Translate this page into:
Comparative evaluation of sublethal doses of different insecticides on the ovipositional behavior of whitefly (Bemisia tabaci) in Brinjal
⁎Corresponding authors. neerudumra23@gmail.com (Neeru Dumra), ashokchoudhary116@gmail.com (Ashok Choudhary)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
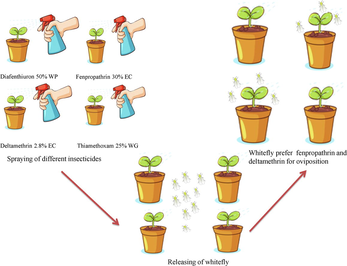
The world's worst invading insect is the whitefly, Bemisia tabaci Gennadius (Hemiptera: Aleyrodidae). For B. tabaci's thorough field control, research on the sublethal impacts of popular insecticides is crucial. We examined the effects of sublethal insecticide concentrations on whiteflies' predilection for oviposition on treated plants of brinjal (Solanum melongena L. cv. Hisar Shyamal) using a multiple-choice test during the 2019 and 2020 growing seasons. The insecticide efficacy was evaluated on the basis of the oviposition preference of B. tabaci on brinjal plants that were raised in pots and repeatedly treated with a distinct dosage of insecticides. The biochemical changes of treated brinjal leaves were also examined in this research, along with their connection to oviposition tendency. The findings showed that whiteflies favored fenpropathrin 30 % EC and deltamethrin 2.8 % EC treated plants for oviposition over diafenthiuron 50 % WP and thiamethoxam 25 % WG. In comparison to untreated control plants, most eggs were deposited on sublethal doses of fenpropathrin 30 % EC treated plants at 50 g a.i./ha and deltamethrin 2.8 % EC at 7.5 g a.i./ha. The findings of biochemical tests showed that with the exception of lesser dosages of fenpropathrin (30 % EC) as well as deltamethrin (2.8 % EC), all insecticidal treatments reduced total sugar and amino acids. Additionally, all the pesticides reduced the overall phenol level and significantly altered the crude protein content. The treated brinjal plant with deltamethrin 2.8 % EC and fenpropathrin 30 % EC attracted whiteflies for oviposition because it provides an improved site for them in terms of nourishment.
Keywords
Pesticides efficacy
Vegetables
Hormoligosis
Biochemical changes
Solanum melongena L
- %
-
percent
- WG
-
Water-dis/sable granules
- WP
-
Wettable powder
- EC
-
Emulsifiable concentrate:
- a.i
-
active ingredient
- CIBRC
-
Central Insecticide Board and Registration Committee
Abbreviations
1 Introduction
One of the most damaging insects of many crop plants, such as ornamentals, cotton, vegetables and many other agricultural products, is the whitefly (Bemisia tabaci Gennadius, Hemiptera: Aleyrodidae) (Nauen et al., 2014; Perring et al., 2018). Both whitefly larvae and adults harm plants by eating beneath the leaves' surface and sucking phloem fluid from sieve passages in the foliage. Whiteflies create honeydew on plant surfaces, which encourages the development of dark, sooty mold from fungi and delays the photosynthetic process (Khan et al., 2015).
The chemical method is the most abundantly used pest management tool to eliminate many pests including whitefly which usually requires high-frequency application of chemicals (Bhushan et al., 2011; Sudarshan and Pijush, 2011). However, the resurgence of whitefly is well documented despite the use of insecticides (Reddy and Srinivas, 2005). Insecticide overuse (Ali et al., 2020; Petrovskaya et al., 2020), suppression of natural enemies (Al–Jorany and Sadik, 2012; Sohrabi et al., 2013), insecticide-induced plant growth (Varshney et al., 2015) and shifts in plant nutrition following insecticide treatment (Shakir et al., 2018) have all been linked to an upsurge in whitefly populations in brinjal, which is one of the most important vegetables grown in India and many other countries. Exposure to pesticides at sublethal concentrations is probably a contributing factor in outbreaks of whitefly populations. Hormoligosis is a process that explains why insect fecundity increases in the presence of sub-lethal pesticide concentrations. Practical concerns, incorrect application techniques, and external factors like weather, wind, and moisture can contribute to this occurrence. If their reproductive processes are encouraged, insect colonies may expand more quickly. Insecticides are applied more frequently as a result of the increased birth rate, which may increase the pressure on insect populations and strengthen their capacity for resistance. To fully comprehend the influence of pesticides on arthropods, it is essential to recognize their sub-lethal impacts (Wu et al., 2022). Although numerous scholars have examined the behavior of whiteflies on brinjal (Perring et al., 2018), little is known regarding the impact of pesticides on whitefly activity (Mokrane et al., 2020). Authors found limited literature that tested the impact of insecticides on egg laying of B. tabaci on cotton (Abdullah et al., 2006; Kaur et al., 2022), whereas no information are available in the case of insecticides with various modes of action on brinjal plant. Studying the lethal as well as sublethal effects of pesticides against whiteflies is crucial because they are the main feeding pest of brinjal that causes substantial crop declines. Additionally, the amount of several biochemical factors of the brinjal plant may be affected by repetitive pesticide applications. These changes might have had a positive or negative effect on B. tabaci's unstudied hormoligosis. For integrated pest management (IPM), a thorough evaluation of an insecticide's sublethal impacts on its target insect is essential (Shi et al., 2022). The present research’s aim is to evaluate how sublethal pesticide dose affects the whitefly ovipositional behavior and biochemical properties of the brinjal.
