Translate this page into:
Comparative evaluation of different extraction methods for antioxidant and anti-inflammatory properties from Osbeckia parvifolia Arn. – An in vitro approach
*Corresponding author. Tel.: +91 422 2428305; fax: +91 422 2425706 drparimel@gmail.com (Thangaraj Parimelazhagan)
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Available online 4 October 2013
Peer review under responsibility of King Saud University.

Abstract
The effect of maceration, Soxhlet and fractionation extraction from whole plant of Osbeckia parvifolia was studied for free radical scavenging and anti-inflammatory activities in vitro. The extracts were quantitatively analyzed for total phenolic, tannin and flavonoid contents using spectrophotometric methods. In vitro free radical scavenging activity of extracts were studied for DPPH• (1,1-diphenyl-2-picryl-hydrazyl), ABTS•+ (2,2′-azinobis (3-ethyl-benzothiozoline)-6-sulfonic acid) scavenging activities, metal ion chelating capability, lipid peroxidation, phosphomolybdenum and FRAP (Ferric reducing/antioxidant power) assays. Protein denaturation and membrane stabilization assays were employed to assess the anti-inflammatory activity of different extracts of O. parvifolia. Quantitative analysis showed that whole plant has high contents of total phenolic, tannin and flavonoid. Antioxidant assessment results registered higher anti-radical property for both macerated and Soxhlet methanol extracts compared to other solvent extracts. Successively extracted methanol extract from Soxhlet apparatus protected protein denaturation and erythrocyte membrane lysis comparable to standard Diclofenac sodium. Whole plant served as a potential source of antioxidant from natural origin and this study also provides a better technique to extract the natural antioxidant and anti-inflammatory substances from O. parvifolia.
Keywords
Anti-denaturation
Antioxidant
Membrane stabilization
Osbeckia parvifolia
Polyphenols
1 Introduction
Oxidative stress refers to an imbalance between the production of free radicals and the antioxidant defense system. Reactive oxygen species (ROS) are various forms of activated oxygen which causes lipid peroxidation and as a consequence of that inflammation may develop (Bahramikia et al., 2009). Inflammation, which is a functionally protective response, can be considered as a complex series of events that develop when the body is injured either by mechanical or chemical agents by a self-destructive process. In many inflammatory disorders there is an excessive activation of phagocytes and production of free radicals which increase vascular permeability, protein denaturation and membrane alteration (Umapathy et al., 2010). This brings the need for antioxidant and anti-inflammatory agents which can prevent oxidative stress and inflammation.
Synthetic antioxidants and Non-steroidal inflammatory drugs (NSAIDs) are commercially available and currently used. However, these chemicals are toxic and their risk to health has increased the demand for natural antioxidants (Liu et al., 2011). Therefore, new antioxidant and anti-inflammatory drugs lacking those effects are being searched all over the world as alternatives to synthetic drugs. On the other hand, the presence of polyphenolic compounds such as phenolic acids, vitamins (α-tocopherols, ascorbic acid) and other substances is widely used as safe natural antioxidants (Jayaprakasha and Rao, 2000). These non-nutritive chemicals have protected the humans from inflammation and oxidative stress related disorders. In addition to medicinal plants, phenolic and non-phenolic compounds are also found in both edible and non-edible plants.
Extraction is an important step involved in the discovery of bioactive components from medicinal plants. Different extraction methods have been used to extract polyphenolic compounds from plant materials. Biological activities of plant extracts showed significant differences depending upon the different extraction methods, emphasizing the importance of selecting the suitable extraction method (Hayouni et al., 2007).
Osbeckia species have antioxidant (Su et al., 1987a,b, 1988; Thabrew et al., 1998), hepatoprotective (Grayer et al., 2008), immunomodulatory (Nicholl et al., 2001), hypoglycemic and anti-hyperglycemic properties (Syiem and Khup, 2006). Osbeckia parvifolia Arn. (Cherkulathi in Tamil) is flowering plant species and widely distributed in South India as an important medicinal plant belonging to the family of Melastomataceae. Whole plant of O. parvifolia (Syn. Osbeckia cupularis) is used to treat inflammation (Anonymous, 1952; Krishnan Nambiar et al., 1985; Nadkarni, 1994; Rashtravardhana, 2006). However, comprehensive scientific investigations are required to access the usefulness of whole plant in treating diseases. There is no report regarding biological activities of O. parvifolia whole plant. Hence the present investigation on whole plant was undertaken to study the effect of different extraction methods on the total phenolic content, in vitro free radical scavenging and anti-inflammatory properties.
2 Materials and methods
2.1 Chemicals
Sodium nitroprusside, 1,1-diphenyl-2-picryl-hydrazyl (DPPH), potassium persulfate, 2,2′-azinobis(3-ethyl-benzothiozoline)-6-sulfonic acid disodium salt (ABTS), 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), Quercetin, Rutin, Butylated Hydroxytoluene (BHT), Butylated Hydroxyanisole (BHA), ferrous chloride, ferric chloride, ethylene diamine tetra-acetic acid (EDTA) disodium salt, 2,4,6-tripyridyl-s-triazine (Fe(III) (TPTZ), N-(1-naphthyl) ethylene diaminedihydrochloride, aluminum chloride, Thiobarbiutric acid (TBA), Diclofenac sodium and Riboflavin were obtained from Merck, Sigma and Himedia (Mumbai, India). All other chemicals and solvents used were of analytical grade.
2.2 Collection and identification of plant material
The whole plant of O. parvifolia was collected during the month of October 2011 from Coonoor district of Nilgiris, Tamil Nadu, India. The taxonomic identity of the plant was confirmed by Botanical Survey of India, Southern circle, Coimbatore, Tamil Nadu. The plant materials were washed under running tap water to remove the surface pollutants and the whole plants were air dried under shade. The dried sample was powdered and used for further studies.
2.3 Preparation of plant extracts
Three different extraction methods were followed to prepare crude extracts from whole plant of O. parvifolia.
2.3.1 Maceration
Powdered plant material (10 g) was taken in a conical flask and extracted with organic solvents (100 mL) such as n-hexane, ethyl acetate, methanol and ethanol in a mechanical shaker with temperature control (Room temperature) at constant stirring rate at 200 rpm. It was left for 24 h and solids were filtered using Whatman No. 1 filter (Raaman, 2006). The extraction was repeated three times until complete extraction.
2.3.2 Successive Soxhlet extraction
Soxhlet equipment was used in this study. Powdered plant material (60 g) was extracted with organic solvents (300 mL) such as n-hexane, ethyl acetate methanol and ethanol in Soxhlet apparatus (Raaman, 2006).
2.3.3 Fractionation
50 g of powdered plant material was extracted with ethanol (300 mL) in Soxhlet apparatus. The ethanol crude extract was freed from solvent by rotary vacuum evaporator. The ethanol extract (10.2 g) was further extracted with organic solvents (300 mL) such as n-hexane, ethyl acetate, methanol and ethanol in Soxhlet apparatus (modified method of Raaman, 2006).
