Translate this page into:
Comparative studies on phenolic, anti-oxidative, biochemical and GC–MS analysis of crude and refined edible oils
⁎Corresponding author. vijaya.settaluri@hct.edu.om (Vijaya saradhi Settaluri),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Fats and oils play a very important role in everyday life. Many oils are available in the market with potential health benefits. Some of these oils if taken in excess are harmful too. Oils can be saturated or unsaturated depending on the nature of fatty acid present in them. The current study was carried out to compare the biological activity of refined and crude oils to understand and analyze their physiochemical analysis and quantitative properties of oils. Three types of oils were selected in this project which are sunflower oil, almond oil and sesame oil. The seeds of the oils were obtained from the local market and the oil were extracted using the Soxhlet extraction method. Three refined oils of the same variety were obtained from the market. Different analytical procedures like measuring the moisture content of oils, total phenolic activity of oils, anti-oxidative properties of oils, iodine number, saponification number of oils, Reichert meisel number, total sterol content, Gas chromatography mass spectroscopy(GC–MS) analysis was performed on all categories of oils. The main purpose of this analysis is to suggest which of the oils crude or refined are good for health and metabolism. From the studies carried out and the results obtained, it was found that the crude oils of all the three varieties have more health benefits than the refined oils.
Keywords
Phytochemicals GC–MS
Analysis
Oils
Saponification
Measurement
1 Introduction
Foods and drinks contain supplements such as carbohydrates, fats, proteins, vitamins, and minerals. A few nourishments or drinks contain a huge amount of 1 supplement, which contains a huge amount of sugar, or nourishment, which contains a defined amount of fat. Dietary fat is important for several body processes. For example, it helps in the synthesis of vitamins in the body and also helps with formation of hormones. For this reason, it is recommended that one must replace foods and drinks high in saturated and Tran’s fat with alternatives that contain additional unsaturated or monounsaturated fats. Meals with a small quantity of fat will enhance the style and conjointly facilitate to help you to be happy and have an extended life. Throughout the day one should consume a different and healthy food every day. By doing this, you will get very low total quantity of dietary fat, particularly unsaturated and monounsaturated fats to meet your daily needs. (Mozaffarian, 2006).
Fats that usually come from animal fats are solid at room temperature and are usually saturated. An increase in the number of saturated fatty acids will increase the levels of low density lipoproteins. Depending on the number of double bonds the Unsaturated fats are of two types, monounsaturated as are found in canola oil, groundnut oil and many others (Campas-Baypoli et al, 2014). when such fats are consumed, they can increase the number of calories, which might be responsible for lowering the steroid alcohol levels. Poly-unsaturated fats exist in the form of omega-3 and omega-6 fatty acids. Omega-6 fatty acids are found in butter substitutes whereas the omega-3 fatty acids are found in fish, especially salmon and sardines. They are found in soybean and sunflower oils (Arshad and Amjad, 2012).
Physical properties of an oil or fat are of essential significance in determining its utility. This is particularly true of the large quantity and kind of oils and fats utilized in varied forms as food. To be sure, the separate words “oil” and “fat” occur in most languages and show that one fundamental physical property-whether the fat is liquid at short temperature has been recognized from the earliest times as of nice importance. The physical state of a fat may vary from a liquid to a viscous fluid to a plastic solid to a weak solid. These two choices fat or oil, build their physical properties which is of utmost interest and have resulted in their use in a wide variety of applications (Timms, 1985).
In this manuscript varying properties pertaining to physical and chemical properties of the fat were studied and were effectively applied to monitor the quality of the oils. In this regard it is important to find out the standards of the oil in order to prevent the usage of impure oil. These physicochemical parameters together with boiling point, density, saponification value, and other quantitative methods are used to find out how good the quality of the oils (Hasan et al., 2016). All edible oils which are used for the preparation of food must meet all the required standards and should pass all the fitness tests before consumption. Once one is sure about the quality and properties of the oils, we can justify whether they are good for human health and can be used for other industrial applications. Oils with lower density are more preferable by consumers. Moisture content of the oils plays a significant role in various applications. High moisture content of oils can be used for manufacture of cosmetics, soaps and oil paints. They are also applied for the purpose of baking, detergents and frying. Depending on the saponification value, the smaller the short chain of fatty acids, and also decrease the average molecule mass of the fatty acids. Rancidity of the fat is indicated by an increase in the acid number of the fat or oil (Sabinus Oscar, 2012).