2 Material and methods
2.1 Research area, trial condition and vegetal material
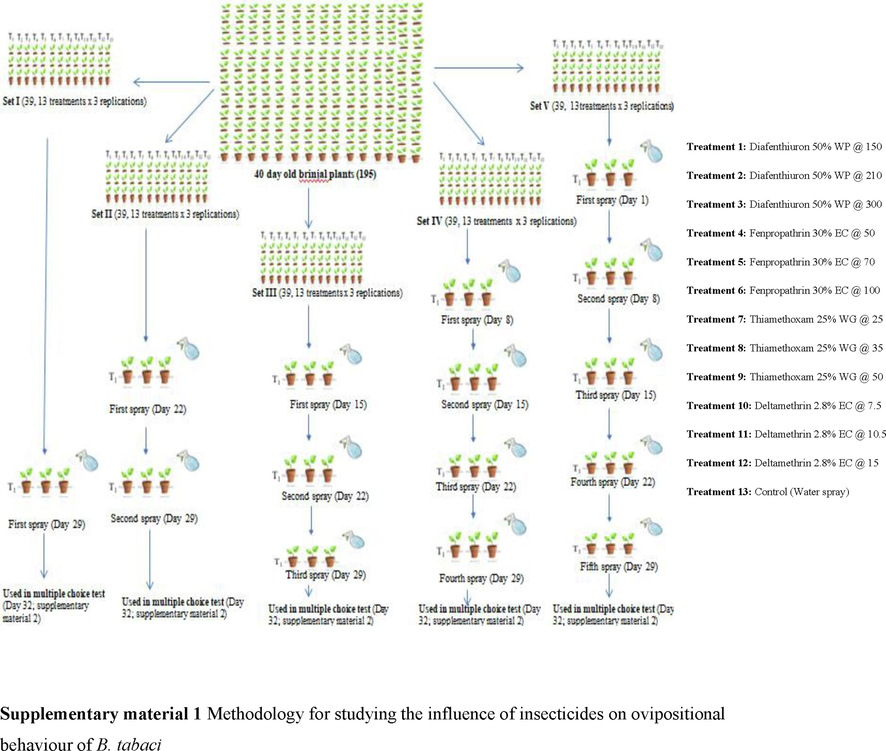
The research was accomplished on Brinjal var. Hisar Shyamal at Entomological Research Farm (HAU), Hisar, in a screenhouse during 2018 and 2019. A brinjal seedling that was 40 days old was placed in pot. These pots were stored in a screenhouse devoid of whiteflies having net. Formalin 0.3 % was sprayed on the screenhouse's walls, roof, and floor to protect the plants from an external whitefly invasion.
2.2 Insect and insecticide
Hisar Shyamal variety of brinjal seedlings were used to sustain B. tabaci populations in screenhouse. Adult whiteflies were sucked out of the field using a 30-centimeter aspirator and discharged onto whitefly free brinjal plants stored in a screenhouse to begin the culture. Various insecticides used in the experiment were fenpropathrin 30 % EC at 50, 70 and 100 g a.i./ha, diafenthiuron 50 % WP at 150, 210 and 300 g a.i./ha, deltamethrin 2.8 %EC at 7.5,10.5 and 15 g a.i./ha along with thiamethoxam 25 % “WP at 25,35 and 50 g a.i./ha (recommendation of CIBRC).
2.3 Leaf cages
The whitefly adults were kept in the leaf cages made of glass. By using a sharp-edged razor to make an opening in the bottom of the cylindrical clear plastic glass, the cylindrical leaf enclosure was created. Adult whiteflies were allowed to enter the cage through a hole having cotton swab plug put in the glass at the bottom.
2.4 Ovipositional preference
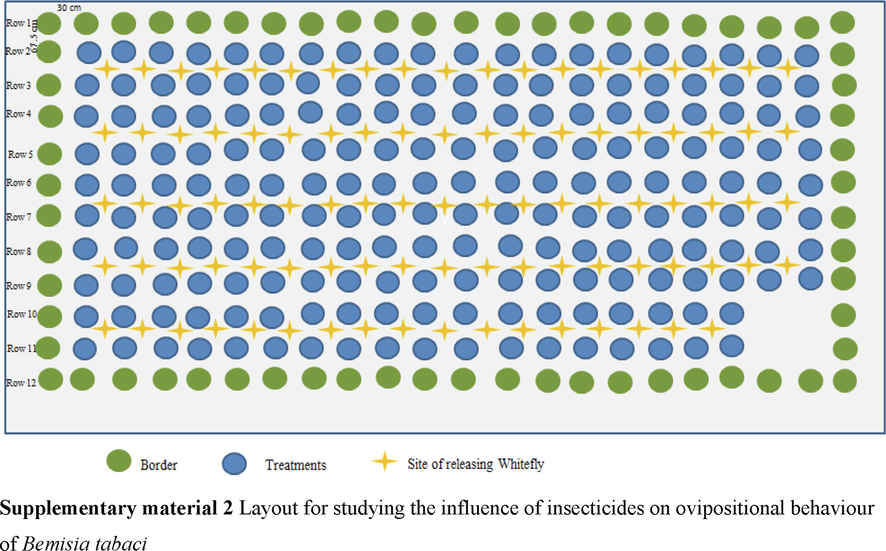
Test plants were kept in a screenhouse without whiteflies and raised in pots. The plants, which were divided into five groups and given a total of five treatments, were each 40 days old. The 1st set received 1 spray, the 2nd set received 2 sprays, the 3rd set received 3 sprays, the 4thset received 4 sprays, and the 5thset received 5 sprays. Seven days passed between each subsequent dose. 196 seedlings were randomly distributed around the open area after 72 h of 1, 2, 3, 4, and 5 applications. The treated seedlings were placed in rows number 2 to 11. The spacing between plants and rows was 30 cm and 67.5 cm, respectively; there were 20 plants in each row, with the exception of the tenth and eleventh rows, which had 18 plants per row. Around the experimental plants was a border row of untreated plants. Only the upper two completely grown leaves remained after the plant's foliage was stripped away. Twenty males and twenty females were discharged at equal intervals in the middle of each of the four plants after being separated from the main population (10 adults per plant). Using a 60X magnifying lens, we counted averaged number of eggs recorded throughout the whole leaf surface. Number of eggs included newly hatched nymphs.
