamanid, did not show significant improvements in the treatment of multidrug-resistant TB patients. Side effects at completion of treatment remained to be significantly lesser (p < 0.05) among short term treatment group as compared to long term treatment group.
Conclusion
The findings suggest that short-term treatment regimens are equally effective as long-term treatment regimens, with the added benefit of presenting fewer side effects.
Keywords
Multidrug resistance
Healthcare
Tuberculosis
Treatment
Communicable diseases
- HIV
-
Human Immunodeficiency Virus
- HRI
-
Health Research Institute
- MDR
-
multidrug-resistant
- NTP
-
National TB Control Program
- PMDT
-
Programmatic Management of Drug-Resistant TB
- SD
-
standard deviations
- SPSS
-
Statistical Package for the Social Sciences
- TB
-
Tuberculosis
- WHO
-
World Health Organization
- XDR
-
extensively drug-resistant
Abbreviations
1 Introduction
Tuberculosis (TB) is a contagious and communicable disease that is ranked among the top 10 causes of global deaths. It surpasses Human Immunodeficiency Virus (HIV) as the leading cause of death by a single infectious agent. In 2019, it was estimated that approximately 10 million people worldwide fell ill with TB. Pakistan ranks 5th among 30 highest TB burden countries. (WHO, 2021)
Prompt diagnosis and early treatment play crucial roles in controlling the further spread of diseases. In addition to the challenges posed by COVID-19, drug-resistant TB remains a significant concern for health authorities. In 2019, approximately 500,000 individuals developed rifampicin-resistant TB, with 78 % of them being classified as multidrug-resistant (MDR) TB. (WHO, 2021) This highlights the magnitude of the issue and underscores the need for effective strategies to combat drug-resistant TB.
Unfortunately, Pakistan ranks fourth among the countries with the highest burden of DR-TB.(NTP, 2015) When isolates of Mycobacterium tuberculosis exhibit simultaneous resistance to isoniazid and rifampicin, at least from first-line anti-TB drugs is referred to as multidrug-resistant TB (MDR-TB). When an MDR-TB strain shows resistance to fluoroquinolones and at least one of the three injectable drugs (amikacin, capreomycin, or kanamycin), it is classified as extensively drug-resistant (XDR) TB. (Gandhi et al., 2006)
Treatment failure cases pose a potential threat (Sadacharam et al., 2007) to achieving these goals of Pakistan's National TB Control Program (NTP) including a high number of notified cases and treatment success rate of 91 % (NTP, 2017). Studies have shown varying rates of treatment failure in different countries. For example, a study in Uganda reported a treatment failure rate of 6.8 % (Namukwaya et al., 2011), while a recent study in Pakistan revealed 4.84 % at the end of the fifth month of anti-TB therapy (Yasin et al., 2016).
The treatment duration for MDR-TB cases is typically 20–24 months. While the World Health Organization (WHO) has recommended a standardized short treatment course of 9–12 months for pulmonary MDR-TB, this approach is not considered safe and effective for pregnant women and those with extra-pulmonary tuberculosis (Lange et al., 2016).
In the short-course regimen, the duration of MDR-TB treatment is pressed to 9–11 month. (NTP). Since 2018, the use of bedaquiline and delamanid, two new anti-TB drugs, has been initiated at selected sites in Pakistan. A recent study reported promising outcomes among patients using either of these drugs, indicating their potential safety and effectiveness (Kim et al., 2018). However, a systematic review regarding both drugs demanded more evidence to see usefulness and effectiveness of these drugs in MDR-TB patients. (D’Ambrosio et al., 2017)
Consequently, a systematic comparison of treatment outcomes between the short-term treatment course of 9–12 months and the long-term treatment course of up to 24 months is essential. Therefore, the objective of the present study was to compare the treatment outcomes between short-term and full-term treatment among patients registered at a programmed management of drug-resistant TB (PMDT) site in a tertiary care hospital of Pakistan.
2 Material and methods
This comparative prospective cohort study was conducted at IMBB, The University of Lahore, in collaboration with the HRI TB Research Centre, King Edward Medical University/Mayo Hospital Lahore. The sample size was statistically calculated as 122 patients. Accounting for a potential 10 % dropout rate, a total of 136 patients (68 in each group) were included in this study.
The present study included adult pulmonary MDR-TB patients, of both genders, who sought treatment from the PMDT site at Mayo Hospital Lahore. Patients were included if they tested positive for rifampicin resistance using GeneXpert, regardless of having a history of previous anti-TB treatment. Patients who had poor treatment compliance, were transferred out or transferred in from other treatment facilities were excluded from the study. Any Patient delays for five or more days to receive monthly drugs for consecutive three months, was labelled as poor compliance.
After obtaining ethical approval from the institutional review board of King Edward Medical University (letter No. 439/RC/KEMU, dated July 4, 2020), informed consent was obtained from all patients participating in the study. Quasi-random sample allocation method was used to divide the patients into two groups based on the type of treatment using consecutive sampling method. Eligibility criteria for short term treatment was used as laid down by the National TB Control Program Pakistan considering MDR/RR TB cases, no prior exposure to second line drugs and no intolerance to any of drugs in regimen etc. (Ghafoor et al., 2017) A trained MDR TB Physician decides to allocate the patients in short and long term treatment groups. Bedaquiline and delamanid were provided to this one of the three PMDT sites in Pakistan. (Munir et al., 2018) These drugs were not being used as standard treatment regimen till completion of this study and MDR TB physician was authorized to select the patients to provide one or both of these drugs on the basis of pre-defined guidelines. (Ghafoor et al., 2017) Data collection was conducted using a pre-designed questionnaire, which included demographic information, medical history, and other relevant details. Patients were followed and monitored till the end of their treatment and the outcomes were recorded accordingly. The rates of patients' cure, treatment completed, treatment failure and death were calculated.
The data collected for this study was entered into the Statistical Package for the Social Sciences (SPSS) software for analysis. Qualitative variables were presented in terms of frequencies and percentages while quantitative variables were presented as mean ± standard deviation (SD). To compare the outcomes of long-term and short-term TB treatments, a Chi-square test was applied, and a p-value of ≤ 0.05 was considered statistically significant. Relative risk for both groups was also calculated.
3 Results
A total of 136 MDR TB patients were initially registered for follow-up, five of whom were lost to follow-up. The remaining 131 patients, consisting of 71 (54.2 %) males and 60 (45.8 %) females, with a female-to-male ratio of 1:1.8, were successfully followed up. There were 66 patients in the long-term treatment group, while 65 were followed up in the short-term treatment group and shown in Fig. 1.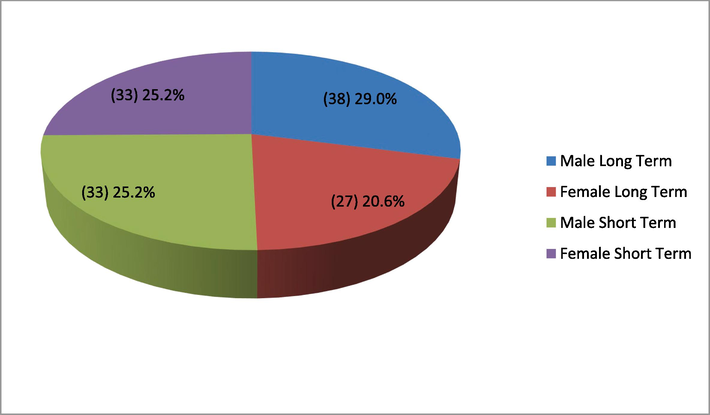
Gender distribution of patients.
Overall mean age of the patients remained 35.08 ± 15.25 in this study. The mean age of female MDR TB patients was 33.10 ± 15.88, which was lower compared to males (36.75 ± 14.60) and difference was insignificant (p-value ≥ 0.174). The mean age of patients receiving short-term treatment was 37.06 ± 15.64, while the mean age of patients in the long-term treatment group was 33.06 ± 14.70. Most patients (62.6 %) were married, and many had either an illiterate (35.9 %) or primary (19.8 %) level of education. Only 3.8 % of respondents had an education level higher than high school. Baseline anthropometric measurements of the patients were also considered in this analysis, as shown in Table 1. Further analysis revealed that 21.4 % of patients were severely underweight, 37.4 % were underweight, 6 % were overweight, and 0.8 % were severely obese, as presented in Fig. 2.
Variable
Category
Total
Short Term (9–11 Months)
Long Term (18–24 months)
Mean
St. Dev.
Mean
St. Dev.
Mean
St. Dev.
Weight (Kg)
47.56
8.29
49.29
11.32
48.42
9.91
Height (Inches)
64.03
3.79
63.60
4.51
63.82
4.15
BMI (Kg/m2)
18.04
3.32
18.84
3.68
18.43
3.51
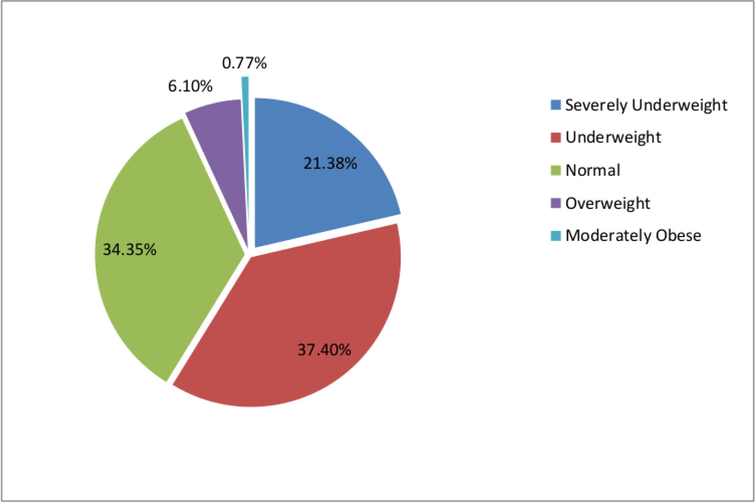
Baseline BMI outcomes among patients.
The smear grading revealed that the highest number of patients (38.9 %) in the 2 + grade and lowest of 3.8 % in the scanty category. Relationship between the smear results and GeneXpert results is presented in Table 2.
Smear Result
GeneXpert Result
Overall
Detected VL
Detected Low
Detected Medium
Detected High
n
%
n
%
n
%
n
%
n
%
Scanty
2
1.5
3
2.3
0
0.0
0
0.0
5
3.8
1+
22
16.8
23
17.6
3
2.3
0
0.0
48
36.7
2+
3
2.3
34
26.0
12
9.2
2
1.5
51
38.9
3+
1
0.8
5
3.8
7
5.3
14
10.7
27
20.6
Total
28
21.4
65
49.6
22
16.8
16
12.2
131
100
Baseline signs and symptoms of the patients were also recorded, fever (96.9 %) showed highest proportion followed by weight loss (97.7 %), fatigue (92.4 %) and anorexia (89.3). History of contact was present among 63.4 %, while 24.4 % had a history of MDR TB contact. The previous history of TB treatment was further explored, revealing that 65 % patients had a history of Category-I treatment and 4 % had a history of Category-II treatment.
After registration, patients were offered various combinations of drugs for short and long-term treatments, as indicated in Table 3. Approximately 70.3 % of patients in the long-term treatment group were offered levofloxacin or ofloxacin, and 34 out of 65 patients (53.1 %) were given bedaquiline. Among those receiving bedaquiline, 5 out of 34 patients (14.7 %) were also administered delamanid in combination. However, these new drugs were not prescribed to patients in short-term treatment group.
Drug Name
Category
Total
Short Term (9–11 Months) (n = 65)
Long Term (18–24 months) (n = 52)
n
%
n
%
n
%
Isoniazid
65
98.5
28
43.8
93
71.5
Rifampicin
0
0.0
2
3.1
2
1.5
Pyrazinamide
66
100.0
62
96.9
128
98.5
Ethambutol
66
100.0
28
43.8
94
72.3
Amikacin
66
100.0
38
59.4
104
80.0
Levo/ofloxacin
6
9.1
45
70.3
51
39.2
Ethionamide
61
92.4
35
54.7
96
73.8
Clofazimine
66
100.0
52
81.2
118
90.8
Bedaquiline
0
0.0
34
53.1
34
26.2
Delamanid
0
0.0
5
7.8
5
3.8
After receiving the drug susceptibility reports, the MDR physician revised the treatment plan for 117 patients (89.3 %). The treatment of almost all patients in the short-term treatment group (65 out of 66) was revised, while 80 % of patients in the long-term treatment group had their treatment plan revised, and the remaining 20 % continued with the initial treatment plan. All the changes in treatment regimen are indicated in Table 4. Notably, linezolid was added to the treatment plan for 26 cases in the long-term treatment group.
Drug Name
Category
Total
Short Term (9–11 months)
Long Term (18–24 months)
n
%
n
%
n
%
Rifampicin
1
1.5
0
0.0
1
0.9
Pyrazinamide
64
98.5
51
98.1
115
98.3
Ethambutol
65
100.0
3
5.8
68
58.1
Amikacin
0
0.0
24
46.2
24
20.5
Levo/Moxifloxacin
64
98.5
44
84.6
108
92.3
Ethionamide
0
0.0
32
61.5
32
27.4
Linezolid
0
0.0
26
50.0
26
22.2
Clofazimine
64
98.5
26
50.0
90
76.9
Cycloserine
0
0.0
50
96.2
50
42.7
Bedaquiline
0
0.0
40
76.9
40
34.2
Delamanid
0
0.0
5
9.6
5
4.3
A total of 98 patients (74.8 %) were cured, and 18 patients (13.8 %) completed their treatment successfully. Comparison of both treatment groups is presented in Table 5. There was no significant difference (p-value > 0.05) among treatment outcomes between the short and long-term treatment groups. Relative risk for short term and long term groups was calculated which shows that the short term treatment is equally effective to the long term treatment regimen (RR = 1.0194). Overall Chi-Square: 8.177p-value: 0.85. Fishers’s Exact Test: 7.673p-value: 0.272.
Treatment outcome
Category
p-value
Short Term (9–11 Months)
Long Term (18–24 months)
n
%
n
%
Cured
50
75.8
48
73.8
0.793
Treatment Completed
9
13.6
9
13.8
0.974
Shifted to LTR
4
6.1
–
–
–
Failure
–
–
4
6.2
–
Died
3
4.5
4
6.2
0.667
Out of 40 patients receiving bedaquiline, 2 (5.0 %) died, and 3 (7.5 %) were categorized as treatment failures. Similarly, out of the 5 patients who received delamanid, 2 (40.0 %) were also categorized as treatment failures, suggesting a limited effectiveness of adding both drugs to the treatment regimen. Side effects at completion of treatment were also explored and significant lesser (p < 0.05) side effects among short term treatment group were observed as compared to long term treatment group as depicted in Table 6.
Side effects
Category
p value
Short Term
(9–11 Months)
Long Term
(18–24 months)
n
%
n
%
Nausea
16
24.2
26
40.0
0.040
Irritation
0
0.0
9
13.8
0.001
Weakness and Lethargy
2
3.0
8
12.3
0.045
Joint Pain
17
25.8
29
44.6
0.019
Hearing Loss
2
3.0
2
3.1
0.679
Vision problem
1
1.5
2
3.1
0.494
4 Discussion
No significant difference was observed in treatment outcomes between the two groups in this study. In the short-term treatment group, 6.1 % of patients who failed to respond to the treatment regimen were shifted to long-term treatment. Conversely, in the long-term treatment group, patients who experienced treatment failure were placed on symptomatic treatment as advised by the physician. Within the short-term treatment group, 3 (4.5 %) patients died, while in the long-term treatment group, 4 (6.2 %) patients died after completing more than half of the treatment.
A study compared drug-resistant and drug-sensitive cases, and reported promising results. (Han et al., 2003) Another study evaluated predictors of unsuccessful interim treatment reported a success rate of only 60 % among MDR-TB cases. A substantial proportion of patients were died (15 %) and 11.3 % were lost to follow-up (Atif et al., 2017) not comparable to the present study, as the criteria used in the present study excluded early defaulters and deaths that occurred before completing at least half of the treatment. An overall success rate of 45.3 % was reported in a study, with a cure rate of 30.2 %, treatment completion rate of 6.6 %, and short-term treatment completion rate of 8.5 %. Additionally, significantly higher rates of treatment failure and default among MDR TB patients compared to XDR TB outcomes were described (Kim et al., 2008), hence not comparable to present findings.
Efforts to reduce the duration of conventional long-term treatment for MDR TB began in 2014, and trials were conducted to study combinations of tolerable drugs based on prior experiences and prospective cohorts. The shorter MDR TB regimen involved the use of seven drugs for 9–12 months, regardless of patients' age and HIV status, which was found to be feasible. (Sotgiu et al., 2016) A meta-analysis on the other hand contradicts the present findings. It showed a lower success rate of 54 % among 9,153 MDR TB patients included in 32 pilot studies conducted in 23 countries (Ahuja et al., 2012).
The use of shorter treatment regimens for MDR-TB patients was initially explored by a few clinicians. However, at that time, only one study had been conducted on a series of MDR-TB patients in Bangladesh (Van Deun et al., 2013). The first randomized clinical trial in various countries during phase III evaluated the safety, efficacy, and economic impact of a short treatment regimen for MDR-TB patients demonstrated high cure rates (>85 %) and effective with significantly fewer side effects. (Trébucq et al., 2019)
The trial of the short treatment regimen showed a favorable outcome as 78.8 %, comparable to the 79.8 % observed with the longer treatment regimen (Trébucq et al., 2019) The results of the present study align closely with the aforementioned trial, as they demonstrated a cure rate of 75.8 % with the short treatment regimen and 73.8 % with the longer treatment regimen, That indicates a potential superiority of the short treatment regimen in terms of patient adherence and treatment completion.
The treatment outcomes were evaluated in patients who received additional drugs, such as bedaquiline and delamanid. Present findings suggest that the addition of bedaquiline and delamanid had limited effectiveness in improving treatment outcomes. It is important to note that bedaquiline and delamanid belong to Group 5 of the World Health Organization (WHO) categorization, which means they are not recommended for routine use due to uncertainties regarding their safety and efficacy in humans, despite showing some activity in animal models (Dheda et al., 2018). In the present study, these drugs were offered to more critical patients, which may have contributed to the compromised treatment results observed. Considering 2(28.6 %) deaths of patients receiving bedaquiline among total 7 deaths is considerable but data is too scarce to apply reasonable statistical tests to find factors like age, gender and severity of disease which could be considered the limitation of this study.
5 Conclusion
This study demonstrates that a short treatment regimen is as effective as long-term treatment, with the added benefit of presenting fewer side effects. However, the effectiveness of isoniazid and rifampicin should not be disregarded until conventional drug susceptibility results are obtained. It is crucial for National TB Control Programs to establish the necessary infrastructure for conducting drug susceptibility testing at the divisional level.
The limited use of newer drugs i.e. bedaquiline and delamanid showed potentially compromised the observations and it is a limitation of this study which require to see a greater sample size for calculation of preside outcomes.
CRediT authorship contribution statement
Muhammad Kashif Munir: Conceptualization, Data curation, Writing-original draft, Investigation, Formal Analysis, Methodology, Software. Muhammad Saqib Saeed: Conceptualization, Data curation, Writing-review and editing, Visualization, Validation, Resources, Project administration. Syed Zeeshan Haider: Writing-review and editing, Formal Analysis, Methodology, Software. Saba Shamim: Conceptualization, Writing-review and editing, Validation, Supervision, Project administration.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Multidrug resistant pulmonary tuberculosis treatment regimens and patient outcomes: an individual patient data meta-analysis of 9,153 patients. PLoS Med.. 2012;9(8):1-16.
- [Google Scholar]
- Predictors of unsuccessful interim treatment outcomes of multidrug resistant tuberculosis patients. BMC Infect. Dis.. 2017;17:1-12.
- [Google Scholar]
- Delamanid and bedaquiline to treat multidrug-resistant and extensively drug-resistant tuberculosis in children: a systematic review. J. Thorac. Dis.. 2017;9:2093-2101.
- [Google Scholar]
- Recent controversies about MDR and XDR-TB: G lobal implementation of the WHO shorter MDR-TB regimen and bedaquiline for all with MDR-TB? Respirology. 2018;23:36-45.
- [Google Scholar]
- Extensively drug-resistant tuberculosis as a cause of death in patients co-infected with tuberculosis and HIV in a rural area of South Africa. Lancet. 2006;368:1575-1580.
- [Google Scholar]
- Protocol for Treating MDR-TB/RR-TB with Shorter Treatment Regimen (STR) NTP Manual. 2017
- [Google Scholar]
- The short-term and long-term treatment outcomes in patients with pulmonary tuberculosis positive for drug-resistant and sensitive strains. Zhonghua jie he he hu xi za zhi= Zhonghua Jiehe he Huxi Zazhi = Chinese J. Tuberculosis Respiratory Diseases. 2003;26:70-73.
- [Google Scholar]
- Treatment outcomes and long-term survival in patients with extensively drug-resistant tuberculosis. Am. J. Respir. Crit. Care Med.. 2008;178:1075-1082.
- [Google Scholar]
- Bedaquiline and delamanid for the treatment of multidrug-resistant tuberculosis: a multicentre cohort study in Korea. Eur. Respir. J.. 2018;51:1702467.
- [Google Scholar]
- Limited benefit of the new shorter multidrug-resistant tuberculosis regimen in Europe. Am. J. Respir. Crit. Care Med.. 2016;194:1029-1031.
- [Google Scholar]
- Meeting the challenge, making a difference: Multidrug resistance tuberculosis in Pakistan. Pak. J. Med. Res.. 2018;57:1-2.
- [Google Scholar]
- Predictors of treatment failure among pulmonary tuberculosis patients in Mulago hospital, Uganda. Afr. Health Sci.. 2011;11:105-111.
- [Google Scholar]
- NTP, 2015. National TB Control Program, Pakistan. Hand book of DR-TB practice. [updated 2015, Cited August, 2021] Available from website: [http://ntp.gov.pk/uploads/Desk_Guide_for_MDR_TB_Physicians.pdf].
- NTP, 2017. National TB Control Program Pakistan. [updated 2017, cited 2021] Available from website: [http://www.ntp.gov.pk/cmsPage.php?pageID=7].
- Status of smear-positive TB patients at 2–3 years after initiation of treatment under a DOTS programme. Indian J. Tuberculosis. 2007;54:199-203.
- [Google Scholar]
- Faster for less: the new “shorter” regimen for multidrug-resistant tuberculosis. Eur. Respir. J.. 2016;48:1503-1507.
- [Google Scholar]
- Short-course regimen for multidrug-resistant tuberculosis: a decade of evidence. J. Clin. Med.. 2019;9:55.
- [Google Scholar]
- Rifampin drug resistance tests for tuberculosis: challenging the gold standard. J. Clin. Microbiol.. 2013;51:2633-2640.
- [Google Scholar]
- WHO, 2021. World health organization. Global tuberculosis report 2020. Geneva. Published 2021. License: Cc by-nc-sa 3.0 igo.
- Prevalence of treatment failure among pulmonary tuberculosis patients in a tertiary care teaching hospital. J. Bacteriol. Mycol.. 2016;3:273-276.
- [Google Scholar]