The combination of curative and fragrant plants is constantly developing due to increasing consumers request and interested in these plants for culinary, restorative, and other anthropogenic applications. As customers are getting to be gradually educated around issues of nourishment, nutrition, and wellbeing, they are moreover getting to be mindful of the benefits and potential applications of restorative and aromatic plants and their metabolites. These plants create an extensive variety of auxiliary metabolites among them. In spite of their rich and complex composition, the utilize of fundamental oils remains wide and limited to the makeup and perfumery areas. It is commendable to create much better, stronger and improved understanding of their chemistry and the organic properties of these extracts and their individual components for new and profitable applications in human wellbeing. (Dhif, 2016).
The iodine value in chemistry is the mass of iodine in grams that consumed by one hundred grams of a chemical substance. Iodine numbers are typically used to verify the quantity of unsaturation in fatty acids. This unsaturation is in the style of double bonds, which react with iodine compounds. The higher the iodine number, the more C⚌C bonds are present in the fat.
Saponification value represents the number of milligrams of potassium hydroxide required to saponify 1 g of fat beneath the conditions. It is a measure of the typical relative chain length of all the fatty acids present. As most of the mass of a fat is in the 3 fatty acids, the saponification price permits for comparison of the average carboxylic acid chain length. The long chain fatty acids found in fats have a low saponification value. The saponification value gives an indication of the nature of the fatty acid’s constituent of fat which in turn depends on the normal molecular weight of the greasy acids constituent of fat. (Mondofacto, 2008).
Acid Number and also called Total Acid Number (TAN) is utilized to test the amount of acidic components in an oil sample. This test decides the sum of dissolvable or about dissolvable acids in a test broken up in a toluene and 2-propanol mixture. Acidic characteristics are caused by numerous chemicals. These can add in natural and inorganic acids, salts of overwhelming metals, esters, phenolic compounds, lactones, resins, salts of alkali and other powerless bases and also corrosive salts of polybasic acids, and addition specialists such as inhibitors and cleansers (ManTech.2016).
Due to the formation of free radicals, there might be some issues with health as their oxidation may form peroxides (Kamath et al, 2015). This can be prevented by the presence of phenols, present in seeds of some oils, which have the potential for preventing health related issue (Aşık and Özkan, 2011). The factors that are responsible for creating an impact on the antioxidant activity is enhanced by the presence of phenolic compounds. Polar compounds like phenols are abundantly found in oils. Among many oils it is observed that olive oils are highly stable as they have increased quantity of phenols (Amelio & Onaoo 2003). The oils that are used in this study can be used for different applications in food industry, in order to improve health and nutrition (Maniak & Targonski 1996).
An inhibitor, is any substance, present at low concentrations compared to reactive substrate; considerably preventing the reaction of the substrates (Hallowell, 1995). Food antioxidant is specially developed to stop reaction of reactive materials like fats (Frankel and Meyer, 2000). Antioxidants terminate chain reactions by removing free radical intermediates and inhibit other reaction by themselves being changed (Saha & Tamrakar, 2011). Antioxidants act as “free radical scavengers”. Antioxidants prevent such changes by retarding the method of reaction or rancidity. Rancidity is a term widely used in the food trade that covers an oversized range of objectionable off-flavor volatile parts generated from the auto-oxidation of unsaturated fatty acids.
The Gas chromatographic- Mass spectroscopic (GC–MS) strategy for investigation was done based on a distributed strategy (Al Owaisi et al., 2014). The oil extracts of sunflower (Žilic et al, 2010) almond and sesame oils (Aglave, 2018) both refined and crude are treated under standard conditions to be loaded into the GC chamber for further analysis.
The oils selected for carrying out the above investigations are sunflower oil, almond oil (Roncero et al, 2016) and sesame oil. The seeds of the above plants were purchased and oils were extracted using the soxtec analysis. Refined oils were directly purchased and analyzed for the determination of Iodine value, saponification value, acid value, total phenolic activity, antioxidant activity and GC–MS analysis.
2 Materials and methods:
2.1 Chemicals and reagents.
1,1-Diphenyl-2-picrylhydrazyl)/ALDRICH, Methanol, Ascorbic acid/ALDRICH, Carbon tetra chloride, Sodium carbonate, Folin ciocalteus reagent, potassium iodide, sodium thiosulfate, iodine crystals, starch, potassium hydroxide, phenolphthalein, UV– Visible Spectrophotometer(Agilent), GC- MS Analyzer.