2.5 Biochemical analysis
Biochemical study of treated brinjal leaves was conducted in the Biochemistry Laboratory, CCSHAU Hisar. For each round of sprays, brinjal plants were harvested three days after treatment by cutting off the 3rdfully formed leaf from the plant's apex on both the treated and untreated plants. For three days, the greens were stored in an oven set to 65 ± 1 °C. Each desiccated leaf was then pulverized and stored for later examination in a tiny airtight plastic container. The total soluble sugar, as well as total free amino acid content in the samples were calculated using the methods of Dubois et al. (1951) and Lee and Takahashi (1966), respectively. Using the Swain and Hillis technique, the total phenol concentration was calculated (Swain and Hills, 1959) and John Kjeldahl's technique was used to determine the amount of crude protein (Kjeldahl, 1883).
2.6 Data analysis
The Levene Test was used to ensure that the ovipositional data choice of B. tabaci eggs had been normal and a two-way ANOVA test was performed in the IBM SPSS Statistic 23 Software. Tukey's Honestly Significance Difference (HSD) was used in a post-hoc comparison to differentiate different treatment means. ANOVA was used to analyze the biochemical component data from a test with a CRD (Completely Randomized Design) and Tukey's HSD (Honestly Significance Difference) was then used to compare the results.
3 Results
3.1 Ovipositional preference
A two-way ANOVA showed that the number of sprays and the various treatments had statistically significant interaction effects for August 2019 (F (194, 48) = 527.888, p = 0.000). In comparison to the untreated control (26.74 eggs), the plant treated with sublethal dosages of fenpropathrin 30 % EC (42.93 eggs) and deltamethrin 2.8 % EC (39.57 eggs) recorded the highest number of eggs. The total number of eggs per leaf was found to be significantly affected by the treatments (p = 0.0001), as determined by a simple main effects analysis. The total number of eggs per leaf has been considerably affected by the number of sprays (p = 0.0001). Likewise, in 2020, a statistically substantial relation between the number of sprays and the effects of the various treatments (F (194, 48) = 425.316, p = 0.000). There were substantial impacts of several treatments on the overall number of eggs per leaves (p = 0.000). The total number of eggs per leaf did not, however, differ substantially as a result of the number of sprays (p = 0.369) (Table 1). The lowest number of eggs (17.84eggs) was observed in plants treated using diafenthiuron 50 % WP at 300 g a.i. / ha concentration. The number of eggs has been comparatively more in sublethal doses of thiamethoxam 25 % WG than the untreated control, but non-significant variation has been found in eggs placed in thiamethoxam 25 % WG treated plant in every dose (25, 35 and 50 g a.i./ha) and untreated control (Mean difference of 25, 35 and 50 g a.i. per ha-control: − 0.750, p = 0.875; −6.706, p = 0.223; and 0.394, p = 0.939) respectively. Fenpropathrin 30 % EC, as well as deltamethrin 2.8 % EC, treated plants have been favored for oviposition using whitefly adults as compared to thiamethoxam 25 % WG and diafenthiuron 50 % WP treated plants, showing that these two compounds have been initiating amendments in the behavior of whitefly that resulted in more attraction to these insecticide treated plants (see Table 2) Data signify mean ± SE of the mean (n = 3). The values shown with the identical letter aren’t statistically distinct from one another (p > 0.05, 2-way ANOVA followed by Bonferroni test).
Treatments
Dose (g a.i./ha)
Number of eggs/leaf after different sprays (Mean + S.E)
2019
Pooled Mean
2020
Pooled Mean
1 spray
2 sprays
3 sprays
4 sprays
5 sprays
1 spray
2 sprays
3 sprays
4 sprays
5 sprays
Diafenthiuron 50 % WP
150
25.73 + 0.82e
31.33 ± 1.07q
21.00 ± 0.71 h
18.69 ± 0.60 s