The different extracts of different extraction methods were concentrated by rotary vacuum evaporator (Yamato RE300, Japan) and then air dried. The extracts obtained (1 mg/mL of respective organic solvents) were used for the assessment for further studies.
2.3.4 Extract yield percentage
The extraction yield is a measure of the solvent’s efficiency to extract specific components from the original material and it was defined as the amount of extract recovered in mass compared with the initial amount of whole plant. It is presented in percentage (%) and was determined for each techniques tested.
2.4 Quantification of total phenolic, tannin and flavonoid contents
The total phenolic content was determined according to the method described by Siddhuraju and Becker (2003). 50 μL triplicates of the extracts (mg/mL of respective organic solvents) were taken in the test tubes and made up to the volume of 1 mL with distilled water. Then 0.5 mL of Folin–Ciocalteu phenol reagent (1:1 with water) and 2.5 mL sodium carbonate solution (20%) were added sequentially in each tube. Soon after vortexing the reaction mixture, the test tubes were placed in the dark for 40 min and the absorbance was recorded at 725 nm against the reagent blank. The results were expressed as gallic acid equivalents (GAE).
Using the same extract, the tannins were estimated after treatment with polyvinyl polypyrrolidine (PVPP) (Siddhuraju and Manian, 2007). One hundred milligrams of PVPP was weighed into a 100 × 12 mm eppendrof tube and to this 500 μL distilled water and 500 μL of the sample extracts were added. The content was vortexed and kept in the freezer at 4 °C for 4 h. Then the sample was centrifuged at 4000 rpm for 10 min at room temperature and the supernatant was collected. This supernatant has only simple phenolics other than the tannins (the tannins would have been precipitated along with the PVPP). The phenolic content of the supernatant was measured and expressed as the content of non-tannin phenolics in percentage of extract. From the above results, the tannin content of the sample was calculated as follows:
Tannins (%) = Total phenolics (%) − Non-tannin phenolics (%)
The flavonoid contents of all the extracts were quantified according to the method described by Zhishen et al. (1999). About 500 μL of different extracts (1 mg/mL of respective organic solvents) was taken in different test tubes and 2 mL of distilled water was added to each of the test tube. A test tube containing 2.5 mL of distilled water served as blank. Then, 150 μL of 5% NaNO2 was added to all the test tubes followed by incubation at room temperature for 6 min. After incubation, 150 μL of 10% AlCl3 was added to all the test tubes including the blank. All the test tubes were incubated for 6 minutes at room temperature. Then 2 mL of 4% NaOH was added to all the test tubes which were made up to 5 mL using distilled water. The contents in all the test tubes were vortexed well and they were allowed to stand for 15 min at room temperature. The pink color developed due to the presence of flavonoid was read UV-spectrophotometrically at 510 nm. Rutin was used as the standard for the quantification of flavonoid. All the experiments were done in triplicates and the results were expressed in Rutin equivalents (RE).
3 In vitro antioxidant assays
3.1 DPPH• scavenging assay
The stable radical DPPH was used to measure the free radical scavenging activity by the method of Blois (1958). Samples at various concentrations (20, 40, 60, 80 μg/mL of extracts with respective organic solvents) were taken and the volume was adjusted to 100 μL with methanol. About 5 mL of a 0.1 mM methanolic solution of DPPH was added to the aliquots of different extracts of plant sample and standards (BHT and Quercetin). 100 μL of methanol in 5 mL of DPPH solution was used as a negative control. All the reaction mixtures were incubated for 20 min at 27 °C. Inhibition of DPPH radical by the plant samples was measured at 517 nm against the blank (methanol).
3.2 ABTS•+ scavenging assay
The total antioxidant activity was determined by ABTS radical cation scavenging assay by the method of Re et al. (1999). ABTS radical cation was produced by ABTS (stable radical) aqueous solution with 2.4 mM potassium persulfate in the dark for 12–16 h. Prior to assay, ABTS solution was diluted in ethanol (1:89 v/v) to give an absorbance of 0.700 ± 0.02 at 734 nm. Triplicates of 10 μL samples (1 mg/mL of respective organic solvents) and Trolox (concentration 0–15 μM) were added to 1 mL of diluted ABTS solution. The reaction mixture was incubated at 30 °C exactly for 30 min and the absorbance was measured at 734 nm against the ethanol (blank).
3.3 Phosphomolybdenum assay
The total antioxidant activity of samples was determined by the green phosphomolybdenum complex formation by the method of Prieto et al. (1999). Triplicates of 100 μL of sample (1 mg/mL of respective organic solvents) and standard (ascorbic acid) were added with 3 mL of reagent solution (0.6 M sulfuric acid, 28 mM sodium phosphate and 4 mM ammonium molybdate). The reaction mixture was incubated at 95 °C for 90 min. The absorbance of the mixture was measured at 695 nm against the reagent blank.
3.4 Ferric reducing/antioxidant power (FRAP) assay
The ferric reducing ability of different extracts was estimated by the method of Pulido et al. (2000). The FRAP reagent was prepared by mixing 2.5 mL of 10 mM TPTZ in 40 mM HCl, 2.5 mL of 20 mM FeCl3.6H2O and 25 mL of 0.3 M acetate buffer (pH 3.6). 900 μL of FRAP reagent was mixed with 10 μL of aliquots of plant extracts (1 mg/mL of respective organic solvents) and incubated at 37 °C. After incubation, ferric reducing ability of plant extracts was measured at 595 nm. The results were expressed as μmol Fe(II) equiv./g extract.
3.5 Metal ion chelating activity
The chelating activity of O. parvifolia was determined by the method of Dinis et al. (1999). 800 μL of samples (1 mg/mL of respective organic solvents) were added to 100 μL solution of 2 mM FeCl2. The reaction was initiated by the addition of 400 μL of 5 mM ferrozine and incubated at room temperature for 10 min. Absorbance of the samples was then measured spectrophotometrically at 562 nm against the deionized water (blank). The metal chelating capacities of the extracts were expressed as mg EDTA equivalents/100 g extract.
3.6 Lipid peroxidation inhibition assay
A modified thiobarbituric acid-reactive substance (TBARS) assay (Ohkowa et al., 1979) was used to measure the lipid peroxide formed, using egg yolk homogenates as lipid-rich media (Ruberto et al., 2000). Molondialdehyde (MDA), a secondary end product of the oxidation of polyunsaturated fatty acids, reacts with two molecules of TBA yielding a pinkish red chromogen. Egg homogenate (500 μL of 10%, v/v in phosphate-buffered saline pH 7.4) and 300 μL of sample (1 mg/mL of respective organic solvents) were added to a test tube and made up to 1.0 mL with distilled water. Then, 50 μL of FeSO4 (0.075 M) and 20 μL of l-ascorbic acid (0.1 M) were added and incubated for 1 h at 37 °C to induce lipid peroxidation. Thereafter, 0.2 mL of EDTA (0.1 M) and 1.5 mL of TBA reagent (3 g TBA, 120 g TCA and 10.4 mL 70% HClO4 in 800 mL of distilled water) were added in each sample and heated for 15 min at 100 °C. After cooling, samples were centrifuged for 10 min at 3000 rpm and absorbance of supernatant was measured at 532 nm (Afanasev et al., 1989). Inhibition (%) of lipid peroxidation was calculated using the equation:
% Inhibition = [(Control OD- Sample OD)/Control OD] × 100
4 In vitro anti-inflammatory activity
4.1 Protein denaturation method
The reaction mixture (0.5 mL; pH 6.3) consisted of 0.45 mL of bovine serum albumin (5% aqueous solution) and 0.05 mL of distilled water. pH was adjusted at 6.3 using a small amount of 1 N HCl. 1000 μg of O. parvifolia methanol extracts (mg/mL of respective organic solvents) was added to the reaction mixture and were incubated at 37 °C for 30 min and then heated at 57 °C for 5 min after cooling the samples, 2.5 mL of phosphate buffer solution was added. Turbidity was measured spectrophotometrically at 600 nm. For negative control 0.05 mL distilled water and 0.45 mL of bovine serum albumin were used. The percentage inhibition of protein denaturation was calculated as follows (Sakat et al., 2010),
4.2 Membrane stabilization method
Alsever’s solution was prepared by 2% dextrose, 0.8% Sodium citrate, 0.05% citric acid and 0.42% sodium chloride dissolved in distilled water, and then the solution was sterilized. Blood was collected from goat. The collected blood was mixed with equal volume of sterilized Alsever’s solution. The blood was centrifuged at 3000 rpm and packed cells were washed with isosaline and a suspension in 10% (V/V) isosaline was made. Reaction mixture (4.5 mL) contains 1 mL phosphate buffer, 2 mL hyposaline, 1 mL of 1000 μg methanol extracts of O. parvifolia (1 mg/mL of respective organic solvents) and 0.5 mL RBC (Red blood cells) suspension. Diclofenac sodium was used as the reference drug. The reaction mixture was served as a control. The assay mixtures were incubated at 37 °C for 30 min and centrifuged and the supernatant solution was estimated using UV analysis at 560 nm. Percent membrane stabilization activity was calculated by the formula (Shinde et al., 1999):
5 Statistical analyses
All the experiments were done in triplicates and the results were expressed as Mean ± SD. The data were statistically analyzed using one way ANOVA followed by Duncan’s test. Mean values were considered statistically significant when p > 0.05.
6 Results
The yield percentage of O. parvifolia is shown in Fig. 1. In maceration, the extract yield percentage of all the extracts was found to be in the order of: methanol > ethyl acetate > ethanol > n-hexane, whereas in Successive Soxhlet extraction it was in the order of: methanol > ethyl acetate > n-hexane ethanol extracts and for fractioned ethanol extracts, it was methanol > n-hexane > ethyl acetate > ethanol. Among the different solvent extractions, the successive Soxhlet method found to have higher recovery over other extraction methods.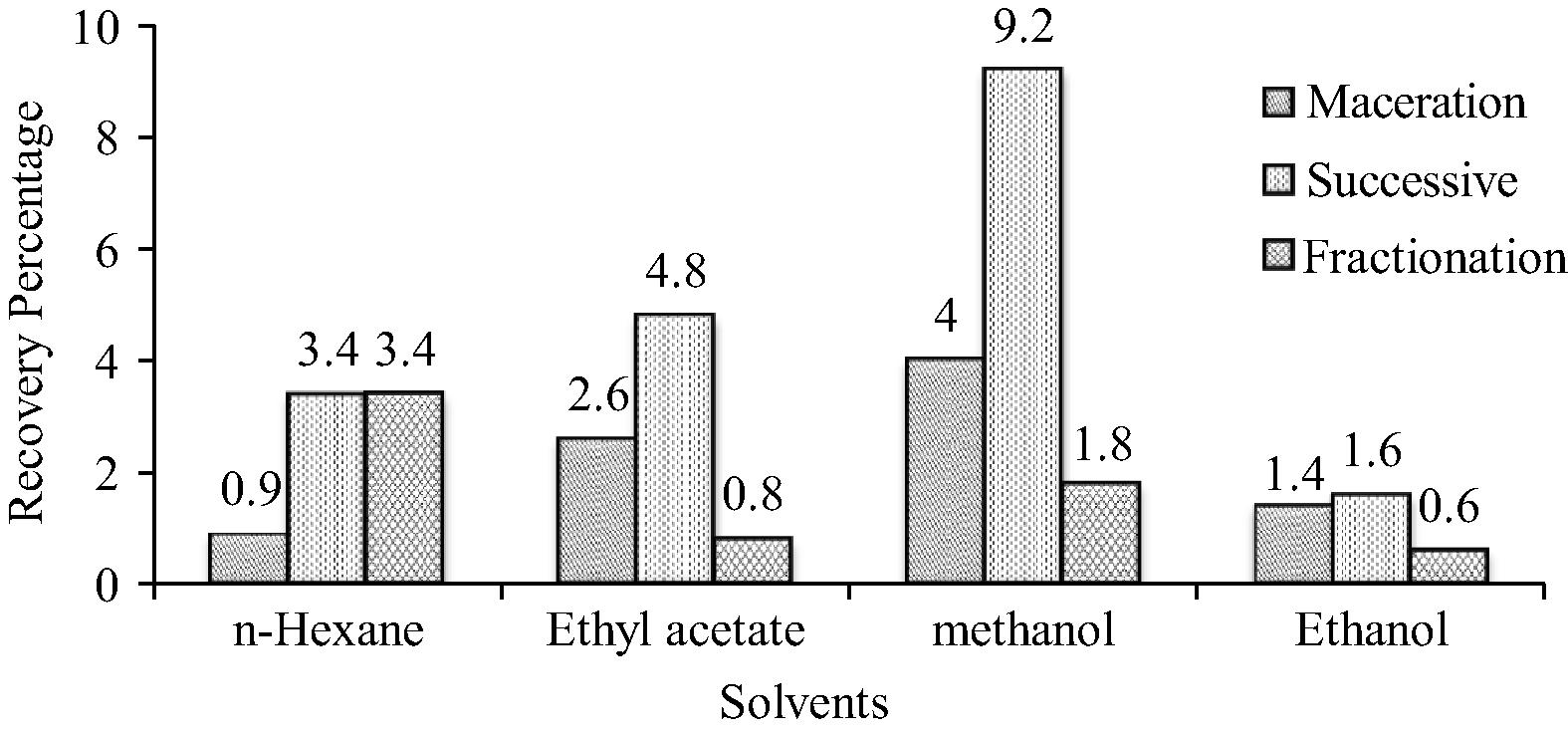
Extract yield percentage of different extracts of O. parvifolia.
The total phenolic, tannin and flavonoid contents of different extracts were analyzed and presented in Table 1. In this study, fractionation ethanol extract (36.72 g GAE/100 g) shows higher total phenolic content while the maceration methanol extract (24.93 g GAE/100 g) has higher tannin content. However, the flavonoid content was found to be higher in successive Soxhlet methanol extract (26.57 g RE/100 g). Among the three extraction methods, the successive Soxhlet extraction method was effective in extracting phenolic constituents. Values are mean of triplicate determination (n = 3) ± standard deviation. Statistically significant at p < 0.05 where a > b > c. GAE – Gallic acid equivalents. RE – Rutin equivalents.
Samples
Total phenolics (g GAE/100 g extract)
Tannins (g GAE/100 g extract)
Flavonoids (g RE/100 g extract)
Maceration
Successive Soxhlet
Fraction
Maceration
Successive Soxhlet
Fraction
Maceration
Successive Soxhlet
Fraction
n-Hexane
9.31 ± 0.17
6.61 ± 0.65
15.97 ± 2.54
1.68 ± 0.56
7.31 ± 0.05
12.44 ± 3.81
7.33 ± 1.11
9.03 ± 0.7
0.45 ± 0.07
Ethyl acetate
32.67 ± 1.17c
33.47 ± 2.84c
20.31 ± 0.45
17.57 ± 0.24
10.88 ± 1.00c
8.15 ± 0.36
9.73 ± 0.4
9.33 ± 0.51
13.80 ± 0.00c
Methanol
30.37 ± 1.97
35.31 ± 1.24a,b
32.96 ± 1.39c
24.93 ± 1.98a
20.32 ± 0.3
22.57 ± 0.89a.b
26.43 ± 0.75a
26.57 ± 0.51a
5.20 ± 0.42
Ethanol
14.45 ± 0.38
222.72 ± 0.73
36.72 ± 2.10a
9.92 ± 0.56
10.67 ± 1.9
20.31 ± 2.20a
17.4 ± 0.44b
26.5 ± 0.85a
1.15 ± 0.07
The free radical scavenging ability of different solvent extracts were measured and shown in Fig. 2. The free radical scavenging activity was measured by stable free radical DPPH.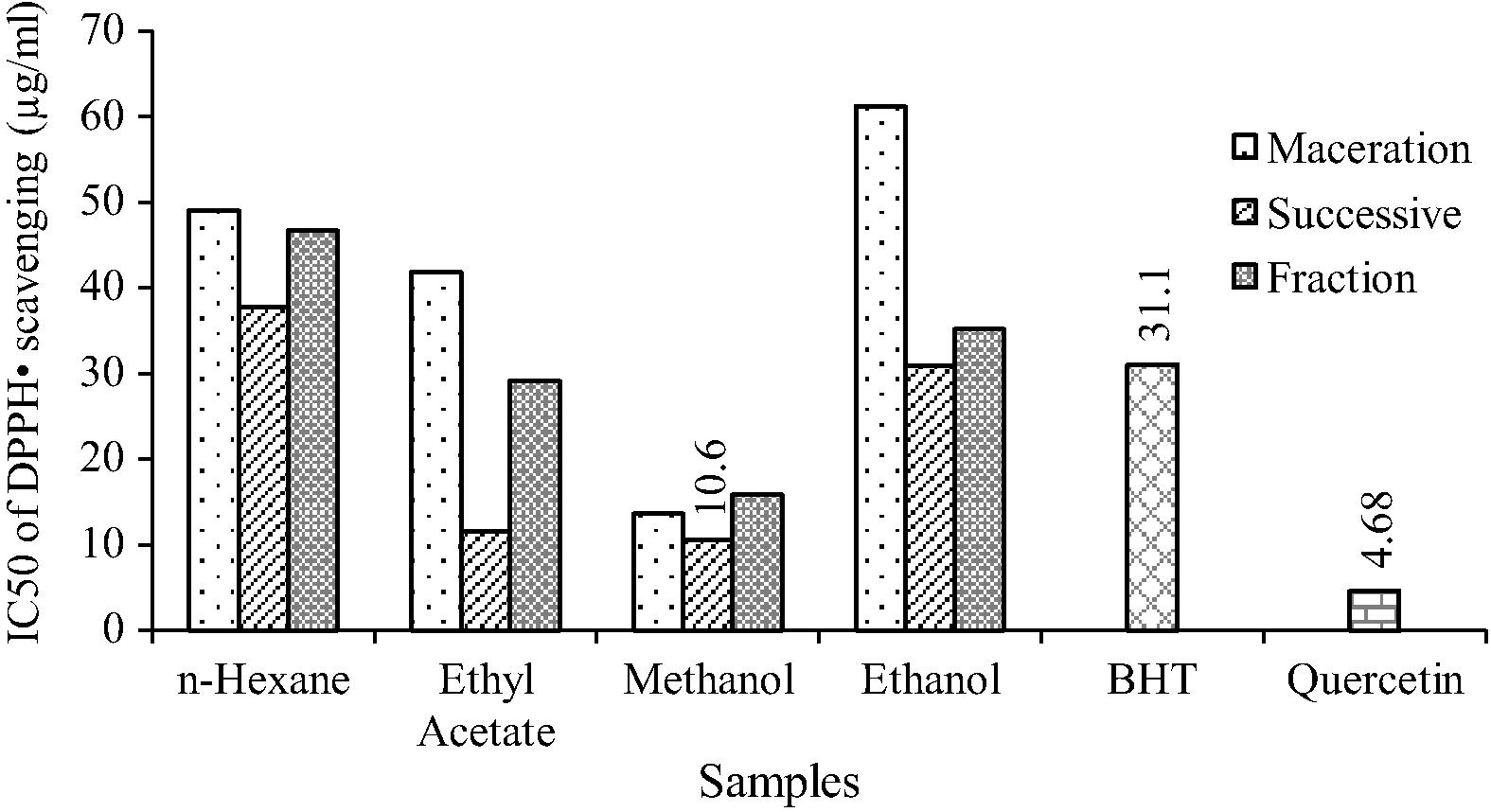
DPPH radical scavenging activity of O. parvifolia.
BHT and quercetin were used as reference compounds. The IC50 value of methanol extracts from different extraction methods follows the order: Successive Soxhlet (10.6 μg/mL) > Maceration (13.6 μg/mL) > Fraction extracts (15.9 μg/mL). The results show that successive Soxhlet methanol extract has better hydrogen donating ability compared to other extracts.
ABTS•+ scavenging assay is a widely accepted model to determine the total antioxidant activity. The results were expressed as μM TEAC/g (Table 2). Among various solvent extracts of O. parvifolia, the successive Soxhlet methanol extract possessed the highest ABTS•+ scavenging activity (14134.4 μM TEAC/g), while the n-hexane extract of fractionated ethanol extract showed the lowest ABTS radical cation scavenging activity (418.5 μM TEAC/g). Values are mean of triplicate determination (n = 3) ± SD. Statistically significant at p < 0.05 where a > b > c. TEAC – Trolox equivalent antioxidant capacity. AAE – Ascorbic acid equivalents.
Samples
ABTS (μmol TEAC/g extract)
Phosphomolybdenum (mg AAE/g extract)
Maceration
Successive Soxhlet
Fraction
Maceration
Successive Soxhlet
Fraction
n-Hexane
1046.2 ± 103.9
1329.7 ± 974.5
418.5 ± 462.8
47.7 ± 3.2
61.8 ± 4.5
30.4 ± 2.0
EthylAcetate
4711.4 ± 466.3
14134.4 ± 1017.0a
3228.7 ± 1250.8
55.1 ± 7.1
173.3 ± 15.1a,b
69.2 ± 4.8
Methanol
7735.4 ± 1190.3c
11754.5 ± 1107.6b
11147.0 ± 442.8b
163.9 ± 10.4a,b
192.3 ± 3.2a
116.8 ± 1.7
Ethanol
8071.0 ± 597.7c
2311.8 ± 1463.8
3501.5 ± 38.6
157.9 ± 17.5a,b
136.9 ± 10.3b,c
108.5 ± 30.8
The total antioxidant activity of different extracts of whole plant was analyzed and shown in Table 2. The results were expressed as mg Ascorbic acid equivalents/g. A better antioxidant capacity was shown by successive Soxhlet methanol extract (192.3 mg AAE/g) and the lower antioxidant activity (30.4 mg AAE/g) was shown in n-hexane (fractionation) extract.
The FRAP assay is determined by the ferric reducing ability of plant crude extracts (Table 3). The successive Soxhlet methanol extract (7075.4 μmol Fe(II) equiv./g) showed higher ferric reducing ability compared to ascorbic acid (6341.84 μmol Fe(II) equiv./g) and quercetin (7461.14 μmol Fe(II) equiv./g). The methanol extract of both maceration and fraction showed moderate ferric reducing ability 5404.1 μmol Fe(II) equiv./g and 4863 μmol Fe(II) equiv./g, respectively. Values are mean of triplicate determination (n = 3) ± SD. Statistically significant at p < 0.05 where a > b > c. Fe(II) – Ferrous sulfate equivalents. EDTA – Ethylene diamine tetra acetic acid equivalents.
Samples
FRAP assay (μm Fe (II)/g extract)
Metal chelating activity (mg EDTA/100 g extract)
Maceration
Successive Soxhlet
Fraction
Maceration
Successive Soxhlet
Fraction
n-Hexane
538.0 ± 25.5
113.1 ± 37.9
305.4 ± 90.0
568.5 ± 43.8
637.1 ± 21.1
755.5 ± 16.0
Ethyl acetate
1211.9 ± 138.3
6895.4 ± 268.0a,b
1861.3 ± 153.8
746.7 ± 43.6
708.3 ± 37.6
939.7 ± 39.5c
Methanol
5404.1 ± 318.3
7075.4 ± 276.0a
4863.0 ± 325.6
1045.5 ± 11.7c
1441.3 ± 42.9a
1072.4 ± 64.6b
Ethanol
3196.1 ± 64.3
6182.5 ± 982.5c
3501.2 ± 542.0
950.7 ± 20.5
1065.2 ± 19.5
639.8 ± 33.0
Ascorbic acid
6341.8 ± 2.1b,c
–
–
–
–
–
Quercetin
7461.1 ± 14.7a
–
–
–
–
–
The metal ion chelating activity extracts were analyzed and shown in Table 3. The decolorization of red color of the reaction mixture depends upon the reduction of ferrous ions by the plant extracts. The results were expressed as mg EDTA equivalents/100 g. The metal chelating activity of methanol extract of maceration, successive Soxhlet and fraction extracts was 1045.5, 1441.3 and 1072.4 mg EDTA equivalents/100 g, respectively. Among the different solvent extracts, the successive Soxhlet methanol extract exhibited better metal ion chelating property.
As shown in Fig. 3, O. parvifolia exhibited a strong inhibitory effect on lipid peroxidation based on the different concentration of samples. The maceration ethyl acetate extract (59.6%) showed higher percentage inhibition at 300 μg concentration. On the other hand, quercetin (58.91%) exhibited higher percentage inhibition than BHT (24.34%).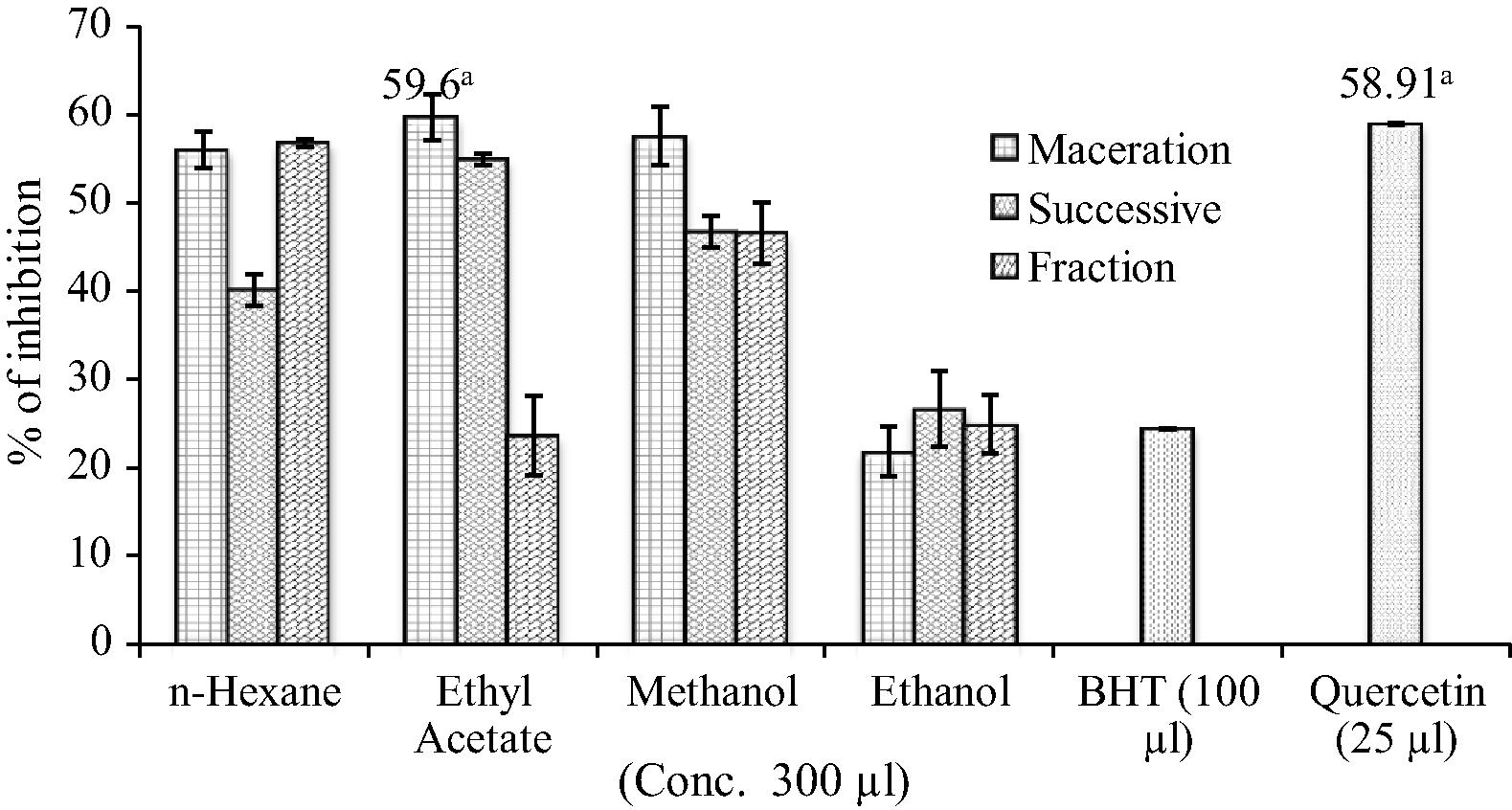
Lipid peroxidation inhibition by O. parvifolia extracts Statistically significant at p < 0.05 where a.
The in vitro anti-inflammatory activity of methanol extracts was studied by protein denaturation and the membrane stabilization method and results are shown in Fig. 4. Methanol extracts of O. parvifolia at a concentration of 1 mg significantly protected the albumin from denaturation and lysis of erythrocyte membrane induced by hypotonic solution which is comparable to the standard Diclofenac sodium. Among the different methanol extracts, successive Soxhlet methanol extract produced 54.3% inhibition of protein denaturation and 59.0% inhibition of RBC hemolysis.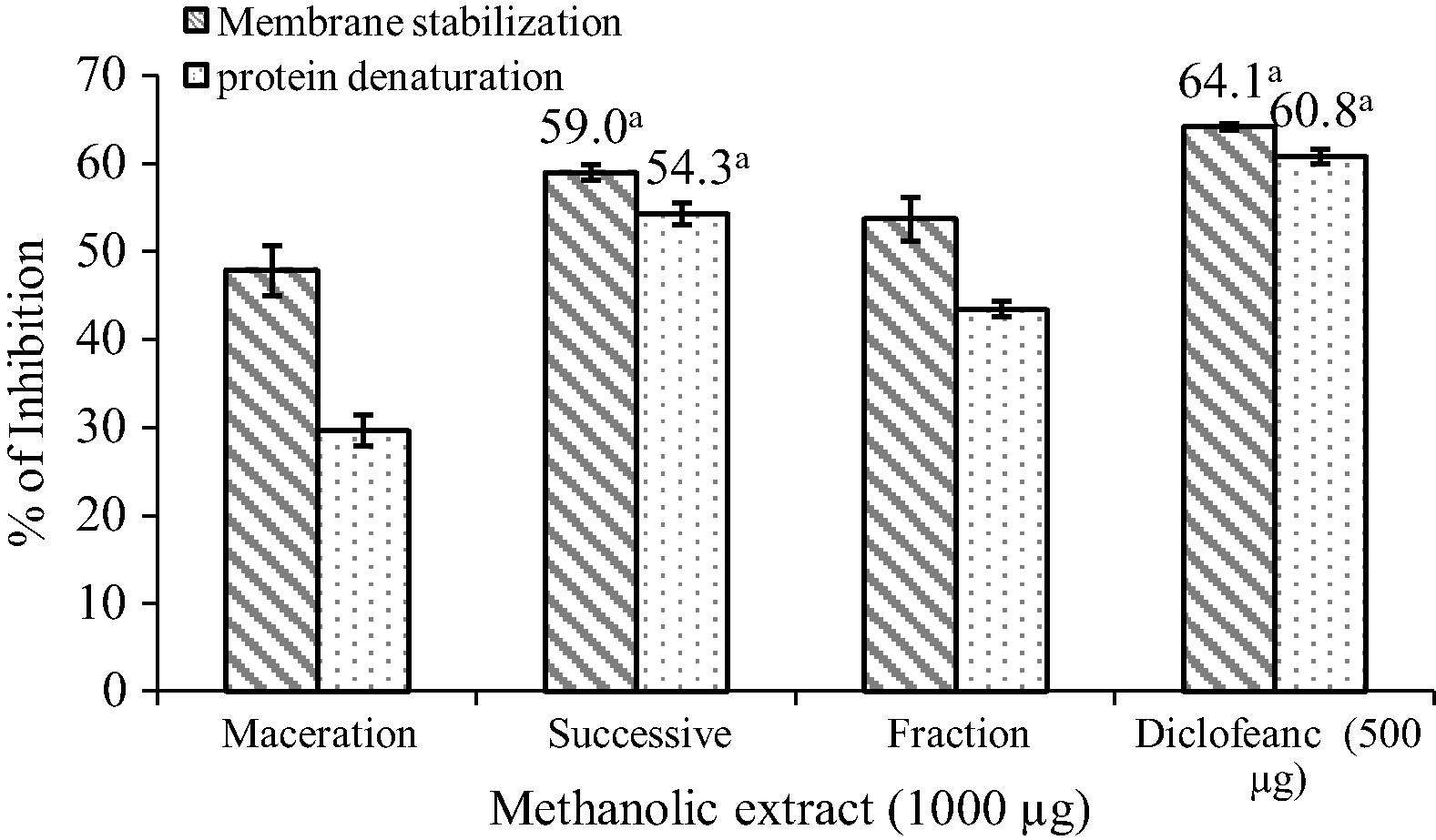
In vitro anti-inflammatory activity of O. parvifolia methanol extracts Statistically significant at p < 0.05 where a.
7 Discussion
In the present study, three extraction methods were used to evaluate the total phenolic contents, antioxidant and anti-inflammatory activity of whole plant extracts. Among the different solvent extraction methods, successive Soxhlet extraction could provide comparable or even better results than the maceration and fractionation for extracting polyphenolic compounds and showed a significant advantage in extraction time and solvent consumption over the other two methods.
Phenolic compounds, tannins and flavonoids have been reported to have multiple biological effects, including antioxidant and anti-inflammatory properties (Amarowicz, 2007). Recent evidences suggest that diets rich in polyphenolic compounds play a significant role against oxidative stress related disorders because of their antioxidant activities. Hence, polyphenolic constituents of whole plant of O. parvifolia may have the property to counteract oxidative stress related disorders. Similarly, previous reports suggest that flavonoids (kaempferol, rutin, quercetin and ellagic acid) and tannins (punicacortein, casuarin) of O. chinensis have good antioxidant potential (Su et al., 1987a,b, 1988).
The DPPH free radical is a stable free radical, which has been widely used for estimating the free radical-scavenging activities of plant extracts/antioxidants. The results of the present investigation demonstrate that a successive Soxhlet-methanol (10.6 μg/mL) extract can significantly decrease in vitro DPPH• concentration, thus suggesting that plant extract contains secondary metabolites with strong antioxidant activity. The antioxidant properties of natural and synthetic antioxidants are believed to be responsible for their beneficial effects during treatment of inflammation disorders. Phenolic compounds of the O. parvifolia extract are possibly involved in their free radical reactions by reducing the stable DPPH radical to a yellowish colored diphenylpicrylhydrazine derivative. Likewise, O. aspera has also been reported to have anti-DPPH• activity (Thabrew et al., 1998).
The ABTS•+ scavenging assay was used to evaluate the total antioxidant activity of the carotenoids, phenolics, and antioxidants (plant extracts), through measuring the reduction of the cation radical as the percentage inhibition (Chen et al., 2008). Trolox, a water soluble vitamin E analog, serves as a positive control inhibiting the formation of the radical cation in a dose dependent manner. However, most of the solvent extracts of O. parvifolia showed high total antioxidant activity. Hagerman et al. (1998) have reported that the high molecular weight phenolics (tannins) have more ability to quench free radicals (ABTS•+). Since, the extracts from different methods of extraction have the ability to scavenge free radicals, thereby preventing lipid oxidation via a chain breaking reaction, they could serve as potential nutraceuticals when ingested along with nutrients.
A spectrophotometric method has been developed for the quantitative determination of antioxidant capacity by the phosphomolybdenum method. The assay is based on the reduction of Mo(VI) to Mo(V) by the sample and the subsequent formation of a green phosphate/Mo(V) complex (Prieto et al., 1999). From the results, antioxidant capacity of the extracts was able to inhibit the Mo complex. From this, total antioxidant capacity (TAA) of O. parvifolia may be due to the presence of phenolics and flavonoids in the plant extracts.
The simple and reliable FRAP assay was used to measure the reducing potential of an antioxidant reacting with a ferric TPTZ complex and producing a colored ferrous TPTZ complex by a reductant at low pH, was adopted (Pulido et al., 2000). Generally, the reducing properties are associated with the presence of phenolic compounds, which exert their action by breaking the free radical chain through donating a hydrogen atom. Therefore, phenolics and flavonoid contents of O. parvifolia may have the ability to disrupt the free radical chain.
Chelation of metal ions has an antioxidant effect because the transition metal iron generates the reactive oxygen species leading to oxidation of unsaturated lipids and promoting oxidative damage at different levels (Meyer and Frankel, 2001). In this assay, plant extracts and standard antioxidant compounds have the ability of chelating the metal ions by capturing ferrous ion before ferrozine. Phenolics and flavonoid contents of successive Soxhlet methanol extract have the ability to scavenge the metal ions which can prevent the cells from lipid oxidation.
The inhibition of Fe2+-induced lipid peroxidation was assayed by the TBARS formation assay. Molondialdehyde is in many instances the most abundant individual aldehyde resulting from lipid peroxidation (Marnett, 1999). In this study, whole plant extracts inhibit the production of molondialdehyde from the lipid peroxidation. Among the different solvent extracts, the maceration ethyl acetate extract shows higher free radical scavenging activity in lipid membranes. Polyphenol and tannin contents of plant extracts could protect the cells from oxidative damage. From the results, polyphenol content of O. parvifolia may inhibit the lipid oxidation and DNA damage.
Methanol and ethanol have been proved as effective solvents to extract phenolic compounds (Siddhuraju and Becker, 2003). Considering the methanol and ethanol extracts, methanol extract is more polar than ethanol. Ethanol extract is also a good solvent extract for obtaining polyphenolic compounds and is safe for human consumption (Shi et al., 2005). From the results, ethanol extract has shown some affinity to extract bioactive compounds even after the use of methanol and it is also evident from the study results. This may be due to extractability of some bioactive compounds in ethanol. Antioxidant results revealed that O. parvifolia whole plant has the ability to scavenge free radicals. Contrasting with different solvent extraction methods, all the methanol extracts showed good antioxidant activity. Therefore, the methanol extracts were selected for further in vitro anti-inflammatory studies.
Denaturation of protein is one of the causes of rheumatoid arthritis that was documented. Production of autoantigen in certain arthritic diseases may be due to denaturation of protein. The mechanism of denaturation probably involves alteration of I electrostatic hydrogen, hydrophobic and disulfide bonding (Williams et al., 2008). From the data obtained, successive Soxhlet methanol extract has the ability to protect the protein membrane from heat and alkali induced protein denaturation comparable to Diclofenac.
To confirm the membrane stabilizing activity of O. parvifolia, the experiments were tested on the erythrocyte membrane. The lysozomal enzymes released during the inflammation produce a variety of disorders. RBC membranes are similar to lysosomal membrane components; the prevention of hypotonicity-induced RBC membrane lysis was taken as a measure of anti-inflammatory activity of drugs and plant extracts (Mounnissamy et al., 2007). The results showed that the methanol extracts protect the erythrocyte membrane against hypotonic induced lysis. The activity was comparable to that of Diclofenac.
Phenolics, flavonoids, tannins and saponins have the ability to bind cations and other biomolecules are able to protect the protein membranes from denaturation and stabilize the erythrocyte membrane (Oyedapo, 2001). Therefore, polyphenolic compounds such as phenolic, tannins and flavonoid contents of methanol extracts could be the possible reason for anti-denaturation property and able to stabilize the lysosome membrane.
This study showed that the extraction is an essential step to find polyphenolic compounds from plant extracts. Comparison of these three extraction methods revealed that they produce similar results, however, there are quantitative differences in total phenolic content, antioxidant and anti-inflammatory activities. Overall, successive Soxhlet extraction was found to be the best in obtaining antioxidant and anti-inflammatory substances from the extracts than the maceration and fractionation.
Soxhlet extraction is a classical technique for the solvent extraction of obtaining polyphenolic compounds from plant sources. Even though, some of the heat sensitive compounds may decompose in the Soxhlet technique (Wan and Weller, 2006). However, thermolabile/thermostable compounds cannot be dehydrolyzed due to the stability of compounds. From the result, thermolabile compounds from the Soxhlet extraction method showed good antioxidant and anti-inflammatory properties compared to other techniques (maceration and fractionation).
8 Conclusion
The present study revealed that different types of extraction methods had a big influence on the antioxidant and anti-inflammatory properties of obtained extracts. These results showed that O. parvifolia could be a potential natural source of antioxidants and could have greater importance as therapeutic agent in preventing or slowing oxidative stress and inflammation related disorders. Further studies are currently underway to assess the in vivo biological activities and to identify the active component responsible for their antioxidant and anti-inflammatory properties.
Acknowledgment
The authors are thankful to Dr. S. Manian, the Head of the Department of Botany, Bharathiar University, Coimbatore, Tamil Nadu and India.
References
- Chelating and free radical scavenging mechanisms of inhibitory action of Rutin and Quercetin in lipid peroxidation. Biochem. Pharmacol.. 1989;38:1763-1769.
- [Google Scholar]
- Tannins: the new natural antioxidants? Eur. J. Lipid Sci. Technol.. 2007;109:549-551.
- [Google Scholar]
- The Wealth of India Raw Materials. Vol vol. 3. New Delhi: Council of Scientific and Industrial Publication and Information Directorate; 1952. p. 113
- Protective effects of four Iranian medicinal plants against free radical-mediated protein oxidation. Food Chem.. 2009;115:37-42.
- [Google Scholar]
- Antioxidants determination by the use of a stable free radical. Nature. 1958;4617:1199-1200.
- [Google Scholar]
- Purification, composition analysis and antioxidant activity of a polysaccharide from the fruiting bodies of Ganoder maatrum. Food Chem.. 2008;107:231-241.
- [Google Scholar]
- Spectrophotometric quantity of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of Vitamin E. Anal. Biochem.. 1999;269:337-341.
- [Google Scholar]
- Phenolic and terpenoid constituents from the Sri Lankan medicinal plant Osbekia aspera. Pharm. Biol.. 2008;46(3):154-161.
- [Google Scholar]
- High molecular weight plant polyphenolics (tannins) as biological antioxidants. J. Agric. Food Chem.. 1998;46:1887-1892.
- [Google Scholar]
- The effects of solvents and extraction method on the phenolic contents and biological activities in vitro of Tunisian Quercus coccifera L. and Juniperus phoenicea L. fruit extracts. Food Chem.. 2007;105:1126-1134.
- [Google Scholar]
- Phenolic constituents from Lichen Phamo tremastuppuem (Nyl-)-Hale and their antioxidant activity. Z. Naturforsch. 2000;55
- [Google Scholar]
- Studies on the Medicinal Plants of Kerala Forests. Thrissur: Kerala Forest Research Institute Peechi; 1985. p. 66
- The antioxidant and free radial scavenging activities of extract and fractions from corn silk (Zea mays L.) and related flavone glycosides. Food Chem.. 2011;126:261-269.
- [Google Scholar]
- Antioxidant activity of hydroxycinnamic acids on human low-density lipoprotein oxidation. Methods Enzymol.. 2001;335:256-265.
- [Google Scholar]
- Evaluation of anti-inflammatory and membrane stabilizing properties of ethanol extract of Cansjerar heedii J. Gmelin (Opiliaceae) Int. Pharm. Technol.. 2007;6(2):235-237.
- [Google Scholar]
- Nadkarni, K.M., 1994. Indian Materia Medica. third ed., vol. 2. Popular Prakasan, p. 889.
- In vitro studies on the the immunomodulatory effects of extracts of Osbeckia aspera. J. Ethnopharmacol.. 2001;78:39-44.
- [Google Scholar]
- Assay for lipid peroxidases in animal tissue by thiobarbituric acid reaction. Anal. Biochem.. 1979;95:351-358.
- [Google Scholar]
- Biological activity of Plyllanthus amarus extracts on pragrow-Dawley rats. Nig. J. Biochem. Mol. Biol. 2001:83-86.
- [Google Scholar]
- Spectrophotometric quantity of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal. Biochem.. 1999;269:337-341.
- [Google Scholar]
- Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay. J. Agric. Food Chem.. 2000;48:3396-3402.
- [Google Scholar]
- Phytochemical Techniques. New Delhi, India: New India Publishing Agency; 2006. p. 10
- Rashtra Vardhana, 2006. Floristic Plants of the World, vol. 2, Serup & Sons, p. 618.
- Antioxidant activity applying an improved ABTS radical cation decolourization assay. Free Radic. Biol. Med.. 1999;26:1231-1237.
- [Google Scholar]
- Antioxidant and Antimicrobial activity of Foeniculum vulgare and Crithmum maritimum essential oils. Planta Med.. 2000;66:687-693.
- [Google Scholar]
- In vitro antioxidant and anti-inflammatory activity of methanol extract of Oxalis corniculata Linn. Int. J. Pharm. Pharmacol. Sci.. 2010;2:146-155.
- [Google Scholar]
- Extraction of polyphenolics from plant material for functional foods-engineering and technology. Food Rev. Int.. 2005;21:139-166.
- [Google Scholar]
- Membrane stabilizing activity – a possible mechanism of action for the anti-inflammatory activity of Cedrus deodara wood oil. Fitoterapia. 1999;70:251-257.
- [Google Scholar]
- Studies on antioxidant activities of Mucuna seed (Mucuna pruriens var. utilis) extracts and certain non-protein amino/imino acids through in vitro models. J. Agric. Food Chem.. 2003;51:2144-2155.
- [Google Scholar]
- The antioxidant and free radical scavenging capacity of dietary phenolic extracts from horse gram (Macrotylo mauniflorum (Lam.) Verdc.) seeds. Food Chem.. 2007;105:950-958.
- [Google Scholar]
- Antioxidative flavonoids isolated from Osbeckia chinensis L. Agric. Biol. Chem.. 1987;51:2801-2803.
- [Google Scholar]
- A novel antioxidative synergist isolated from Osbeckia chinensis L. Agric. Biol. Chem.. 1987;51:3449-3450.
- [Google Scholar]
- Study of the traditionally used medicinal plant Osbeckia chinensis for hypoglycemic and anti-hyperglycemic effects in mice. Pharm. Biol.. 2006;44(8):613-618.
- [Google Scholar]
- An experimental evaluation of Albu casetosa aqueous extract on membrane stabilization, protein denaturation and white blood cell migration during acute inflammation. J. Med. Plants Res.. 2010;4:785-795.
- [Google Scholar]
- Recent advances in extraction of nutraceuticals from plants. Trends. Food Sci. Technol.. 2006;17:300-312.
- [Google Scholar]
- The in vitro anti-denaturation effects induced by natural products and non-steroidal compounds in heat treated (Immunogenic) bovine serum albumin is proposed as a screening assay for the detection of anti-inflammatory compounds, without the use of animals, in the early stages of the drug discovery process. West Indian Med. J.. 2008;7:327.
- [Google Scholar]
- The determination of flavonoid contents on mulberry and their scavenging effects on superoxide radical. Food Chem.. 1999;64:555-559.
- [Google Scholar]