2.1.1 Iodine value of oils
0.5 g of oils solution was taken in a conical flask. 25 ml of Hanus solution was added and mixed with oils. Allowed to stand in dark place for 30 min. 10 ml of 15 % KI was added and boiled and then cooled using to room temperature. Titrated with 0.1 mol/L sodium thiosulfate to obtained the blank value. When the color of the solution turned yellow Color 1 ml of starch which given blue color was added and, then titrated again until color become colorless. (Iodine value = (B-S) *N*12.69/weight of sample) (Titrator, 1959).
2.1.2 Saponification value of oils
1 g of oil sample was taken into a conical flask and solvent solution comprising of (Ethanol and ether) was added. 25 ml of potassium hydroxide (KOH) was added and mixed together, then heated the flask 30 min for the backflow of ethanol. The contents were cooled immediately after heating. The resulting solution was Titrated with 0.5 mol/L HCl by using one drop of phenolphthalein as indicator. The saponification value of the crude and refined oils were calculated.
2.1.3 Acid value of oils
5 g of oils solution was added into conical flask with solvent solution (Ethanol and Ether) and mixed well. Sample was keep on water path to warm for 5 to 6 min to completely dissolve the oils. 1 ml of phenolphthalein was used as inductor which given colorless. Titrated with KOH until the color become pink. the acid value was calculated (Acid value = 5.6 * titrate value/ weight of oil).
2.1.4 Total phenolic activity
The amount of total phenolic activity in the extract was determined according to the Folin ciocalteu procedure 0.0.5 ml of oil was introduced into test tubes add 0.5 of carbon tetra chloride (CCL4) was added to it. 0.5 ml of Folin-ciocalteus reagent and 3 ml of sodium carbonate were added. The tubes were mixed and allowed to stand for 30 min. Absorption at 765 nm was measured by using spectrophotometer. The total phenolic content was expressed as Gallic acid equivalents (GAE) in milligrams per gram dry materials.
2.1.5 Total antioxidant activity
2 ml of 1 mmol/L of 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical solution, prepared in methanol was added to test tubes. The solution was rapidly mixed and allowed to stand in dark at 37°C for 30 min. Ascorbic acid was used as standard. The blank was prepared in similar way without oils solution or ascorbic acid. Absorbance of each solution was measured at 517 nm used UV–visible spectrophotometer. The percentage of radical scavenging activity calculate by used formula: Free radical scavenging activity % (Ac-As/Ac*100) (Amzad Hossain and Shah, 2015).
2.1.6 GC–MS analysis
The GC MS method for analysis was done based on a published method (Al Owaisi et al., 2014). The oil extracts of sunflower, almond and sesame oils both refined and crude are treated under standard conditions to be loaded into the GC chamber for further analysis. The published GC–MS procedure was followed 15, using HPGC (Model 6890 series). The apparatus is equipped with flame ionization detector and injector (H P 7683). The MS transfer line was established at 250 °C using (30 × 0.25 mm, 1.0 um). The chromatographic conditions were fixed: oven temperature was kept at 50 °C at a rate of 2 °C/min, using helium gas (99.9 %) as a carrier gas at a constant flow rate of 22 cm/s. 1ug/ml concentration of extracts was injected (split ratio 1:30). For MS analysis coupled Agilent M Spectrometer (model 5973) was used with the help of NISTO8 Library software. Mass spectra were taken at 70 eV/200 °C (1 scan/s). The results of analysis obtained by the procedure were systematically analyzed for further interpretations.
3 Results and discussion
3.1 Iodine value of crude and refined oils:
Iodine number was determined for all the crude and refined oils. The highest value of Iodine number in sunflower crude oil while the lowest percentage in sesame refined oil which higher iodine number good for our body while low iodine number have more saturated fatty acids. It can however be noted that the crude oils have higher iodine values, indicating a higher degree of unsaturation, compared to refined oils which are relatively high in saturated fatty acids. When the oils were heated leads to an substantial decrease in the iodine value of the selected oils. The results of analysis are indicated in Fig. 1.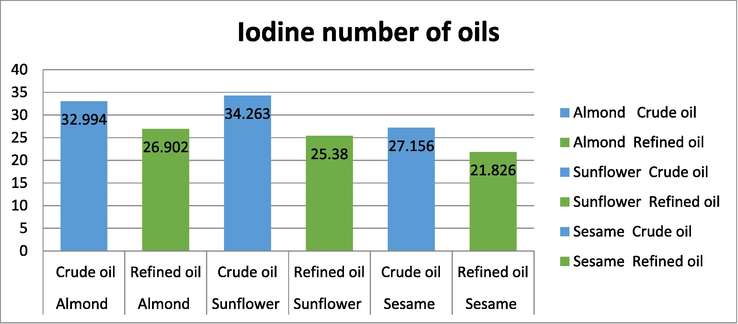
Iodine value of oils.
3.2 Saponification value of oils:
Saponification value was determined for all the crude and refined oils. The highest value of saponification value was found in refined almond oil and the lowest value was found in crude sesame oil. It can however be noted that the crude oils have lower saponification values when compared to the refined oils. The results of analysis are indicated in Fig. 2. Based on the values, it is evident that the crude oils with low saponification number have more long chain fatty acids and high molecular weight, which is usually considered good for health. On the other side when the oils are refined, the saponification value increases very much, which indicates the presence of more short chain fatty acids with low molecular weight. Lower the saponification number, the oil has better quality and properties.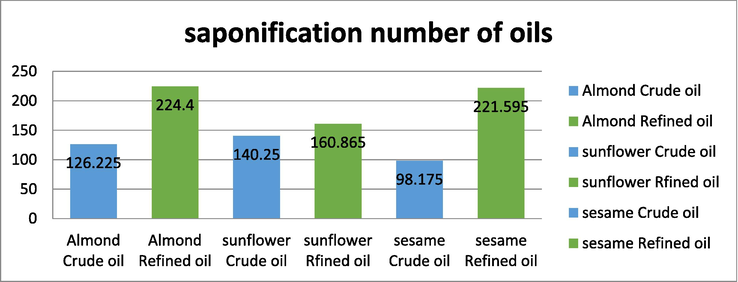
Saponification value of oils.
3.3 Acid number of fats and oils
In the Figure (Fig. 3), it can be observed after analysis that the highest value of Acid number is in refined sesame oil while the lowest percentage in crude almond oil. Fats and oils get rancid when they are exposed to unnatural agents. Lower the acid number less rancid is the fat and higher the acid number, the fat starts giving a foul smell. Such fats are not fit for consumption and could end up in severe health related issues. One can observe that though for all the oils the acid number is in permissible limits, but still the crude oils have relatively very low acid values when compared to the refined oils.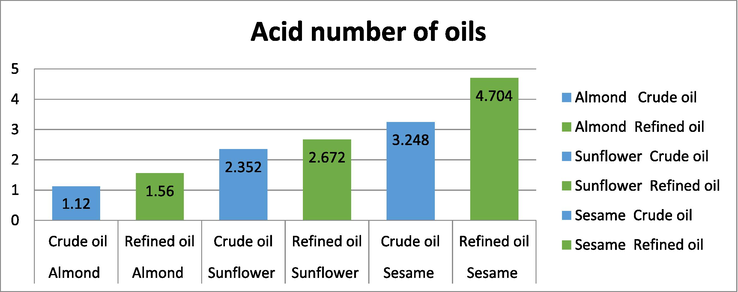
Acid value of crude and refined oils.
3.4 Phenolic activity of oils:
Polyphenols are a category of widely distributed groups of metabolites found in plants. They are found to have wide variety of different of different roles in biological system. The reaction between Folin-Ciocalteu and phenolic compounds results in the formation of blue color complex (Shafique Ahmad, 2004) which allow quantification, using gallic acid as standard. The phenolic activity was determined for all the oils, both crude and refined. Comparatively the phenolic activity was found to be higher for refined oils when compared to the crude oils. It was found to be highest for refined sunflower oil and was found to be least for crude almond oil. The high content of phenolic activity in refined sunflower oil indicates that the oils is good for consumption. However, the results may vary from one country to another based on the variation in the genotypes and geographical locations (see Fig. 4).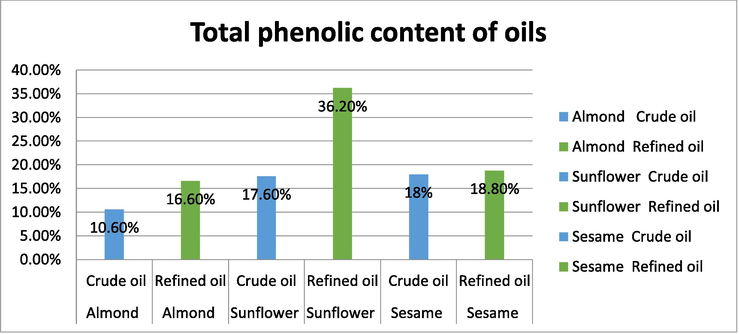
Total Phenolic activity of oils.
3.5 anti-oxidative property of crude and refined oils
Antioxidants are substances which prevent the formation of free radicals and prevent the oxidation of unsaturated fatty acids to peroxides. The mechanism of antioxidant activity is due to the unpaired electron of DPPH which forms a pair with the electron of hydrogen donated by free radical scavenging antioxidants present in oils, thus converting the purple colored odd electron DPPH to its reduced form which is yellow. The degree of decolorization is measured by UV–Visible spectrophotometer at a wavelength of 515 nm. The above figure related to the antioxidant activity of crude and refined oils (Fig. 5) indicates that the crude oils have got very high anti-oxidative properties and hence are best for consumption when compared to the crude oils which have relatively low antioxidant activity. Among the oils considered for study, crude almond oil has an antioxidant activity of 82.5 % followed by crude sesame oil and then crude sunflower oil. All the refined oils of the three varieties had an antioxidant activity of less than 50 %. (Masoom Raza Siddiqui, 2017).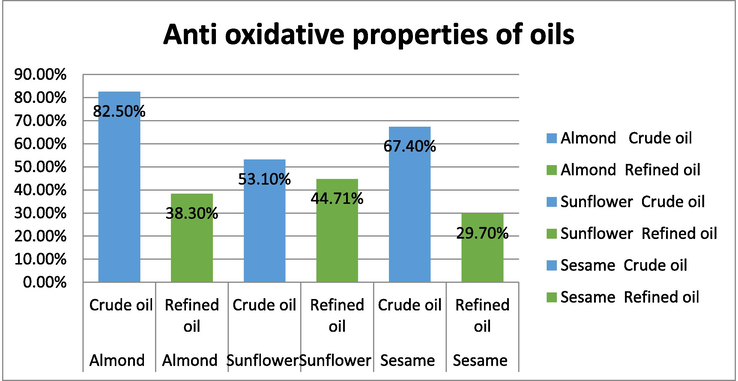
anti-oxidative properties of crude and refined oils.
3.6 GC–MS analysis of crude and refined oils
GC -MS analysis of the crude oil samples showed a good variation in the presence of unsaturated and polyunsaturated fatty acids. In crude almond oil the highest percentage was of 9-octadecadienic acid while the lowest percentage was shown by Tetradecanoic acid. In sunflower crude oil, the fatty acid that exhibited the highest percentage was 9,12-octadecadienic acid, while the lowest percentage of fatty acid was recorded for Dodecanoic acid. Similarly, GC–MS analysis of crude sesame oil showed that the fatty acid with the highest percentage was 9,12-octadecadienic acid and the fatty acid with the least percentage was oleic acid. The above results show that the crude oils have relatively higher percentage of unsaturated fatty acids (Table 1).
Crude almond oil
Crude Sunflower oil
Crude Sesame oil
Fatty acid
Relative%
Fatty acid
Relative%
Fatty acid
Relative%
Dodecanoic acid
0.295
Dodecanoic acid
0.481
Hexadecanoic acid
12.267
Hexadecanoic acid
5.085
Hexadecanoic acid
25.36
9,12-octadecadienic acid
70.893
8,11-octadecadienic acid
0.395
Tetradecanoic acid
0.531
9-octadecadienic acid
5.573
9-octadecadienic acid
44.412
9-octadecadienic acid
0.565
Oleic acid
0.233
Linoleic acid
2.986
9,12-octadecadienic acid
62.037
Phthalic acid
0.397
Cis-vaccenic acid
0.791
8-otadecednoi acid
4.496
Dodecanoic acid
0.481
Piperonylcyanoacetic acid
4.104
Tetradecanoic acid
0.268
GC -MS analysis of the ^refined oil samples also showed the presence of unsaturated and polyunsaturated fatty acids, however when compared to the crude oils the relative percentage was less. In refined almond oil the highest percentage was of oleic acid while the lowest percentage was shown by octadecadienoic acid. In sunflower refined oil, the fatty acid that exhibited the highest percentage was 9-octadecadienic acid, while the lowest percentage of fatty acid was recorded for 9,12-octadecadienic acid. Similarly, GC–MS analysis of refined sesame oil showed that the fatty acid with the highest percentage was 6-octadecadienic acid and the fatty acid with the least percentage was 9-octadecadienic acid. The results have been shown in Table 2.
Refined almond oil
Refined Sunflower oil
Refined Sesame oil
Fatty acid
Relative%
Fatty acid
Relative%
Fatty acid
Relative%
Hexadecanoic acid
0.499
9,12-octadecadienic acid
7.853
Hexadecanoic acid
10.131
9-octadecadienic acid
13.886
9-octadecadienic acid
12.738
6-octadecadienic acid
75.717
6-octadecadienic acid
0.431
Cis-vaccenic acid
6.929
Cis-vaccenic acid
1.714
9-octadecadienic acid
0.287
Dichloroacetic acid
16.263
Oleic acid
20.866
Hexadecanoic acid
0.499
9-octadecadienic acid
13.886
Given below is a graphical representation of the GC- MS profiles of crude and refined oils.
4 Conclusion
Three oils in crude and refined form were selected to determine their quality namely almond, sunflower and sesame oils. The crude almond oil is rich in unsaturated fatty acid which is good for our body. The high iodine value in sunflower crude oil. The almond refined oil has highest value. The highest value of anti-oxidative in crude oil then the refined oil. The refined oils contain high acid numbers while crude oil have less acid number. the heat effect in the results of iodine number. Among these types of oils, the crude oils are more good than the refined oils which shows poor quality. The GC_MS analysis also exhibited the presence of more unsaturated fatty acids in crude oils when compared to the refined oils as the results show, the crude oil good for our body and health while the refined oil is not good for our body. The crude oil which directly used after extraction from seeds is more useful than the refined oil which utilized from marketplace (see Figs. 6-11).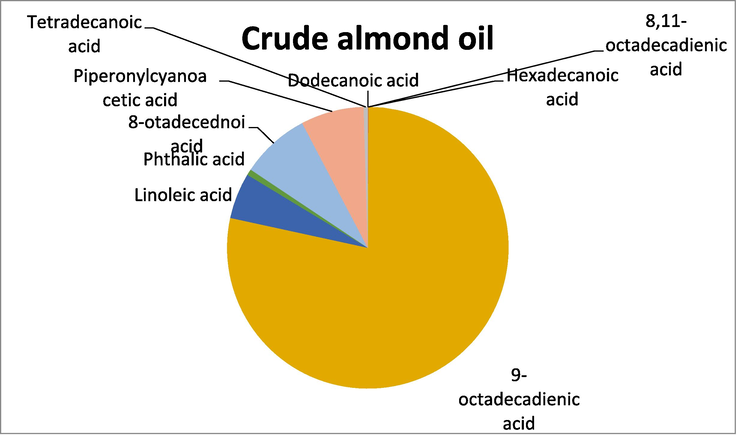
GC–MS analysis of crude almond oil.
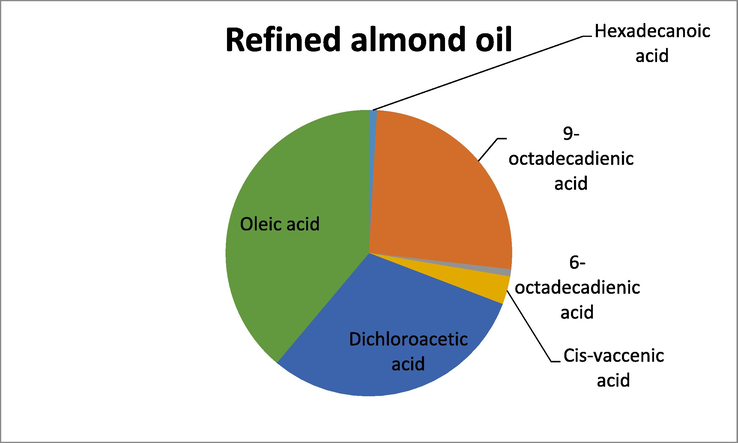
GC–MS analysis of Refined almond oil.
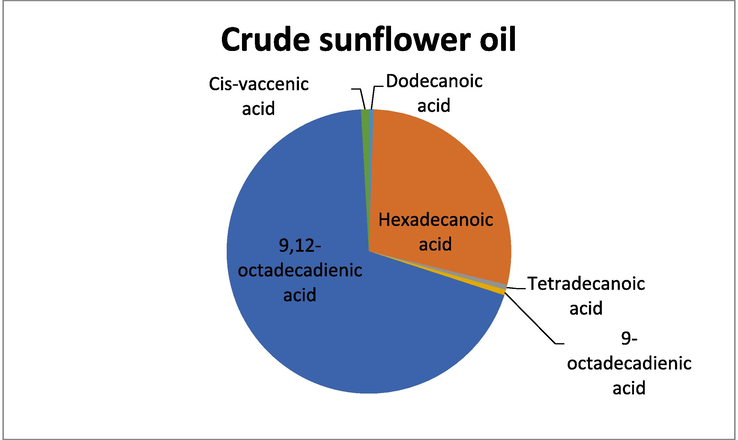
GC–MS analysis of crude sunflower oil.
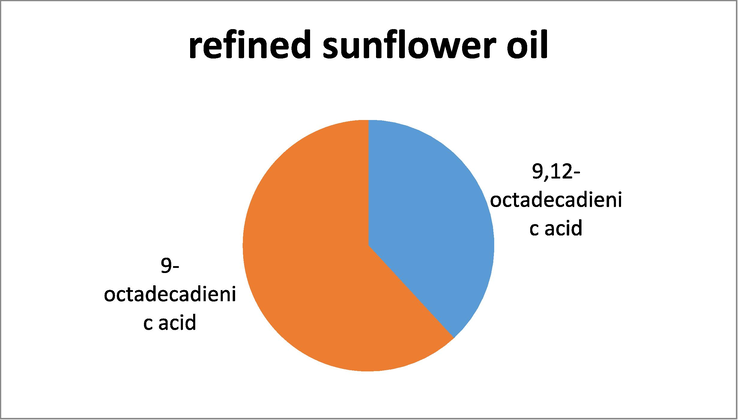
GC–MS analysis of Refined sunflower oil.
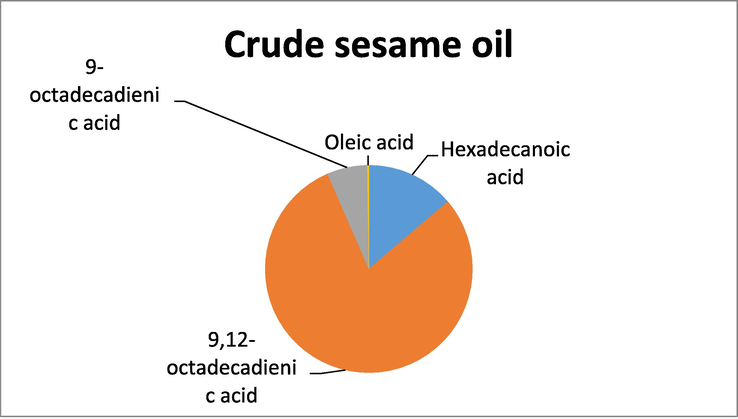
GC–MS analysis of crude sesame oil.
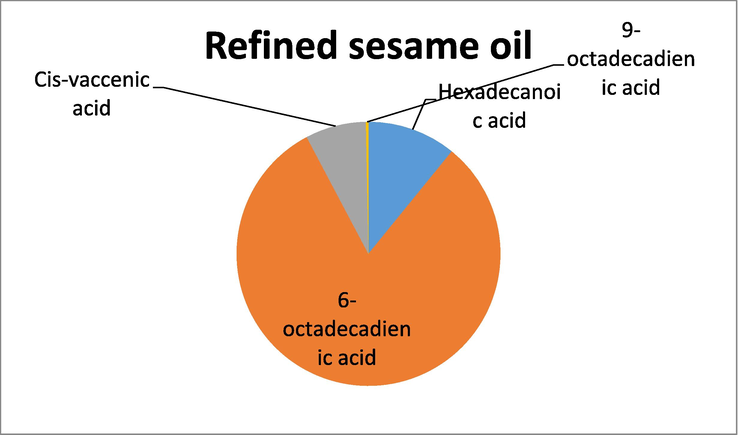
GC–MS analysis of refined sesame oil.
Funding
This work was supported by University of Technology and Applied Sciences-Higher College of Technology, Muscat, Sultanate of Oman. This study was also financially supported by the Researchers Supporting Project number (RSP-2021/371), King Saud University, Riyadh, Saudi Arabia.
Acknowledgements
The authors are very much thankful to the help and support rendered by the technical staff of the applied Biology department for the completion of the project. The author would also like to thank the management of University of Technology and Applied sciences, for their never-ending encouragement in publishing quality work. The authors would also like to acknowledge the funding support by the Researchers Supporting Project number (RSP-2021/371), King Saud University, Riyadh, Saudi Arabia. Sincere thanks and appreciation are also due to all the staff members of the Applied Sciences Department. The authors also thank the Ministry of Higher Education, Innovation, and research for providing required support. The authors are also thankful to biology lab technicians.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Physiochemical characteristics of sesame seeds. 2018;6(1):64-66.
- Amelio, M., & Onaoo, O. 2003. Chemical-physical characteristics of olive oils Technical course for Olive oil tasters, 1–26.
- GC-MS analysis, determination of total phenolics, flavonoid content and free radical scavenging activities of various crude extracts of Moringa peregrina (Forssk.) Fiori leaves. Asian Pacific Journal of Tropical Biomedicine 2014
- [Google Scholar]
- A study on the total phenols content and antioxidant activity of essential oil and different solvent extracts of endemic plant Merremia borneensis. Arabian J. Chem.. 2015;8(1):66-71.
- [Google Scholar]
- Medicinal Use of Sunflower Oil and Present Status of Sunflower in Pakistan : A Review Study. 2012;31(2):99-106.
- Physical, Chemical and Antioxidant Properties of Olive Oil Extracted from Memecik Cultivar. 2011;9(2):13-18.
- Biochemical composition and physicochemical properties of Moringa oleifera seed oil. Acta Alimentaria. 2014;43(4):538-546.
- [Google Scholar]
- Essential Oils’ Chemical Characterization and Investigation of Some Biological Activities: A Critical Review. . 2016 Sep 22;3(4):25. PMID: 28930135; PMCID: PMC5456241. Medicines (Basel) 2016
- [CrossRef] [Google Scholar]
- The problems of using one-dimensional methods to evaluate multifunctional food and biological antioxidants. J. Sci. Food Agric. 2000
- [Google Scholar]
- Study on Physicochemical Properties of Edible Oils Available in Bangladeshi Local Market. Archives of Current Research International. 2016;6(1):1-6.
- [Google Scholar]
- Determination of total phenolic content and total antioxidant activity in locally consumed food stuffs in Moodbidri, Karnataka. India. Advances in Applied Science Research. 2015;6(6):99-102.
- [Google Scholar]
- Masoom Raza Siddiqui, Zeid A. AlOthman, Nafisur Rahman, Analytical techniques in pharmaceutical analysis: A review,Arabian Journal of Chemistry,Volume 10, Supplement 1, 2017,Pages S1409-S1421,ISSN 1878-5352, http://doi.org/10.1016/j.arabjc.2013.04.016.
- [Antioxidant naturally occur in food]. Przem. Ferm. Owoc. Warz. 4, 7-9 [in Polish]. Przem. Ferm. Owoc. Warz 1996
- [Google Scholar]
- Trans fatty acids and cardiovascular disease. N Engl J Med. 2006;13(354):1601-1613.
- [CrossRef] [Google Scholar]
- Virgin almond oil: Extraction methods and composition. Grasas y Aceites. 2016;67(3):e143.
- [Google Scholar]
- Sabinus Oscar O. EZE. 2012. Physico-chemical properties of oil from some selected underutilized oil seeds available for biodiesel preparation. Afr. J. Biotechnol. 11(42), 10003–10007.
- Spectrophotometric Determination of Ampicillin, Amoxycillin, and Carbenicillin Using Folin-Ciocalteu Phenol Reagent. J. Anal. Chem.. 2004;59:119-123.
- [CrossRef] [Google Scholar]
- <Physical Properties of Oils and Mixtures of Oils.pdf>. 1985;62(2):241-242.
- Titrator, A. P. 1959. Iodine value of Oil and Fat Redox titration by Automatic Potentiometric Titrator, 2–5.
- Characterization of sunflower seed and kernel proteins. Helia. 2010;33(52):103-114.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2022.102432.
Appendix A
Supplementary material
The following are the Supplementary data to this article:







