Translate this page into:
Comparative study of phytochemical screening, antioxidant and antimicrobial capacities of fresh and dry leaves crude plant extracts of Datura metel L
*Corresponding author dramzadh@gmail.com (Mohammad Amzad Hossain)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Available online 23 July 2013
Peer review under responsibility of King Saud University.

Abstract
The aim of this work is to investigate and compare the phytochemical screening, antioxidant and antimicrobial activities of different crude extracts from dry and fresh leaves of Datura metel L. Different organic solvents including methanol, chloroform, hexane, ethyl acetate and butanol were used to prepare the crude extracts from the fresh and dry leaves. Antioxidant and antimicrobial activities of different crude extracts from dry and fresh leaves of D. metel were determined by DPPH method and agar disc diffusion method with minor modification. In vitro phytochemical screening for all crude extracts from both dry and fresh leaves was tested and shown positive result for alkaloid, flavonoid, saponin and tannin compounds. However, all the crude extracts did not show positive results for steroids and triterpenoid compounds. The antioxidant activity results of both fresh and dry crude extracts showed that when gradually increasing the samples concentration there was an increase in the absorbance. Therefore the antioxidant activity of dry crude extracts as equivalent to DPPH (2, 2-diphenyl-1-picrylhydrazyl) was in the order of butanol > chloroform > ethyl acetate extract > methanol > hexane extract. However, the order of antioxidant activity for fresh organic crude extracts to DPPH was in order of methanol > hexane > chloroform > ethyl acetate extract > butanol. The methanol crude extract and its derived fractions from dry and fresh leaves showed small and moderate antibacterial potential with one gram positive (Staphylococcus aureus) and three gram negative(Escherichia coli, Klebsiella pneumoniae and Pseudomonas aeruginosa) bacteria in the range of 0–17%. In conclusion, all organic crude extracts from both fresh and dry leaves could be used as potential sources of new antioxidant and antimicrobial agents.
Keywords
Datura metel
Soxhlet extractor
Biochemical screening
Antioxidant activity
Antimicrobial activity
1 Introduction
Herbal medicine is the study and use of medicinal properties of plants. Native Americans traditionally used about 2500 of the approximately 20,000 plant species that are native to North America (Akerele, 1993). About 80% of the population worldwide use traditional medicine, which has compounds derived from medicinal plants (Manandhar, 2000).
The name of Datura comes from Sanskrit Dustura (Dorman and Deans, 2002) or Dahatura. There are many different species in theDatura genus. It is commonly known as Thorn apple belonging to the family Solanaceae. Locally it is known as Mernha. Datura metel (D. metel) is a flowering plant. The plant grows up to 3 feet high. Its leaves are covered with short and soft grayish hairs. The leaves are about 10–20 cm long and 5–18 cm broad. It is widely found in Asia, Africa, England, India and other tropical and subtropical regions (Kokate et al., 2008; Harbone, 1999).
The phytoconstituents such as flavonoids, phenols, tannins, saponins and sterols are found in D. metel (Donatus and Ephraim, 2009). Some other phytochemicals have also been found in D. metel and the main phytoconstituents include alkaloid (Yussuf, 1991; Donatus and Ephraim, 2009). Globally it is considered as a poisonous plant when taken in large doses. It can cause delirium, coma, and even death due to high percentage of alkaloids (Yussuf, 1991; Donatus and Ephraim, 2009). It should not have more than 0.20% of alkaloids (Kokate et al., 2008). It is one of the most important medicinal herbs used worldwide due to its anti-inflammatory property (Harbone, 1999).
Primarily this plant is used as an intoxicant and hallucinogen (Yussuf, 1991; Donatus and Ephraim, 2009). The whole plant, especially the leaves and seeds are used for the treatment of an aesthetic, antispasmodic, anodyne, antiasthmatic, antitussive, bronchodilator, animal bites hallucinogenic, hypnotic and mydriatic (Satyavati et al., 1977; Duke and Ayensu, 1984). Sometimes its seeds are added to the wine and beer to increase intoxication (Richard, 1992; Satyavati et al., 1977). In Indochina and Africa, the powered leaves or seeds are often mixed cannabis and smoked to relieve asthma, and rheumatism (Richard, 1992; Satyavati et al., 1977). Mexican women use it to relieve the pain of childbirth. Traditionally it is also used for the treatment of wound healing and burn wounds (Satyavati et al., 1977; Duke and Ayensu, 1984). In India, it is popular and widely used for the treatment of epilepsy, hysteria, heart diseases, insanity, fever with catarrh, cough, convulsion, diarrhoea, skin diseases etc. (Chopra et al., 1986; Nguyen and Doan, 1989). More recently the crushed datura leaves are used to relieve pain (Chopra et al., 1986; Nguyen and Doan, 1989). Several reports have been carried out with antimicrobial activity against bacteria, bacterial pathogens and fungi (Sakthi et al., 2011; Ali and Shuab, 1996). Several scientific studies and the results on antimicrobial, antioxidant and phytochemical screening on ethanol and hydro alcoholic crude extracts of this plant have been reported earlier (Okwu and Morah, 2007; Okoli et al., 2007). But our study has been planned to determine the antioxidant activity and phytochemical compounds of different organic crude extracts from both dry and fresh leaves of D. metel. The antimicrobial activity of different crude extracts from both fresh and dry leaves against some selective pathogenic bacteria locally available for possible development of new drugs for the prevention and treatment of infectious diseases caused by bacterial pathogens. Therefore, the aim of this present work is to investigate and compare the phytochemical screening, antioxidant and antimicrobial activities of different crude extracts from dry and fresh leaves of D. metel native to Sultanate of Oman.
2 Materials and methods
2.1 Materials
The chemicals used in this present study such as hexane, chloroform, ethyl acetate and acetic anhydride were purchased from Scharlau, European Union. Butanol and DPPH (2, 2-diphenyl-1-picrylhydrazyl) were obtained from Sigma–Aldrich, Germany. Methanol was obtained from Emsure, Germany. Ammonia was obtained from Appli Chem, Germany. Sodium hydroxide and sulphuric acid were obtained from Ohilip Harris, England. Filter papers were used in the disc from Whatman, GE Healthcare companies, China, Catalogue number: 1001090. The bacterial strains such as Staphylococcus aureus (S. aureus), Escherichia coli (E. Coli), Klebsiella pneumoniae (K. pneumoniae) and Pseudomonas aeruginosa (P. aeruginosa) were obtained from Nizwa Hospital, Nizwa, Sultanate of Oman. The UV spectroscopy (UV-1800 Shimadzu spectrophotometer, Japan) was used for measuring the absorbance of the samples.
2.2 Plant sample
The leaves of D. metel sample were collected from Tanuf, Nizwa, Sultanate of Oman. The leaves were harvested on September 21, 2012. The samples were packed instantly in polyethylene bags to avoid decomposition of some bioactive compounds.
2.3 Preparation of samples
The leaf samples were washed carefully with water to remove dust and foreign materials. Then the washed leaves were divided in two parts. One part of the leaf samples (200 gm) were dried under shade at temperature (25 °C) for 7 days. The other parts consisting of fresh samples (200 gm) were cut into small pieces for the extraction process. After drying the leaf samples (150 gm) were ground into a powder form using a grinder for 30 s.
2.4 Extraction procedure for dry leaf powder samples
The dry leaf powder samples (150 gm) were extracted with methanol solvent (350 ml) for 3 days using Soxhlet extractor until complete extraction. After extraction, the sample was filtered with filter paper (Whatmann 41). The methanol solvent was evaporated using a rotary evaporator under pressure for 30 min resulting in a semi solid crude extract (9.31 g). The dry methanol crude extract (0.34 g) was transferred into test tube for antioxidant activity, antimicrobial and phytochemical screening. The methanol crude extract (9.0 gm) was suspended in water (100 ml) and shaken until the crude extract dissolved. The solution was transferred into a separatory funnel and extracted successively and separately with 30 ml and 20 ml of hexane, chloroform, ethyl acetate and butanol, respectively. After extraction all crude extracts were put inside the fume hood for the solvents to evaporate. After the solvent was completely evaporated the hexane crude extracts (0.22 g), chloroform crude extracts (0.12 g), ethyl acetate crude extracts (0.15 g) butanol crude extracts (0.36 g) and residual methanol fractions (0.44 g) were obtained.
2.5 Extraction procedure for fresh leaf samples
The small pieces of fresh leaf samples (200 gm) were extracted using the maceration method with methanol solvent (300 ml) for 3 days. After complete extraction, the sample was filtered with filter paper and the solvent was evaporated using a rotary evaporator under pressure for 30 min resulting in a semi solid crude extract (5.58 g). About (0.34 g) of methanol crude extract was transferred in a test tube for a different study. The methanol crude extract was suspended in water and then extracted successively and separately with hexane, chloroform, ethyl acetate and butanol. After extraction, all crude extracts were put inside the fume hood for few days. After the solvent evaporates, the hexane crude extracts (1.68 g), chloroform (0.11 g), ethyl acetate (0.32 g) and butanol (0.29 g) and residual methanol fractions (0.21 g) were obtained.
2.6 Preliminary phytochemicals screening
The stock solution was prepared from each of the crude extracts such as hexane, chloroform, ethyl acetate, butanol and methanol extracts (100 mg); and was dissolved in 10 ml of its own mother solvents. The obtained stock solutions were subjected to preliminary phytochemical screening (Harborne, 1998; Kokate, 2000).
2.6.1 Test for alkaloids
The dry powder samples (1 gm) were taken in a test tube and an ammonia solution (3 ml) was added to it. They were allowed to stand for few minutes. Then chloroform (10 ml) was added to the test tube samples which was shaken and then filtered to remove the powder samples. The chloroform was evaporated using a water bath and Mayer’s reagent (2 ml) was added. A cream coloured precipitate was immediately produced which indicates the presence of alkaloids.
2.6.2 Test for flavonoids
A few drops of diluted sodium hydroxide solution were added to the stock solution of D. metel (0.5 ml). An intense yellow colour appeared in the plant crude extract, which became colourless upon the addition of a few drops of diluted H2SO4 acid. This shows the presence of flavonoids.
2.6.3 Test for saponins
The stock solution from each crude extract of D. metel (0.5 ml) was diluted with distilled water (20 ml) and then the test tube was shaken by hand for 15 min. The formation of a foam layer on the top of the test tube showed the presence of saponins.
2.6.4 Test for steroids
The powder samples of D. metel (1 gm) were dissolved in chloroform (10 ml) and added concentrated sulphuric acid (1 ml) into the test tube by wall sides. The colour of the upper layer turned red and the sulphuric acid layer showed yellow with green fluorescence. This indicated the presence of steroids.
2.6.5 Test for tannins
The stock crude extract solution (0.5 ml) was dissolved in chloroform (5 ml) and added acetic anhydride (1 ml). Finally sulphuric acid (1 ml) was added carefully to the solution along the wall sides of the vessel. A green colour was formed, showing the presence of tannins.
2.6.6 Test for triterpenoids
The dry crude plant extract (5 mg) was dissolved in chloroform (2 ml) and then acetic anhydride (1 ml) was added to it. One millilitre of concentrated sulphuric acid was added to the solution. The formation of reddish violet colour shows the presence of triterpenoids.
2.7 Antioxidant activity
The estimation of free radical scavenging activity of different dry and fresh crude extracts of D. metel was described by Blois (1958) with minor modification. The different dry and fresh crude extracts of D. metel at different concentrations (12.5, 25, 50, 100 and 200 ppm equivalent to 12.5, 25, 50, 100 and 200 μg, respectively) were taken in separate test tubes. One millilitre of DPPH (2,2-diphenyl-1-picrylhydrazyl) solution (0.1 Mm) was dissolved in methanol and added each to test tube and shaken vigorously. After the addition of DPPH solution, all of the test tubes were shaken gently and allowed to stand at 27 °C in a dark place for 45 min. The blank and positive controls were prepared as the same way without any extract. Ascorbic acid was used as standard at the concentration of 50 ppm. The absorbance of the prepared samples was measured using UV spectroscopy at a wavelength of 517 nm. Each method in this experiment was replicated three times. Radical scavenging activity of the tested crude extract samples was estimated as an inhibition percentage and was calculated by using the following formula,
Measurement of radical scavenging activity (%)
2.8 Antibacterial activity assay
The antibacterial potential test was carried out using the agar disc diffusion method (Kokate, 2000). Negative controls were prepared by using the same solvents employed to dissolve the samples. Inhibition zones were measured and compared with the standard reference antibiotic amoxicillin. Each extract was subjected to serial dilution by using dimethyl sulphoxide (DMSO) as a solvent to give 2 mg/ml, 1 mg/ml, 0.5 mg/ml, and 0.25 mg/ml solutions. The concentration of amoxicillin standard used for this study was at 1 mg/ml. Each prepared concentration of the different extracts was tested for its antimicrobial activity against one gram (+) bacteria (S. aureus) and three gram (−) bacteria (E. coli, K. pneumoniae and P. aeruginosa) on nutrient agar plates using disc diffusion method. Whatman No. 1 sterile filter paper discs (6 mm diameter) were impregnated with methanol extracts or subfractions of D. metel and placed on the inoculated agar. The concentration of amoxicillin standard used for this study was at 1 mg/ml. All the plates were incubated at 37 °C for 24 h. Evaluation of antibacterial activity was measured showing the diameter of the zones of inhibition against the tested bacteria. Each method in this experiment was replicated three times.
3 Results
The dry and fresh powder samples of D. metel were extracted with methanol solvent using Soxhlet extractor and the maceration method. After the complete extraction, the methanol solvent was evaporated using a rotary evaporator producing semi solid mass crude extracts. The methanol crude extract was dissolved in water and extracted successively with hexane, ethyl acetate, chloroform and finally butanol.
3.1 Phytochemical screening
Biochemical screening results showed that alkaloids, flavonoids, saponins and tannins were present in the fresh and dry leaf crude extracts of D. metel. But, all the crude extracts from both leaf samples did not show any colour change for triterpenoid and steroid test. However, methanol crude extracts from fresh and dry leaves showed negative test for steroids, tannins and triterpenoids but showed positive test for alkaloids, saponins and flavonoids. Both hexane crude extracts and chemicals such as alkaloids, steroids, flavonoids and triterpenoids were absent except for saponins and tannins. Ethyl acetate crude extract from both leaves showed positive test for alkaloids and saponins. The chloroform crude extracts from both leaves also showed positive test for alkaloids, saponins and tannins. The butanol crude extracts, a group of chemical constituents such as saponins, tannins, steroids, and triterpenoids were absent except alkaloids and flavonoids (Table 1). + = Presence; − = absence.
Inference
Extracts
Biochemicals
Alkaloids
Steroids
Flavonoids
Saponins
Tannins
Triterpenoids
Hexane extract
Fresh leaves
−
−
−
+
+
−
Dry leaves
−
−
−
+
+
−
Chloroform extract
Fresh leaves
+
−
−
+
+
−
Dry leaves
+
−
−
+
+
−
Ethyl acetate extract
Fresh leaves
+
−
−
+
−
−
Dry leaves
+
−
−
+
−
−
Butanol extract
Fresh leaves
+
−
+
−
−
−
Dry leaves
+
−
+
−
−
−
Methanol extract
Fresh leaves
+
−
+
+
−
−
Dry leaves
+
−
+
+
−
−
3.2 Antioxidant capacity
The antioxidant activity of hexane, ethyl acetate, chloroform, butanol and methanol crude extracts from the dry and fresh leaf samples of D. metel at different concentrations (12.5, 25, 50, 100 and 200 ppm) showed activity ranging from 42–54% for dry samples and 47–71% for fresh samples. The absorbance was gradually increased with increasing concentrations of organic crude extracts from dry and fresh samples (Figs. 1 and 2).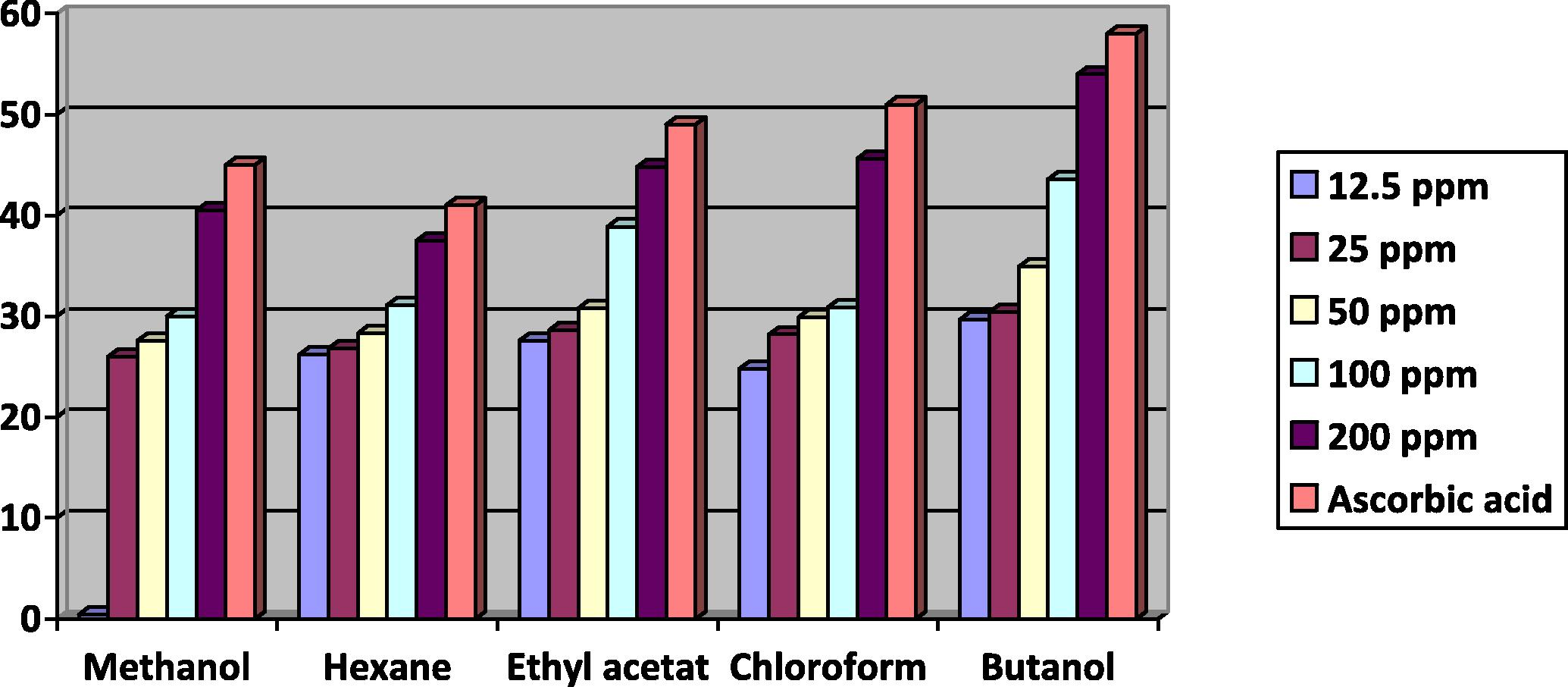
Antioxidant capacity of different crude extracts from dry leaf samples of Datura metel.
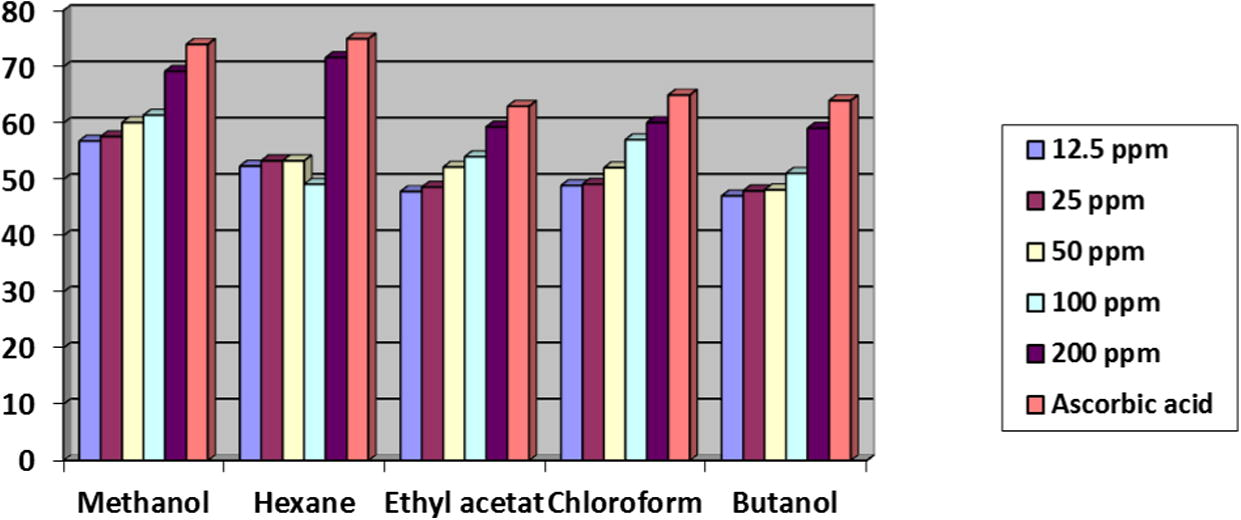
Antioxidant capacity of different crude extracts from fresh leaf samples of Datura metel.
The antioxidant activity of different dry leaf crude extracts as equivalent to DPPH was in the order of butanol > chloroform > ethyl acetate extract > methanol > hexane extract. However, the trend of antioxidant activity for fresh crude extracts was in the order of methanol > hexane > chloroform > ethyl acetate extract > butanol. Therefore, the antioxidant activity results for all crude extracts from fresh leaves are higher than that from dry leaves (Figs. 1 and 2).
3.3 Antimicrobial activity
In vitro antibacterial activity of methanol crude extracts and their derived fractions from dry and fresh leaf crude extracts against the four bacteria employed quantitatively assessed the presence or absence of inhibition zones. The different crude extracts from dry and fresh leaves of D. metel exhibited antibacterial potential against one gram positive (S. aureus) and three gram negative(E. coli, P. aeruginosa andK. pneumoniae) bacteria at four concentrations of 2 mg/ml, 1 mg/ml, 0.5 mg/ml and 0.25 mg/ml with dimethyl sulphoxide (DMSO). All dry crude extracts of D. metel revealed a small antibacterial potential against E. coli at all four concentrations (Table 2). However, the fresh crude extracts inhibit moderate antibacterial potential against E. coli at all four concentrations (Table 2). nd = Not detected.
Crude Extract
Concentration
E. colia (mm)
S. aureus (mm)
P. aeruginosa (mm)
K. pneumoniae (mm)
Fresh leaves
Dry leaves
Fresh leaves
Dry leaves
Fresh leaves
Dry leaves
Fresh leaves
Dry leaves
Hexane
2 mg/ml
11 ± 0.11
7 ± 0.30
12 ± 0.20
8 ± 0.33
16 ± 0.22
6 ± 0.17
nd
nd
1 mg/ml
15 ± 0.23
10 ± 0.44
nd
nd
13 ± 0.51
8 ± 0.41
7 ± 0.32
7 ± 0.27
0.5 mg/ml
9 ± 0.18
8 ± 0.35
nd
nd
12 ± 0.27
6 ± 0.28
8 ± 0.18
8 ± 0.10
0.25 mg/ml
8 ± 0.44
8 ± 0.28
10 ± 0.31
7 ± 0.32
nd
nd
nd
nd
Standard
30 ± 0.22
30 ± 0.10
26 ± 0.13
26 ± 0.34
7 ± 0.54
7 ± 0.23
8 ± 0.41
8 ± 0.28
2 mg/ml
12 ± 0.43
6 ± 0.33
16 ± 0.20
12 ± 0.21
14 ± 0.09
6 ± 0.10
6 ± 0.22
6 ± 0.10
Ethyl acetate
1 mg/ml
11 ± 0.08
11 ± 0.30
11 ± 0.16
10 ± 0.21
13 ± 0.22
8 ± 0.34
7 ± 0.54
7 ± 0.17
0.5 mg/ml
9 ± 0.23
7 ± 0.25
7 ± 0.15
8 ± 0.31
12 ± 0.41
6 ± 0.24
nd
nd
0.25 mg/ml
8 ± 0.12
6 ± 0.21
nd
nd
7 ± 0.12
7 ± 0.55
9 ± 0.20
9 ± 0.32
Standard
30 ± 0.11
30 ± 0.23
20 ± 0.52
20 ± 0.22
7 ± 0.41
7 ± 0.56
7 ± 0.29
7 ± 0.08
2 mg/ml
nd
nd
nd
nd
10 ± 0.52
8 ± 0.21
8 ± 0.09
8 ± 0.09
Chloroform
1 mg/ml
13 ± 0.41
11 ± 0.25
12 ± 0.37
16 ± 0.32
8 ± 0.41
6 ± 0.22
7 ± 0.22
7 ± 0.22
0.5 mg/ml
9 ± 0.23
8 ± 0.27
nd
nd
6 ± 0.41
6 ± 0.41
7 ± 0.12
7 ± 0.12
0.25 mg/ml
8 ± 0.30
8 ± 0.37
11 ± 0.20
8 ± 0.26
nd
nd
7 ± 0.45
7 ± 0.14
Standard
30 ± 0.31
30 ± 0.25
8 ± 0.45
8 ± 0.23
8 ± 0.41
8 ± 0.59
8 ± 0.05
8 ± 0.15
2 mg/ml
17 ± 0.22
7 ± 0.23
12 ± 0.33
6 ± 0.34
10 ± 0.61
6 ± 0.21
7 ± 0.17
7 ± 0.29
Butanol
1 mg/ml
12 ± 0.17
7 ± 0.28
9 ± 0.12
7 ± 0.34
8 ± 0.29
7 ± 0.49
8 ± 0.23
8 ± 0.54
0.5 mg/ml
9 ± 0.20
6 ± 0.28
9 ± 0.09
9 ± 0.31
8 ± 0.37
8 ± 0.18
nd
nd
0.25 mg/ml
9 ± 0.55
9 ± 0.39
8 ± 0.22
8 ± 0.12
7 ± 0.49
7 ± 0.23
nd
nd
Standard
10 ± 0.22
10 ± 0.37
7 ± 0.61
7 ± 0.27
8 ± 0.12
8 ± 0.34
9 ± 0.11
9 ± 0.19
Methanol
2 mg/ml
16 ± 0.38
8 ± 0.12
16 ± 0.37
6 ± 0.44
17 ± 0.08
8 ± 0.17
6 ± 0.15
6 ± 0.39
1 mg/ml
12 ± 0.19
6 ± 0.44
12 ± 0.55
6 ± 0.31
14 ± 0.12
8 ± 0.23
6 ± 0.28
6 ± 0.43
0.5 mg/ml
13 ± 0.26
7 ± 0.25
10 ± 0.13
7 ± 0.33
8 ± 0.71
8 ± 0.12
7 ± 0.03
7 ± 0.33
0.25 mg/ml
8 ± 0.13
8 ± 0.56
8 ± 0.22
8 ± 0.34
7 ± 0.12
7 ± 0.42
8 ± 0.61
8 ± 0.10
Standard
10 ± 0.22
10 ± 0.24
7 ± 0.33
7 ± 0.17
11 ± 0.09
11 ± 0.32
7 ± 0.13
7 ± 0.32
4 Discussion
Phytochemical constituents in the plant samples are known to be biologically active compounds and they are responsible for different activities such as antioxidant, antimicrobial, antifungal, and anticancer. (Hossain and Nagooru, 2011; Suresh and Nagarajan, 2009). All secondary metabolite components displayed antioxidant and antimicrobial properties through different biological mechanisms. Most of the secondary metabolite components were isolated and identified in the polar plant crude extracts (Gonzalez-Guevara et al., 2004). The biochemical screening of hexane, ethyl acetate, chloroform, butanol and methanol crude extracts from fresh and dry powder leaf samples of D. metel used in this study revealed that the crude extracts contained alkaloids, flavonoids, saponins and tannins (Table 1). The phytochemical screening of methanol fresh and dry leaf crude extracts studied showed the presence of active chemical constituents such as alkaloids, flavonoids and saponins (Table 1). Saponins were also present in other dry leaf crude extracts of D. metel. The most effective bioactive compounds alkaloids and flavonoids were found in polar methanol and butanol crude extracts. Tannins are another active compound found to be present in hexane and chloroform extracts. Therefore, the detected different bioactive compounds in different crude extracts from dry and fresh leaves of D. metel may be responsible for the antioxidant and antibacterial activities. Several reports are available on flavonoid groups which exhibited high potential biological activities such as antioxidant, anti-inflammatory, antimicrobial, anti-angionic, anticancer and anti-allergic reactions (Anyasor et al., 2010; Chao et al., 2002; Igbinosa et al., 2009; Thitilertdecha et al., 2008). Saponins are also bioactive constituent which involved in plant defence system because of their antimicrobial activity (Barile et al., 2007; Ayoola et al., 2008). Tannins and their derivatives are phenolic compounds considered to be primary antioxidants or free radical scavengers (Barile et al., 2007; Ayoola et al., 2008; Akharaiyi, 2011; Varahalarao and Kaladhar, 2012; Sekar et al., 2012).
The antioxidant activity through free radical scavenging activity (DPPH) method of five different crude extracts from fresh and dry leaves of D. metel at 12.5 to 200 μg/mL concentrations was determined and compared (Figs. 1 and 2). The principle of antioxidant activity is their interaction to produce oxidative free radicals. The role of DPPH method is that the antioxidants react with the stable free radical. During the free radical reaction, DPPH (α,α-diphenyl-β-picrylhydrazyl) is converted into α,α-diphenyl-β-picrylhydrazine with colour change. The rate of colour change gradually decreases to indicate the scavenging potentials of the sample antioxidant. The crude extracts of D. metel contain flavonoid, saponins, tannins, phenolics and aromatic compounds. All these bioactive compounds were able to discolour DPPH solution by their hydrogen donating ability (Barile et al., 2007; Ayoola et al., 2008; Blois, 1958; Akharaiyi, 2011; Varahalarao and Kaladhar, 2012; Sekar et al., 2012). From the results it appears that the five crude extracts from the dry and fresh leaves of Datura possess hydrogen donating capabilities and it will act as an antioxidant. The results of free radical scavenging potentials of dry leaf crude extracts were found to be in the order of butanol extract > chloroform > ethyl acetate extract > methanol > hexane extract. However no similar trends were obtained for all crude extracts from fresh leaf samples of D. metel. The antioxidant activity for all crude extracts from fresh samples was higher compared to all crude extracts from dry samples. The activity difference obtained from dry and fresh crude extract samples might probably be due to the extraction procedures, samples processing or drying. During the processing of samples some active volatile compounds may have been destroyed or evaporated from the dry samples. That may be why the antioxidant activity of all dry crude extracts is lower than the fresh samples.
The antimicrobial activity of the fresh and dry plant crude extracts was estimated using standard conventional methods against S. aureus, E. coli, P. aeruginosa andK. pneumoniae. The dry methanol crude extract of D. metel and its fractions revealed comparatively small antibacterial potential against gram-positive and gram-negative bacteria at the concentrations of 2 mg/ml, 1 mg/ml, 0.5 mg/ml and 0.25 mg/ml with their respective zones of inhibition of 0–11 mm (Table 2). However, the fresh methanol crude extract of D. metel and its fractions revealed a moderate antibacterial potential against the employed bacterial strains and all working concentrations with their respective zones of inhibition of 0–17 mm (Table 2). The methanol fresh crude extract showed moderate antibacterial potential against S. aureus, E. coli and P. aeruginosa bacteria, at the concentrations of 2 mg/ml, 1 mg/ml and 0.5 mg/ml (Table 2). However, all crude extracts from dry samples showed small activity against all employed bacterial strains. Butanol crude extract from fresh samples showed moderate activity against S. aureus, E. coli, P. aeruginosa andK. pneumoniae at concentrations 2 mg/ml and 1 mg/ml but dry crude extract samples showed small potential at most of the concentrations against all bacterial strains. However, the bacterium K. pneumoniae did not show any potential activity at the concentration of 0.5 mg/ml and 0.25 mg/ml. The chloroform crude extracts from fresh and dry samples did not show any activity against E. coli and S. aureus at the concentration of 2 mg/ml. P. aeruginosa andK. pneumoniae with chloroform crude extracts showed small activity at all concentrations. The hexane and ethyl acetate subfractions showed moderate antibacterial potential against most of the tested bacteria. But, the hexane crude extract did not show any activity against P. aeruginosa andK. pneumoniae tested bacterial strains at the concentration 0.25 mg/ml. The ethyl acetate crude extracts also did not show any activity against S. aureus andK. pneumoniae tested bacterial strains at the concentrations of 1 mg/ml and 0.5 mg/ml and 2 mg/ml and 0.25 mg/ml. The control inhibits the growth of all the tested bacteria. Almost similar antioxidant and antimicrobial activities’ results on D. metel crude extracts were reported by other authors (Barile et al., 2007; Ayoola et al., 2008). Generally, the antimicrobial activity of plant crude extracts depends on the dose and the type of bacterial strains employed. Also this antibacterial actions could be related to their chemical components in the crude extracts (Barile et al., 2007; Ayoola et al., 2008; Akharaiyi, 2011; Varahalarao and Kaladhar, 2012; Sekar et al., 2012). The bioactive compounds such as tannins and flavonoids components were present in the crude extracts. However, these bioactive compounds were inducing the antioxidant and antimicrobial activities. The amount of active components in the crude extract may be diluted or increased their concentrations by fractionation (Anyasor et al., 2010; Barile et al., 2007; Igbinosa et al., 2009; Akharaiyi, 2011; Varahalarao and Kaladhar, 2012; Sekar et al., 2012) because they have the ability to inactivate microbial activity, enzymes, cell envelope transport proteins, and so forth (Barile et al., 2007; Ayoola et al., 2008; Akharaiyi, 2011; Varahalarao and Kaladhar, 2012; Sekar et al., 2012). Further studies are designed for the isolation and identification of individual active compounds and also in vivo studies are needed for better understanding of their mechanism of action as an antioxidant.
5 Conclusion
The present antimicrobial study of different crude extracts of D. metel showed that the methanol crude extract from fresh leaves shows highest activity against the employed bacteria. Similarly the methanol crude extract from fresh leaves showed the highest antioxidant activity. Phytochemical screening showed that the antioxidant and antibacterial activities of the crude extracts of D. metel depend on the presence of phytochemicals such as alkaloids, steroids, flavonoids and tannins. This plant crude extracts could serve as potential sources of new antimicrobial and antioxidant agents. Further research is needed towards isolation and identification of active principles present in the extracts which could be used for pharmaceutical use.
Acknowledgements
The authors are grateful to Prof. Dr. NafsiahBinti Shamsudin, Dean, College of Pharmacy and Nursing, University of Nizwa, Sultanate of Oman for her continuous encouragement during the work and all laboratory facilities. The authors are also grateful to University of Nizwa, Nizwa, Sultanate of Oman for providing all chemicals and other expenses from their internal fund to carry out this project. Thanks to Khaloud Ali Said Al-Alawi and Ahlam Rashed Alabri, Lab Technicians, Natural Product Lab, University of Nizwa for their continuous help during the experiment.
References
- Summary of WHO Guidelines for the assessment of herbal medicines. Herbal Gram. 1993;22:13-28.
- [Google Scholar]
- Antibacterial, phytochemical and antioxidant activities of Datura metel. International Journal of Pharmacology Technology Research. 2011;3(1):478-483.
- [Google Scholar]
- Characterization of the chemical constituents of Datura metel Linn. Indian Journal of Pharmaceutical Science. 1996;5(6):243-245.
- [Google Scholar]
- Phytochemical constituents and antioxidant activities of aqueous and methanol stem extracts of Costus afer Ker Gawl. (Costaceae) African Journal of Biotechnology. 2010;9(31):4880-4884.
- [Google Scholar]
- Phytochemical screening and antioxidant activities of some selected medicinal plants used for malaria therapy in Southwestern Nigeria. Tropical Journal of Pharmaceutical Research. 2008;7(3):1019-1024.
- [Google Scholar]
- Phytochemical screening and antimicrobial assessment of Abutilon mauritianum, bacopa monifera and Datura stramonium. Phytochemistry. 2007;68:596-603.
- [Google Scholar]
- Antioxidants determination by the use of a stable free radical. Nature. 1958;4617:1199-1200.
- [Google Scholar]
- Flavonoids in herbs: biological fates and potential interactions with xenobiotics. Journal of Food Drug Analysis. 2002;10(4):219-228.
- [Google Scholar]
- Glossary of Indian Medicinal Plants. New Delhi: Council of Scientific and Industrial Research; 1986.
- Isolation, characterization and antibacterial activity of alkaloid from Datura metel Linn leaves. African Journal of Pharmacy and Pharmacology. 2009;3(5):277-281.
- [Google Scholar]
- Antimicrobial agents from plants: antibacterial activity of plant volatile oils. Journal of Applied Microbiology. 2002;88:308-316.
- [Google Scholar]
- Handbook of Medicinal Herbs. Boca Raton, FL: CRC Press; 1984.
- Phytochemical screening and in vitro antiherpetic activity of four Erythtroxylum species. Acta Farmaceut Bonaer. 2004;23(4):506-509.
- [Google Scholar]
- Harbone J.B., ed. Phytochemical Dictionary. London: Taylor and Francis; 1999.
- Phytochemical Methods, A guide to Modern Techniques of Plant analysis (second ed.). London: Chapman and Hall; 1998. (pp. 54–84)
- Biochemical profiling and total flavonoids contents of leaves crude extract of endemic medicinal plant Corydyline terminalis L. Kunth. Pharmacognosy Journal. 2011;3(24):25-29.
- [Google Scholar]
- Antimicrobial activity and phytochemical screening of stem bark extracts from Jatropha curcas (Linn) African Journal of Pharmacy and Pharmacology. 2009;3(2):058-062.
- [Google Scholar]
- Kokate, K.K., Purohit, A.P., Gokhale, S.B., 2008. Pharmacognosy, Forty second edition, Vallabh Prakashan, India (pp. 13–44).
- Practical Pharmacognosy. Vallabh Prakashan; 2000. (pp. 218)
- Plants and People of Nepal. USA: Timber Press; 2000. (pp. 50)
- Medicinal Plants in Vietnam, World Health Organization, Regional Office for the Western Pacific, Manila. Hanoi: Institute of Materia Medica; 1989.
- Potentials of the leaves of Aspilia Africana (compositae) in wound care; an experimental evaluation. BMC Complement Alternative Medicine. 2007;7:24-30.
- [Google Scholar]
- Isolation and characterization of flavanone glycoside 4l, 5, 7 trihydroxy flavanone rhamnoglucose from Garcinia kola seed. Journal of Applied Sciences. 2007;7(2):306-309.
- [Google Scholar]
- Plants of the Gods. Alfred Van Der Marck, NewYork: McGraw Hill; 1992. (pp. 599)
- Antibacterial evaluation and phytochemical screening of Datura metel leaf extracts against bacterial pathogens. International Journal of Pharmaceutical & Biological Archives. 2011;2(4):1130-1136.
- [Google Scholar]
- Medicinal Plants of India. New Delhi: Indian Council for Medical Research Publication; 1977. (vol. 1. p. 333–334)
- Screening of Phyllanthus amarus, Acalypha indica and Datura metel for its antimicrobial activity against selected pathogens. International Journal of Pharmaceutical & Biological Archives. 2012;3(5):1231-1235.
- [Google Scholar]
- Preliminary phytochemical and antimicrobial activity analysis of Begonia malabarica Lam. Journal of Basic & Applied Biology. 2009;3(1&2):59-61.
- [Google Scholar]
- Antioxidant and antibacterial activities of Nephelium lappaceum L.extracts. Food Science and Technology. 2008;41:2029-2035.
- [Google Scholar]
- Antimicrobial study of plant extracts of Datura metel L. against some important disease causing pathogens. Asian Pacific Journal of Tropical Disease 2012:S94-S97.
- [Google Scholar]
- Phytochemical and antimicrobial studies. International Journal of Pharmacognosy. 1991;29:252-258.
- [Google Scholar]