12.77 ± 0.43n
21.90 ± 0.85
28.73 + 0.94 g
34.77 + 1.16 g
25.25 + 0.83 h
21.00 + 0.72i
24.39 + 0.86d
26.82 + 0.71
Diafenthiuron 50 % WP
210
22.01 + 0.74 h
22.00 + 0.72n
17.73 + 0.59v
20.35 + 0.69z
17.07 + 0.56 h
19.83 + 0.410
32.73 + 1.11 h
30.72 + 1.00 l
20.31 + 0.67c
21.00 + 0.69q
18.31 + 0.60 l
24.61 + 0.84
Diafenthiuron 50 % WP
300
19.00 + 0.61 g
18.00 ± 0.57p
15.33 ± 0.49e
9.29 ± 0.30e
13.00 ± 0.42z
14.92 ± 0.47
28.34 + 0.92f
18.31 + 0.62p
14.31 + 0.45p
11.50 + 0.37f
16.75 + 0.54o
17.84 + 0.73
Fenpropathrin 30 % EC
50
39.00 + 1.83j
27.03 + 1.24c
38.31 + 1.71 s
49.33 + 2.29z
61.00 + 2.89 h
42.93 ± 1.67
50.72 + 2.35 k
49.71 + 2.34j
33.72 + 1.52w
45.00 + 2.12u
66.73 + 3.20j
49.17 + 1.76
Fenpropathrin 30 % EC
70
37.79 + 1.08i
26.00 + 0.74r
33.71 + 0.94i
43.30 + 1.21 s
54.32 + 1.53 h
39.02 ± 1.43
45.34 + 1.28i
42.76 + 1.21q
30.31 + 0.86v
48.29 + 1.34r
57.30 + 1.61y
44.80 + 1.30
Fenpropathrin 30 % EC
100
55.74 + 2.17c
35.00 + 1.38c
29.39 + 1.15e
28.15 + 1.10d
26.47 + 1.02 t
34.95 ± 1.44
51.71 + 1.96j
42.00 + 1.61q
40.73+ 1.59r
41.00 + 1.59 h
25.75 + 1.02r
40.2 3+ 1.30
Thiamethoxam 25 % WG
25
32.33 + 0.44 k
24.78 + 0.34n
21.31 + 0.30r
22.31 + 0.30f
18.34 + 0.25o
23.81 ± 0.57
29.00 + 0.41d
30.35 + 0.43d
38.31 + 0.55q
30.33 + 0.43o
24.00 + 0.33d
30.39 + 0.62
Thiamethoxam 25 % WG
35
31.71 + 1.32 l
28.27 + 1.17r
27.17 + 1.13o
20.78 + 0.87c
22.89 + 0.96m
26.16 ± 0.70
36.00 + 1.45 l
26.00 + 1.11r
23.34 + 0.98c
29.73 + 1.23f
20.00 +0.85u
27.01 ± 0.84
Thiamethoxam 25 % WG
50
24.84 + 0.78m
20.14 + 0.61 s
18.45 + 0.56w
22.00 + 0.67j
19.32 + 0.58e
20.95 ± 0.42
30.72 + 0.94 l
25.76 + 0.80 s
23.72 + 0.73 g
20.13 + 0.62x
28.00 + 0.84 g
25.66 + 0.61
Deltamethrin 2.8 % EC
7.5
37.71 + 0.92f
34.78 ± 0.86o
29.31 ± 0.68n
36.72 ± 0.91r
59.31 ± 1.48aa
39.57 ± 1.39
47.34 + 1.19e
51.33 + 1.27o
47.00 + 1.19f
50.00 + 1.23i
62.34 + 1.54r
51.60 + 0.88
Deltamethrin 2.8 % EC
10.5
36.75 + 0.60d
32.00 ± 0.52 m
27.00 ± 0.42u
34.33 ± 0.58x
53.33 ± 0.85 h
36.68 ± 1.17
42.72 + 0.71d
41.31+ 0.67m
38.00 + 0.63u
41.00 + 0.68c
55.76 + 0.94j
43.75 + 0.89
Deltamethrin 2.8 % EC
15
31.73 + 0.74e
29.77 ± 0.68n
23.33 ± 0.54e
26.71 ± 0.63y
43.12 ± 1.02 t
30.93 + 0.90
44.31 + 1.05e
33.72 + 0.77n
32.35 + 0.75 l
29.73 + 0.69u
28.31 + 0.65u
33.68 + 0.78
Control
24.00 + 0.58c
26.70 ± 0.64j
29.30 ± 0.72 t
21.00 ± 0.51q
28.70 ± 0.70 aa
26.74 ± 0.47
27.71 + 0.66c
29.70 + 0.73 h
26.00 + 0.64 t
28.71 + 0.70r
30.00 + 0.72d
28.42 + 0.38″
2019
2020
Source
F
P value
df
F
P value
Df
Treatments
536.58
0.010
12
1885.50
0.000
12
Number of sprays
53.33
0.000
4
1.08
0.369
4
Treatments x Number of sprays
527.88
0.000
48
425.31
0.000
48″
Sugar
20.26
0.000
1
35.17
0.000
1
Crude Protein
6.83
0.010
1
5.92
0.016
1
Phenol
11.77
0.001
1
22.20
0.000
1
Amino acid
44.28
0.000
1
0.04
0.840
1
3.2 Hormoligosis in host plant
3.2.1 Total sugar content
In 2019, the maximum sugar concentration was discovered in a lesser dosage of fenpropathrin 30 % EC (3.49 %) after five sprays, whereas the lowest sugar level has been observed in diafenthiuron 50 % WP at 210 g a.i./ha (1.95 %) after three sprays (Fig. 1). Sugar level of brinjal leaves varied greatly (from 2.23 % to 3.26 %). The minimum dosage of deltamethrin, 2.8 % EC (3.26 %), was shown to have the highest sugar concentration in brinjal leaves. All treatments except for lower doses of deltamethrin 2.8 % EC and fenpropathrin 30 % EC had lower sugar level as compared to untreated control (2.80 %). In 2020, The data analysis revealed that the sub-optimal dosage of deltamethrin 2.8 % EC had greater sugar content (3.14 %). Sugar levels in brinjal leaves were shown to decrease after repeated applications of insecticides, with diafenthiuron 50 % WP (2.16 % at 300 g a.i./ha) as the least efficient (see Fig. 2).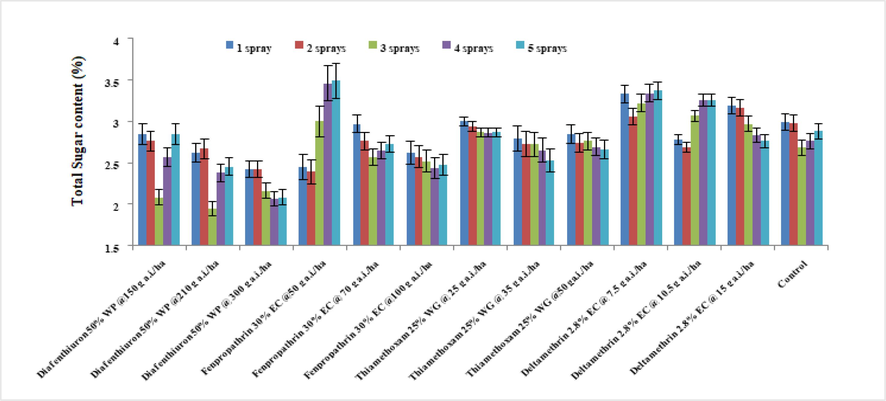
Average of the total sugar levels of treated brinjal leaves after various sprays during 2019 (mean ± SE).
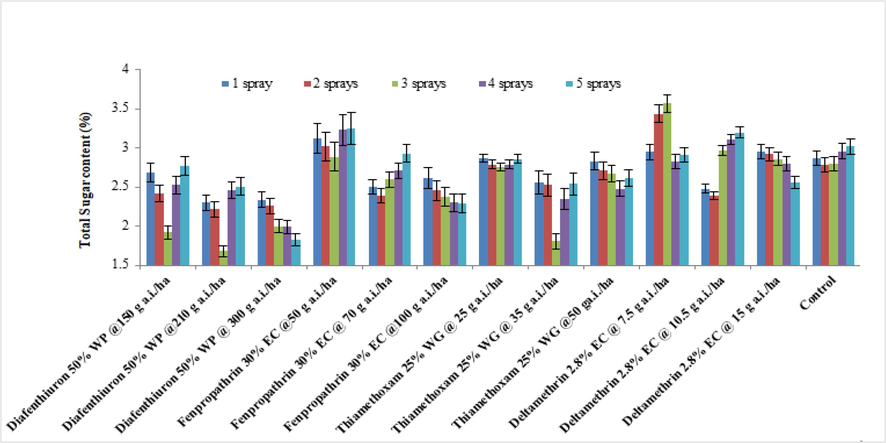
Average of the total sugar levels of treated brinjal leaves after various sprays during 2020 (mean ± SE).
3.2.2 Total free amino acid
The level of free amino acids in brinjal leaves differed significantly across different insecticides at various dosages in 2019 and 2020. Except for fenpropathrin 30 % EC at 50 g a.i./ha after three sprays as well as deltamethrin 2.8 % at 7.5 g a.i./ha after four sprays, total amino acids in insecticidal treated plants were generally lower in 2019 than in untreated controls (Fig. 3). The lowest dosage fenpropathrin 30 % EC (1.49 mg/g), has been observed to have the greatest concentration of amino acids, followed using a lower dose of deltamethrin 2.8 % EC (1.35 mg/g), In comparison to the untreated control, the other treatments showed significantly lower quantities of amino acids (1.34 mg/g). Similar results were attained in 2020 (Fig. 4). The combined data from the two years indicated that, except for lower doses of deltamethrin 2.8 %EC along with fenpropathrin 30 % EC, all test insecticides caused a decrease in the amino acid level of brinjal leaves when compared to the untreated control (1.41 mg/g).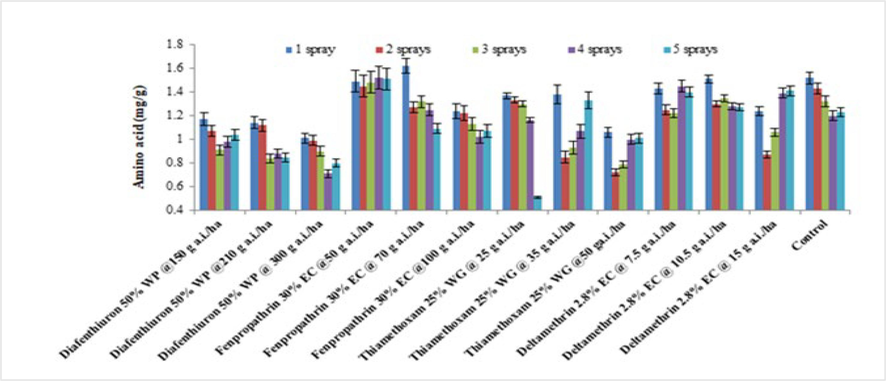
Average of the total amino levels of treated brinjal leaves after various sprays during 2019 (mean ± SE).
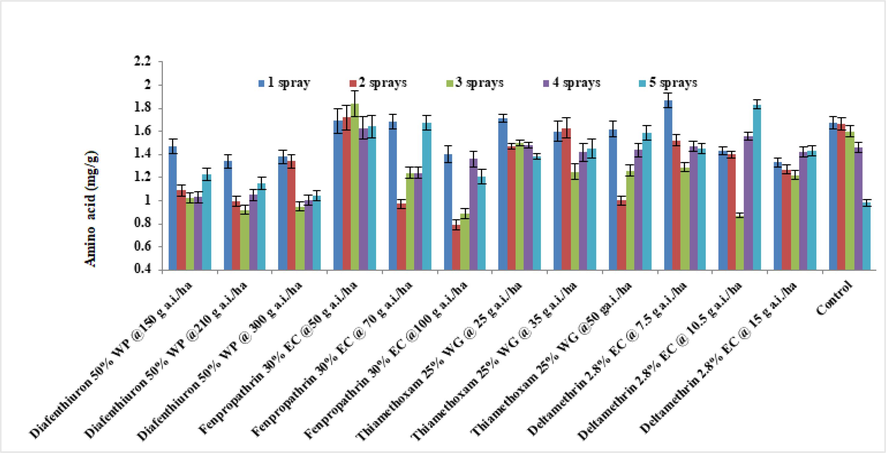
Average of the total amino acid levels of treated brinjal leaves after various sprays during 2020 (mean ± SE).
3.2.3 Total phenol
The Fig. 5 depicts how different pesticide treatments affected the total phenol levels of brinjal leaves throughout 2019. The overall findings showed that the maximum phenol content of brinjal was produced by using the minimum dosage of deltamethrin 2.8 % (1.32 mg/g), which was similar to the untreated control (1.37 mg/g). At 150 g a.i/ha, diafenthiuron 50 %WP had the lowest phenol concentration ever (1.04 mg/g). Similar results were obtained in 2020 (Fig. 6).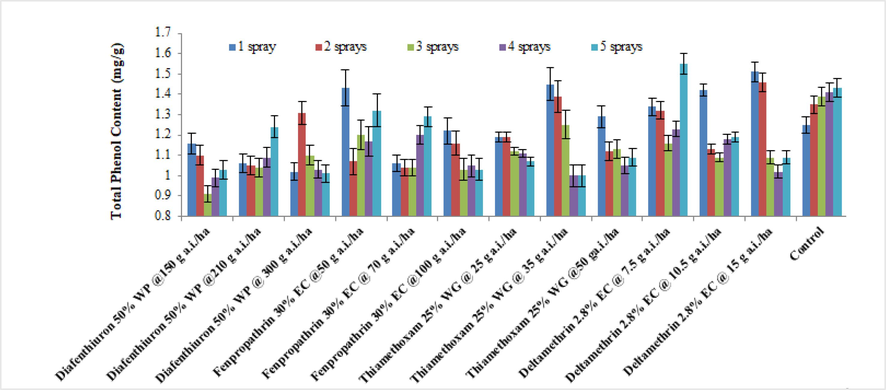
Average of the total phenol content of treated brinjal leaves after various sprays during 2019 (mean ± SE).
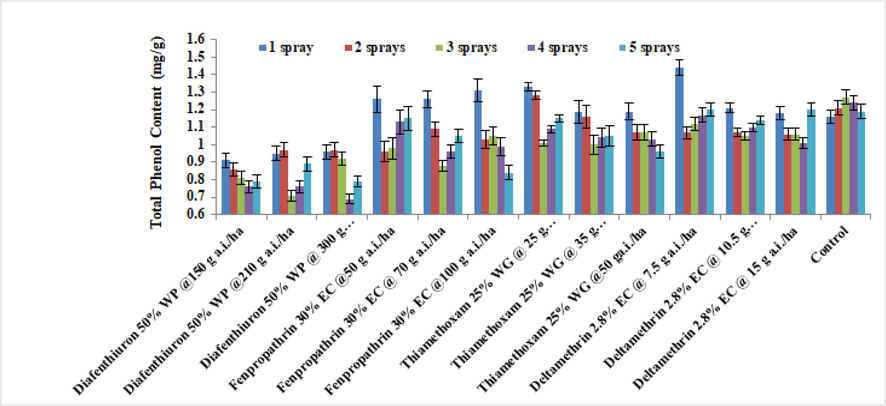
Average of the total phenol content of treated brinjal leaves after various sprays during 2020 (mean ± SE).
3.2.4 Crude protein
The data indicating the effect of various pesticide treatments on crude protein of brinjal leaves in 2019 (Fig. 7). Thiamethoxam 25 % WG at 50 g a.i./ha (1.21 %) noted the minimum crude protein level after four sprays, whereas fenpropathrin 30 % EC at 100 g a.i./ha noted the highest crude protein after three sprays (2.32 %). A very similar trend was seen in the year 2020 (Fig. 8). Combining data from both seasons showed that the total content of protein ranged between1.68 and 2.07 %, with deltamethrin 2.8 % EC at 15 g a.i./ha (1.68 %) having the lowest level. The treatment of plant with diafenthiuron 50 % WP at 300 g a.i./ha had the highest total protein content (2.16 %). The crude protein level of brinjal leaves is stimulated by a reduced deltamethrin 2.8 % EC quantity.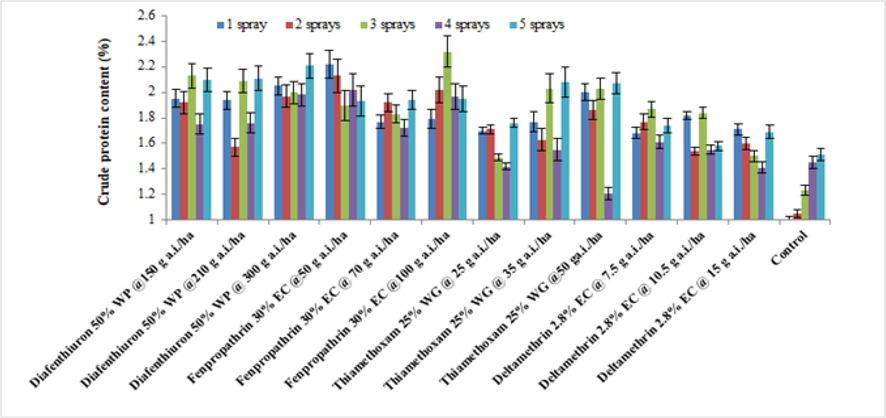
Average of the crude protein content of treated brinjal leaves after various sprays during 2019 (mean ± SE).
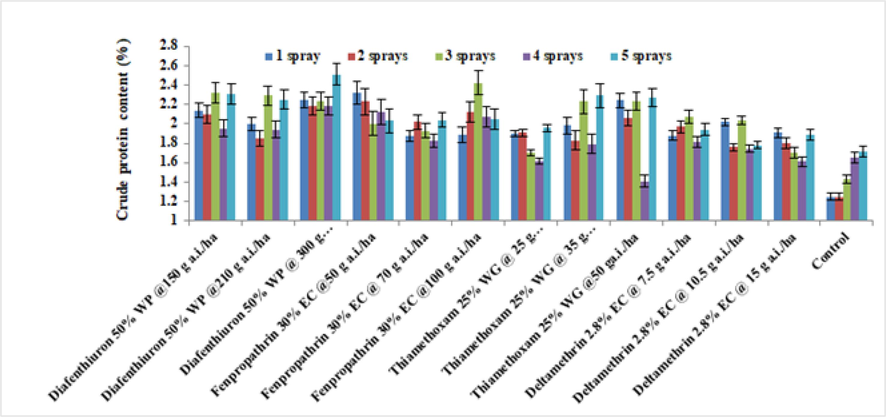
Average of the crude protein content of treated brinjal leaves after various sprays during 2020 (mean ± SE).
4 Discussion
The current research evaluated the effects of four pesticides that came from three distinct chemical families and gave experimental proof of their sublethal effects on B. tabaci adults. Interestingly, even when used at sublethal doses, deltametrin and fenpropathrin had substantial sublethal impacts on reproduction. Contrarily, diafenthiuron and thiamethoxam only had an adverse effect on reproduction when B. tabaci were subjected to fatal levels of those insecticides. For these two insecticides no stimulatory effects were recorded at the lowest doses. In 2019, a greater number of eggs was reported in sublethal dose of fenpropathrin treated plant, while in 2020 is the maximum value was recorded in plants treated with a sublethal dose of deltamethrin. This might be explained by meteorological conditions, which in 2019 were more fenpropathrin friendly than in 2020 which was favourable for deltamethrin, although both insecticides belongs to synthetic pyrethroid group.
Hormoligosis is a condition that happens to both insects and natural enemies of insects after exposure to lowest amounts of a few insecticides, like synthetic pyrethroids (Calabrese and Blain, 2005). Increased reproduction of insect on insecticide treated foliage may be due to shift in the nutrient status of the leaf (Riley, 1988) or a direct impact of the insecticide on the whitefly. The findings demonstrate that the impacts of insecticide can significantly differ based on several variables, including the outcome under consideration (mainly fatal, physiological, and behavioral), the chemical family of the insecticide, and the insecticide dosage under consideration.
Interestingly, even when used at sublethal doses, deltametrin and fenpropathrin had substantial sublethal impacts on reproduction. In 2019, a greater number of eggs were reported in sublethal dose of fenpropathrin treated plant, while in 2020 is the maximum value was recorded in plants treated with sublethal dose of deltamethrin. This might be explained by meteorological conditions, which in 2019 were more fenpropathrin friendly than in 2020.
According to Abdullah et al. (2006), whitefly adults favored all three dosages of fenvalerate (“25, 38 & 50 g a.i./ha”) for oviposition, followed by acephate and other treatments. Dhaliwal et al. (2000) reported 1.25 to 2.63 fold rise in oviposition of Helicoverpa armigera in treatment of deltamethrin to cotton. The fertility of Nilaparvata lugens and Myzus persicae decreased after feeding on leaves that are treated with sublethal doses of imidacloprid (Widiarta et al., 2001; Bao et al., 2009). Reproduction related traits are one of the most important aspects of a species' life history that has traditionally received the most attention when studying insect population sizes (Biondi et al., 2012; Zappalà et al., 2012). Tamilselvan et al. (1990), found that the sugar content of cotton plant treated with fenvalerate 20EC increased with increasing treatment frequency. Untreated cotton plants, as shown by Sithanantham et al. (1973), have more total sugar than treated cotton plants. The pesticide dosages in the present study that had the greatest sugar content also had the highest ovipositional preference rate. According to the findings, one of the reasons contributing to the resurgence of whiteflies may be the sugar level of these treatments (Dominick and Sundaram, 1992). Insects that feed on host plants with higher amino acid concentrations have greater nutrition, which in turn stimulates plant reproduction, which in turn increases insect fecundity (Asrorov et al., 2015). The crude protein content of brinjal leaves is stimulated by a reduced deltamethrin 2.8 % EC quantity (Zama and Hotzios, 1986). Kaur et al. (2022) found a substantial favorable association between biochemical factors like total sugar and reducing sugars and the predilection of whiteflies for oviposition. Pesticides enhance plant’s nutritional value, that in turn encourages greater insect reproduction (Suri and Singh, 2011).
5 Conclusion
All insecticidal interventions had a sizable impact on how the adults of B. tabaci made its choice of appropriate habitats. Both optimum as well as suboptimal dosages of insecticides elicited behavioral hormoligosis. Whitefly may have preferred those plants for oviposition because of the compensation of both may have given a better nutritional balance that was superior to other treatments. It is essential to find alternative ways to control arthropods (AlShabar et al., 2021; Baazeem et al., 2022; Khalaf et al., 2023). These alterations could play a role in B. tabaci’s hormoligosis. More research is needed to properly understand the interactions how brinjal’s fitness as a host for B. tabaci’s feeding, mating, and shelter niches will be impacted by modifications in the host plant brought by the usage of pesticides.
Declarations
Ethics approval
Not applicable.
Consent to participate
All authors consent to participate in the manuscript publication
Consent for publication
All authors approved the manuscript to be published.
Declaration of Funding: No external funding.
Author Contributions
N.D. and K.R. conceptualized the research; N.D. conducted the experiments. L.K.K, S.S.Y., SM, YKS and UK analyzed the data and helped in writing of the manuscript. AA, SMP and AC edited the language of the manuscript. All authors read and approved the final draft for submission.
Acknowledgement
We are grateful to the Head, Department of Entomology and Biochemistry Laboratory, CCSHAU Hisar for giving the needed technical aid during the investigation. The authors would like to extend their sincere appreciation to the Researchers Supporting Project Number (RSPD2024R695), King Saud University, Riyadh, Saudi Arabia
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Behavioral hormoligosis in oviposition preference of Bemisia tabaci on cotton. Pestic. Biochem. Physiol.. 2006;84:10-16.
- [CrossRef] [Google Scholar]
- insecticidal activity of eucalyptus sp. volatile oil against backswimmer insect Anisops sardea. Iraqi J. Agric. Sci.. 2020;51:470-482.
- [Google Scholar]
- Efficiency of some insecticides to protect potato tubers from attack by some soil insects. Iraqi J. Agric. Sci.. 2012;43
- [Google Scholar]
- AlShabar, S.H., Timm, A., Khalaf, L., 2021. Population variation of Polyphagotarsonemus latus (Banks) in Baghdad province, central Iraq. IEEE, pp: 138–141.
- Comparative analysis of free amino acids and nitrogen in cotton leaves treated with different classes insecticides. Agric. Res.. 2015;4:277-282.
- [CrossRef] [Google Scholar]
- Identification and environment-friendly biocontrol potential of five different bacteria against Aphis punicae and Aphis illinoisensis (Hemiptera: Aphididae. Front. Microbiol.. 2022;13:961349
- [Google Scholar]
- Sublethal effects of four insecticides on the reproduction and wing formation of brown planthopper, Nilaparvata lugens. Pest Manag. Sci.. 2009;65:170-174.
- [CrossRef] [Google Scholar]
- Efficacy and economics of pest management modules against brinjal shoot and fruit borer (leucsinodes orbonalis. The Bioscan. 2011;6:639-642.
- [Google Scholar]
- Using organic-certified rather than synthetic pesticides may not be safer for biological control agents: Selectivity and side effects of 14 pesticides on the predator Orius laevigatus. Chemosphere. 2012;87:803-812.
- [CrossRef] [Google Scholar]
- The occurrence of hormetic dose responses in the toxicological literature, the hormesis database: an overview. Toxicol. Appl. Pharmacol.. 2005;202:289-301.
- [CrossRef] [Google Scholar]
- Oviposition Response of Helicoverpa armigera (Hubner) on Cotton under Insecticidal Applications in Hirsutum Cotton. Insect Environ.. 2000;5:157-158.
- [Google Scholar]
- Effect of insecticides on the biochemical nature of the host plant and its relation to resurgence of whitefly, Bemisia tabaci on cotton. Pestology. 1992;16:7-10.
- [Google Scholar]
- Dubois, M., Gilles, K., Hamilton, J.K., Rebers, P.A., Smith, F.A., 1951. Colorimetric Method for the determination of Sugar. Nature 168, 167–167. https://doi.org/10.1038/168167a0.
- Behavioural hormoligosis in oviposition preference of Bemisia tabaci (Gennadius) in Bt cotton. Int. J. Trop. Insect Sci.. 2022;42:2163-2171.
- [CrossRef] [Google Scholar]
- Occurrences of wheat curl mite Aceria tosichella Keifer 1969 (Eriophyidae) and the associated viruses, (WSMV, HPWMoV, TriMV) in Iraq. Iraqi J. Agric. Sci.. 2023;54:837-849.
- [CrossRef] [Google Scholar]
- Proximate chemical composition of brinjal Solanum melongena L. J. Entomol. Zool. Stud.. 2015;3:303-306.
- [Google Scholar]
- New Method for the determination of Nitrogen. Chem. News. 1883;48:101-102.
- [CrossRef] [Google Scholar]
- An improved colorimetric determination of amino acids with the use of ninhydrin. Anal. Biochem.. 1966;14:71-77.
- [CrossRef] [Google Scholar]
- Behavioral effects induced by organic insecticides can be exploited for a sustainable control of the Orange Spiny Whitefly Aleurocanthus spiniferus. Sci. Rep.. 2020;10:15746.
- [Google Scholar]
- Whitefly special issue organized in two parts. Pest Manage. Sci.. 2014;70:1438-1439.
- [CrossRef] [Google Scholar]
- Whiteflies: Biology, ecology, and management. In: WakilG W., Brust E., Perring T.M., eds. Sustainable Management of Arthropod Pests of Tomato. ElsevierAmsterdam, Cambridge, MA, USA; 2018. p. :73-110.
- [Google Scholar]
- Modelling a targeted use of pesticide procedure for pest populations with heterogeneous spatial distributions. Ecol. Model.. 2020;427:109059
- [Google Scholar]
- Efficacy of insecticides against brinjal shoot and fruit borer, Leucinodes orbonalis (Guen. Pestology. 2005;29:31-33.
- [Google Scholar]
- Riley, T.J. 1988. Plant stress from arthropods: insecticide and acaracide effects on insect, mite, and host plant biology. 187–204.
- Pesticide induced oxidative stress and antioxidant responses in tomato (Solanum lycopersicum) seedlings. Ecotoxicology. 2018;27:919-935.
- [Google Scholar]
- Shi, Y., Chen, H., Wu, S.X., F, H.M., Yang, L.L.R., Liao, X., Li, M., 2022. Sublethal effects of nitenpyram on the biological traits and metabolic enzymes of the white-backed planthopper, Sogatella furcifera (Hemiptera: Delphacidae. Crop Protection 155, 105931.
- Some changes in the biochemical status of cotton plants due to systemic insecticidal protection, in relation to resurgence of the aphid Aphis gossypii Glov. Madras Agric. J.. 1973;60:512-518.
- [Google Scholar]
- Lethal and Sublethal effect of imidacloprid and buprafezin on the sweet potato whitefly parasitoid Eretmocerus Mundus (Hymenoptera: Aphelinidae. Crop Prot.. 2013;45:98-103.
- [Google Scholar]
- Management of Leucinodes Orbonalis guenee on eggplants during the rainy season. India J. Plant Protection Res.. 2011;51:325-328.
- [Google Scholar]
- Insecticide induced resurgence of the white backed planthopper Sogatella furcifera (Horvath) (Hemiptera: Delphacidae) on rice varieties with different levels of resistance. Crop Prot. 2011
- [Google Scholar]
- The phenolic constituents of Prunus domestica. I. The quantitative analysis of phenolic constituents. J. Agric. Sci.. 1959;10:63-68.
- [CrossRef] [Google Scholar]
- Influence of deltamethrin on the biochemical parameters of cotton ad biology of the whitefly, Bemisia tabaci (Gennadius. Pestology. 1990;14:17-19.
- [Google Scholar]
- Varshney, S., Khan, M.I.R., Masood, A., Per, T.S., Rasheed, F., Khan, N.A., 2015. Contribution of plant growth regulators in mitigation of herbicidal stress. J. Plant Biochem. Physiol. 3–2.
- Effects of sublethal doses of imidacloprid on the fecundity of green leafhoppers, Nephotettix spp. (Hemiptera: Cicadellidae) and their natural enemies. Appl. Entomol. Zool.. 2001;36:501-507.
- [CrossRef] [Google Scholar]
- Wu, H.M., Feng, H.L., Wang, G.D., Zhang, L.L., Zulu, L., Liu, Y.H., Zheng, Y.L., Rao, Q., 2022. Sublethal Effects of Three Insecticides on Development and Reproduction of Spodoptera frugiperda (Lepidoptera: Noctuidae. Agronomy 12, 1334. https://doi.org/10.3390/.
- Comparative effects of cyometrinil and flurozole on selected metabolic process of isolated soyabean leafy cells. J. Plant Growth Regul.. 1986;5:59-72.
- [Google Scholar]
- Efficacy of sulphur on Tuta absoluta and its side effects on the predator Nesidiocoris tenuis. J. Appl. Entomol.. 2012;136:401-409.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2023.103070.
Appendix A
Supplementary material
The following are the Supplementary data to this article: