Translate this page into:
Comparative evaluation of cadmium-induced oxidative stress in camel and bovine erythrocytes
⁎Corresponding author at: Department of Biochemistry, College of Science, King Saud University, P.O. Box 2455, Riyadh 11451, Saudi Arabia. haseeb@ksu.edu.sa (Haseeb A. Khan)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objectives
Erythrocytes or red blood cells (RBCs) are the most abundant cells in blood. They transport oxygen from lungs to tissues and bring back carbon dioxide from tissues to lungs. Being non-nucleated and lacking the cellular organelles, they have been widely used for in vitro toxicity studies. The present study was carried out to study the comparative in vitro toxicity of cadmium (Cd) on RBCs of camel and cow.
Methods
Both cow and camel whole blood samples were exposed to 0, 10, 50 and 100 μM of cadmium chloride (CdCl2) and the tubes were incubated at 37 °C for 24 h. The erythrocytes from both the species were separated and used for biochemical determinations for markers of oxidative stress.
Results
Cd caused a concentration dependent decrease in bovine glutathione (GSH) content and superoxide dismutase (SOD) activity. However, in the case of camel RBCs, the decrease in GSH content and SOD activity were observed at medium and high concentrations only. Cd exposure also resulted in significant inhibition of glutathione peroxidase (GPx) and glutathione S-transferase in both bovine and camel RBCs. The GPx in camel RBCs was inhibited only at a high concentration of Cd. Various concentrations of Cd inhibited the catalase in bovine RBCs only, without any significant inhibition of catalase in camel RBCs. Medium and high concentrations of Cd caused an increase in lipid peroxidation in bovine RBCs. In the case of camel RBCs, none of the used concentrations of Cd caused lipid peroxidation.
Conclusions
The present study concludes that in vitro incubation of camel and bovine RBCs with Cd causes an impairment of their antioxidant system and therefore increases their susceptibility to hemolysis. However, the camel RBCs appear to have an increased resistance to oxidative stress generated by cadmium exposure.
Keywords
Cadmium
Oxidative stress
Antioxidant enzymes
Red blood cells
Cow
Camel
1 Introduction
Cadmium (Cd) is a ubiquitous and non-biodegradable contaminant in the environment that poses serious health risks. Cadmium is released in the environment as a result of geogenic and anthropogenic processes (Demchenkov et al., 2021). Industrial applications of Cd include its use in manufacture of plastics, pigments, enamels, ceramics, and steel plating. Cadmium is released into the environment through various natural and human activities like mining, smelting, refining, Cd-containing fertilizers and atmospheric deposition of combustion products (Hayat et al., 2019). As a result, Cd is one of the most mobile heavy metals in the environment (Kubier et al., 2019). Living organisms are exposed to Cd through water, air, and soil, which results in Cd toxicity. Cd is also released as byproduct of various industrial processes (Hayat et al., 2019). Cadmium is a toxic and nonessential element for the living organisms. It is an environmental threat that can ultimately cause disorders in plants and animals. It can accumulate in different organs of the human body due to its persistent nature. Cadmium exposure is known to cause disorders of glucose metabolism, breast and lung cancer, cerebral infarction and cardiac failure (Khan et al., 2017).
Erythrocytes are non-nucleated and most abundant cells in the in the blood. They function to transport oxygen from lungs to the tissues and carbon dioxide from the tissues to the lungs. Red blood cells can enzymatically synthesize vasodilator viz., nitric oxide (Kleinbongard et al., 2006) and also produce hydrogen sulfide, a signaling molecule that acts to relax vessel walls. The cardio protective effects of garlic on RBC membrane have been attributed to red blood cells converting its sulfur compounds into hydrogen sulfide (Benavides et al., 2007). Red blood cells also play a part in the body's immune response. When the RBCs are lysed by pathogens such as bacteria, their hemoglobin releases free radicals which disintegrate the bacterial cell wall and ultimately kills them (Jiang et al., 2007). Cadmium shortens the half-life of RBCs in the blood stream. Cadmium also causes anemia in humans and laboratory animals which is accompanied by hemolysis (Horiguchi et al., 2011). Cadmium uptake into rat red blood cells occurs by passive transport and the process is modulated by the alterations of sulfhydryls of red blood cells (Garty et al., 1986). Many in vivo and in vitro studies suggest oxidative stress to be one of the most important mechanisms of cadmium toxicity (Matović et al., 2015). Erythrocytes have an average diameter of 5–6 μm in cattle, which is small compared to other species. Bovine erythrocytes have a relatively long life span of 130–160 days (Brockus, 2011). Camels have a very high level of red blood cells, and these blood cells are oval in shape.
The cell membranes of erythrocytes contain many polyunsaturated fatty acids. Therefore, exposure of erythrocytes to reactive oxygen species (ROS) makes them highly susceptible to oxidative damage (Abdallah et al., 2011). Erythrocytes have been used to study the toxicity of xenobiotics due to their functional and structural simplicity (Farag and Alagawany, 2018). The erythrocytes contain many antioxidants and antioxidant enzymes. The alterations in the antioxidant capacities of RBCs demonstrate the ability of cadmium or other xenobiotics to inflict oxidative damage to RBCs. Therefore, the erythrocytes can serve as an in vitro model to evaluate the toxicity of xenobiotics (Farag and Alagawany, 2018). In the present study, in vitro effects of Cd on oxidative stress and antioxidant defense indices in bovine and camel erythrocytes were investigated.
2 Materials and methods
Fresh blood samples from cow and camel were collected from a local slaughter house. The heparinized whole blood samples were kept in an ice box and transported to lab. The blood samples were distributed into individual tubes for cadmium exposure. All the experiments were performed in triplicate. Both cow and camel whole blood samples were exposed to 0, 10, 50 and 100 μM of cadmium chloride (CdCl2) and the tubes were incubated at 37 °C for 24 h. After completion of the cadmium exposure time, the samples were centrifuged at 1,000 × g for 10 min at 4 °C. The upper layer was removed without disturbing the buffy coat of RBCs. The erythrocytes were suspended in ice cold distilled water and centrifuged at 10,000 × g for 10 min to pellet and remove the erythrocyte membranes. The supernatants were stored at 80 °C for biochemical analyses.
We used commercially available kits from MyBioSource Inc., San Diego, CA, USA for measuring the activities of antioxidant enzymes including, superoxide dismutase (SOD, Cat No: MBS841580), catalase (CAT, Cat No: MBS841637), glutathione peroxidase (GPx, Cat No: MBS2540472) and glutathione-S-transferase (GST, Cat No: MBS2540411). The non-enzymatic markers of oxidative stress including reduced glutathione (GSH, Cat No: MBS841550) and Lipid peroxidation (LPO, Cat No: MBS2540553) were also measured by colorimetric kits from MyBioSource Inc., USA, according to manufacturer’s instructions. The hemoglobin assay kit was obtained from Sigma-Aldrich, USA and used for measuring the hemoglobin (Hb) levels following the protocol mentioned in the kit insert.
The results are presented as means ± standard error of means (SEM) and expressed as per gram of Hb. The data were analyzed by one-way analysis of variance (ANOVA) using the SPSS software package version 11. Dunnett’s test was used for comparing the differences among different groups. P values < 0.05 were considered as statistically significant.
3 Results
The activities of superoxide dismutase (SOD) in unexposed RBCs from cow and camel blood were 1031.06 ± 51.31 U/g Hb 969.33 ± 15.32 U/g Hb which was significantly reduced by Cd exposure in a concentration dependent manner in cow RBCs, whereas in camel RBCs, the high concentration (100 µM) of Cd did not further reduce SOD levels as compared to medium concentration of Cd (50 µM) (Fig. 1). The activities of glutathione-S-transferase (GST) in cow and camel RBCs were found to be 21.41 ± 1.65 U/g and 19.77 ± 1.36 U/g, respectively (Fig. 2). Exposure to different concentrations of Cd significantly reduced the activities of GST in cow and camel RBCs. However, the effects of medium (50 μM) and high (100 µM) concentrations of Cd were not significantly different form the effect of low (10 μM) concentration of Cd (Fig. 2). The activities of glutathione peroxidase (GPx) in the RBCs of cow (39.06 ± 3.11 U/g Hb) and camel (39.80 ± 5.41 U/g Hb) were almost same (Fig. 3). Exposure of medium and high doses of Cd significantly reduced GPx activity in RBCs of cow; however, only high concentration of Cd was able to significantly reduce the GPx activity in the RBCs of camel (Fig. 3). The antioxidant enzyme catalase (CAT) activity in cow RBCs was found to be 142.67 ± 7.06 U/g Hb, which was significantly reduced by exposure of different concentrations of Cd; however, this reduction was not concentration dependent as low and medium concentration exerted similar effects (Fig. 4). The activity of CAT in camel RBCs was 140.67 ± 2.85 U/g Hb, which was not affected by the exposure to any of the three concentration of Cd (Fig. 4).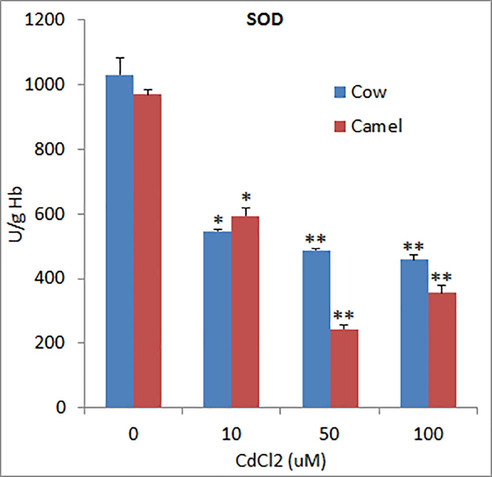
Effects of different concentrations of Cd on superoxide dismutase (SOD) activities in cow and camel RBCs. *P < 0.05 and **P<0.01 versus respective control groups.
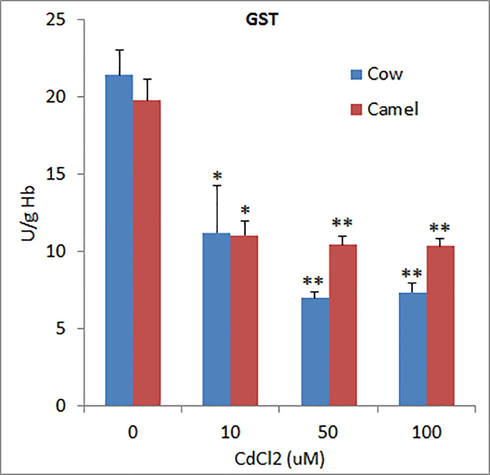
Effects of different concentrations of Cd on glutathione-S-transferase (GST) activities in cow and camel RBCs. *P < 0.05 and **P<0.01 versus respective control groups.
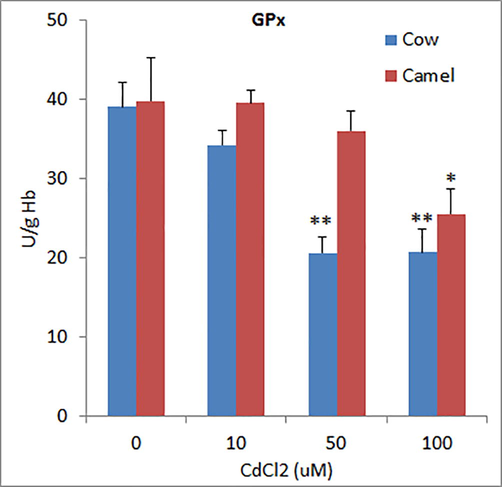
Effects of different concentrations of Cd on glutathione peroxidase (GPx) activities in cow and camel RBCs. *P < 0.05 and **P<0.01 versus respective control groups.
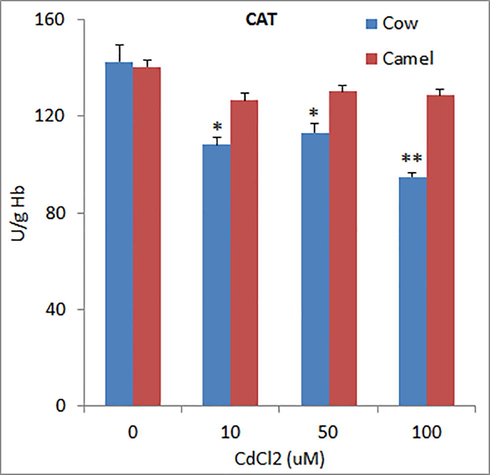
Effects of different concentrations of Cd on catalase (CAT) activities in cow and camel RBCs. *P < 0.05 and **P < 0.01 versus respective control group.
The levels of reduced glutathione (GSH) in RBCs of cow and camel were 16.45 ± 2.21 µmol/g Hb and 17.97 ± 0.64 µmol/g Hb, respectively (Fig. 5). All the concentrations of Cd significantly depleted GSH levels in cow RBCs in a concentration dependent manner. However, in camel RBCs, the lower concentration of Cd (10 μM) did not affect GSH levels whereas medium and high concentrations of Cd significantly and concentration dependently depleted GSH levels (Fig. 5). The levels of MDA (marker of lipid peroxidation) in the RBCs of cow and camel were found to be 8.31 ± 0.63 mmol/g Hb and 11.54 ± 0.87 mmol/g Hb, respectively (Fig. 6). In cow RBCs, exposure of low (10 μM) concentration of Cd did not affect MDA levels whereas medium (50 μM) and high (100 µM) concentrations of Cd significantly increased MDA levels. In camel RBCs, low and medium concentrations of Cd did not affect MDA levels, whereas high concentration of Cd slightly and insignificantly increased MDA levels (Fig. 6).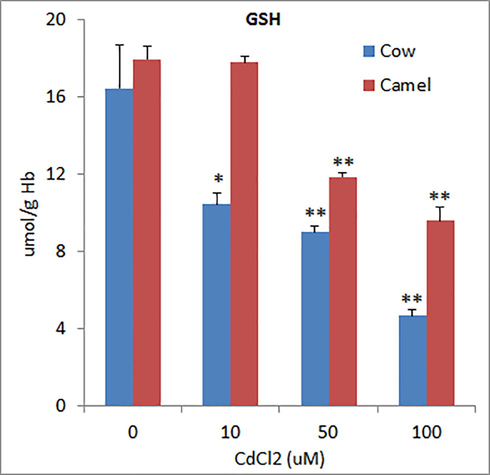
Effects of different concentrations of Cd on glutathione (GSH) levels in cow and camel RBCs. *P < 0.05 and **P < 0.01 versus respective control groups.
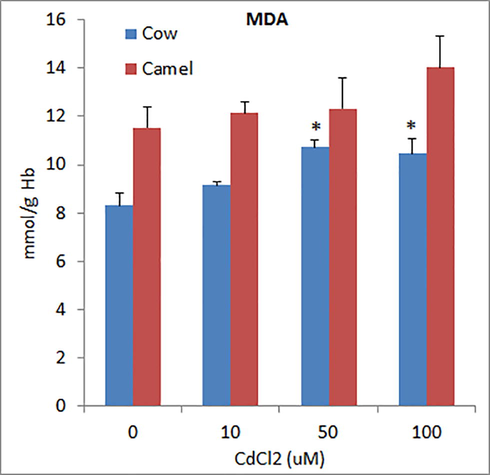
Effects of different concentrations of Cd on malondialdehyde (MDA) levels in cow and camel RBCs. *P < 0.05 versus respective control group.
4 Discussion
Oxidative stress reflects an imbalance between oxidant and antioxidant levels (Celi, 2011) and increases the risk of metabolic and infectious diseases by causing dysfunction in inflammatory responses (Mavangira and Sordillo, 2018; Sordillo and Raphael, 2013). Cadmium is one of the ubiquitously distributed toxic elements in the environment. Cadmium enters the biological system commonly through ingestion and inhalation (Pan et al., 2010; Khan et al., 2012). Cadmium has a long half-life in the biological systems and is distributed in the body by binding to hemoglobin in erythrocytes (Rashid et al., 2013). Exposure to Cd induces the generation of reactive oxygen species (ROS) which causes mitochondrial dysfunction and apoptosis, both in vitro and in vivo (Ahmad et al., 2018).
The reactive oxygen species are generated in a cell during various metabolic reactions. The ROS at low concentration function in cell signaling. However, at high concentration they can damage various macromolecules like proteins, lipids, nucleic acids etc. Superoxide dismutase (SOD), glutathione peroxidase (GPx), and catalase (CAT) are 3 important antioxidant enzymes in erythrocytes. These three enzymes are commonly used as an index of antioxidant status (Yang et al., 2014). Studies of Mousa et al. (2006) have shown the presence of various antioxidants/antioxidant enzymes such as glutathione, ascorbic acid, glutathione peroxidase, catalase, and superoxide dismutase in camel RBCs. Similarly other studies have demonstrated the presence of these antioxidant enzymes in bovine RBCs (Razavi et al., 2011; Salem et al., 2016). The mature RBCs of the adult bovine are biconcave in shape have a width of 5–6 μm, and have minimal central pallor and relatively long lifespan of approximately 130 days (Wood and Quiroz-Rocha, 2010). Cadmium has a solubility of 4.3 g/100 ml in water (25 °C), it is soluble in blood and therefore causes harmful systemic effects (Adachi et al., 2007). Mladenović et al. (2014) have demonstrated that exposure of cadmium caused a significant decrease in RBC hemoglobin content.
Cadmium exposure caused a decrease in the antioxidant enzymes including SOD (Fig. 1), GST (Fig. 2), GPx (Fig. 3) and CAT (Fig. 4) in both bovine and camel RBCs. Studies of Uchida et al. (2004) have shown that long term exposure to Cd resulted in decrease in erythrocytic SOD and CAT activities which was accompanied by renal tubular dysfunctions in inhabitants of Cd-polluted Jinzu River basin, Japan. Blood GPx levels were found to be decreased in battery factory workers who had inhaled Cd from batteries for 10 years (Wasowicz et al., 2001). Bansal and Bhatnagar (1996) have postulated that Cd makes erythrocytes vulnerable to oxidative damage by altering their antioxidant systems. Cadmium has been reported to alter the activities of various antioxidant enzymes by promoting the expression of stress genes (Wang and Templeton, 1998). The presence of SOD has been reported in the camel and bovine RBCs (Bengoumi et al., 1998). The reduction in antioxidant enzyme activities in erythrocytes may be due to inactivation of the enzymes by superoxide anions (Hodgson and Fridovich1975; Kono and Fridovich, 1982). Moitra et al. (2014) have reported similar results in human RBCs. Gutiérrez-Salinas et al. (2013) have observed similar effects of sodium fluoride on erythrocytes viz., increased lipid peroxidation and inhibition of SOD, GPx and CAT. Other environmental and industrial toxicants have also been shown to exert their toxic effects via generation of potentially toxic free radicals leading to oxidative stress (Al Deeb et al., 2000; Tariq et al., 2002; Ahmad Khan et al., 2004; Al Asmari et al., 2006; Al Asmari et al., 2014; Reddy et al., 2015).
Glutathione S-transferase (GST) is a ubiquitous enzyme which is involved in detoxification reactions. Other functions of GST include protection against oxidative damage to lipids in erythrocytes (Chikezie and Chidoka, 2011). The presence of GST in camel and bovine erythrocytes has been confirmed by previous studies (Chafik et al., 2019; Güvercin et al., 2008). The camel enzyme is unique in that it has a lower molecular weight, heterodimeric structure, higher optimum temperature, relatively lower optimum pH, lower content of selenium and higher affinity for hydrogen peroxide at low reduced glutathione concentration when compared to other mammals (Chafik et al., 2019). In this study in vitro incubation of camel and bovine erythrocytes caused significant inhibition of erythrocyte GST. Goto et al. (1992) have shown that the GST in erythrocytes of sheep directly correlated with the intracellular GSH level. In the present study also, Cd caused a decrease in erythrocyte GST activity which was accompanied with decreased GSH content. Bocedi et al. (2016) have shown higher expression in erythrocytic GST in cows from polluted areas. Our study however shows that Cd inhibits the erythrocytic GST in both camels and cow.
The tripeptide glutathione in its reduced form protects the cells against oxidative stress and xenobiotics. In the present study, Cd exposure of camel and cow RBCs resulted in significant decrease in reduced glutathione (GSH) concentration. Cadmium-GSH complexes are thought to be involved in the dynamic exchange of cadmium with other molecules inside the cell (Maret and Moulis, 2013). Mukhopadhyay et al. (1988) have shown a reduction in blood GSH levels in response to cadmium. Studies of Shekar et al. (2006) have demonstrated a decrease in GSH content after exposure of human RBCs to Cd. Other studies have shown that Cd exposure of mice resulted in an increase in lipid peroxidation and a decrease in GSH, GST, CAT and SOD in mice (Ahmad et al., 2018). The GSH along with ascorbic acid prevents oxidation of Hb and formation of its ferryl intermediate (Simoni et al., 2009). The decrease in the GSH concentration of bovine and camel RBCs as observed in this study (Fig. 5) would make their RBCs more susceptible to the oxidative stress generated by Cd. Decrease in erythrocyte GSH levels has been correlated to decrease in total antioxidant potential of plasma (Rizvi et al., 2006).
Oxidative stress lowers the antioxidant capacity of erythrocytes and makes them susceptible to hemolysis (Maurya et al., 2015). The erythrocytes of camels are unique when compared to other mammals in being elliptical (Moore, 2000), presence of increased amounts of short and unsaturated fatty acids in the membrane, and increased protein to lipid ratio (Al-Quarawi and Mousa, 2004). In this study, the exposure of RBCs from cow to Cd resulted in the generation of oxidative stress which was evident by an increased lipid peroxidation in RBCs (Fig. 6). Oxidative stress is known to be the cause of Cd toxicity (Conterato et al., 2013). Cadmium replaces redox active metals such as iron, copper etc. and thereby affects free radicals formation (Valko et al., 2006). Membranes are the earliest targets for the metal ions due to their fatty acid content. The mature RBCs of the adult bovine are biconcave in shape (Barger, 2010). In this study, exposure of bovine RBCs to Cd caused significant generation of oxidative stress only at medium and higher concentrations. This may be due to unique properties of ruminant RBCs. The ruminant RBCs have very low levels or absence of phosphatidylcholine (PC) along with high sphingomyelin levels. Secondly, only 2% of it is present on the outer bilayer phosphatidylethanolamine (PE) (Florin-Christensen et al., 2001). Camel RBCs were not significantly affected in terms of oxidative stress when exposed to Cd. This may be attributed to the difference in dietary habits of the two species (Revskij et al., 2019).
In conclusion, the present study demonstrates that in vitro incubation of camel and bovine RBCs with Cd causes an impairment of their antioxidant system and therefore increases their susceptibility to hemolysis. However, the camel’s RBCs appear to have an increased resistance to oxidative stress generated by cadmium exposure.
Acknowledgments
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for funding the Research Group No. RGP-1435-066.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Dimethoate-induced oxidative stress in human erythrocytes and the protective effect of vitamins C and E in vitro. Environ. Toxicol.. 2011;26(3):287-291.
- [Google Scholar]
- Strong acute toxicity, severe hepatic damage, renal injury and abnormal serum electrolytes after intravenous administration of cadmium fluoride in rats. J. Occup. Health. 2007;49(3):235-241.
- [Google Scholar]
- Nano-composites chitosan-curcumin synergistically inhibits the oxidative stress induced by toxic metal cadmium. Int. J. Biol. Macromol.. 2018;108:591-597.
- [Google Scholar]
- Time-course of lipid peroxidation in different organs of mice treated with Echis pyramidum snake venom. J. Biochem. Mol. Toxicol.. 2006;20:93-95.
- [Google Scholar]
- Effects of Echis Pyramidum snake venom on hepatic and renal antioxidant enzymes and lipid peroxidation in rats. J. Biochem. Mol. Toxicol.. 2014;28(9):407-412.
- [Google Scholar]
- Exacerbation of iminodipropionitrile-induced behavioral toxicity, oxidative stress and vestibular hair cell degeneration by gentamicin. Neurotoxicol. Teratol.. 2000;22:213-220.
- [Google Scholar]
- Lipid concentrations in erythrocyte membranes in normal, starved, dehydrated and rehydrated camels (Camelus dromedarius), and in normal sheep (Ovis aries) and goats (Capra hircus) J. Arid. Environ.. 2004;59:675-683.
- [Google Scholar]
- Cadmium induced lipid peroxidation and antioxidant enzymes in vitro in human erythrocytes. Fresenius Environ. Bull.. 1996;5:460-462.
- [Google Scholar]
- Erythrocyte morphology, in Schalm’s VeterInary Hematology (6th edition.). Ames, Iowa, USA: Wiley-Blackwell; 2010. p. :144-151.
- Hydrogen sulfide mediates the vasoactivity of garlic. PNAS. 2007;104(46):17977-17982.
- [Google Scholar]
- Comparative relationship between copper-zinc plasma concentrations and superoxide dismutase activity in camels and cows. Vet. Res.. 1998;29:557-565.
- [Google Scholar]
- Erythrocyte glutathione transferase: a general probe for chemical contaminations in mammals. Cell Death Discov.. 2016;2(1)
- [CrossRef] [Google Scholar]
- Brockus, C.W., 2011. Erythrocytes. In: Duncan and Prasse’s veterinary laboratory medicine: clinical pathology, ed. Latimer, KS , 5th ed., pp. 3–44. Wiley, Chichester, UK.
- Characterization of an interesting selenium-dependent glutathione peroxidase (Se-GPx) protecting cells against environmental stress: The Camelus dromedarius erythrocytes Se-GPx. Biocat. Agr. Biotech.. 2019;18:101000.
- [CrossRef] [Google Scholar]
- Glutathione-S-transferase activity of human erythrocytes incubated in aqueous solutions of five antimalarial drugs. Free Radic. Antioxidant.. 2011;1(2):26-30.
- [Google Scholar]
- Biomarkers of oxidative stress in ruminant medicine. Immunopharmacol. Immunotoxicol.. 2011;33(2):233-240.
- [Google Scholar]
- Blood thioredoxin reductase activity, oxidative stress and hematological parameters in painters and battery workers: relationship with lead and cadmium levels in blood. J. Appl. Toxicol.. 2013;33(2):142-150.
- [Google Scholar]
- Usage of atomic force microscopy for detection of the damaging effect of CdCl2 on red blood cells membrane. Ecotoxicol. Environ. Saf.. 2021;15(208):111683.
- [Google Scholar]
- Erythrocytes as a biological model for screening of xenobiotics toxicity. Chem. Biol. Interact.. 2018;5(279):73-83.
- [Google Scholar]
- A unique phospholipid organization in bovine erythrocyte membranes. Proc. Natl. Acad. Sci. U. S. A.. 2001;98(14):7736-7741.
- [Google Scholar]
- The relationship between reduced glutathione level and glutathione S-transferase activity in sheep erythrocytes. Jpn. J. Vet. Res.. 1992;40:99-104.
- [Google Scholar]
- In vitro effect of sodium fluoride on malondialdehyde concentration and on superoxide dismutase, catalase, and glutathione peroxidase in human erythrocytes. Sci. World J.. 2013;2013:1-7.
- [Google Scholar]
- Determination of some kinetic and characteristic properties of glutathione S-transferase from bovine erythrocytes. Protein Pept. Lett.. 2008;15:6-12.
- [Google Scholar]
- Bangash N in Chapter 7 - Environmental Hazards of Cadmium: Past, Present, and Future, Editor(s): Mirza Hasanuzzaman. In: Cadmium Toxicity and Tolerance in Plants. Elsevier; 2019. p. :163-183.
- [CrossRef] [Google Scholar]
- The interaction of bovine erythrocyte superoxide dismutase with hydrogen peroxide: inactivation of the enzyme. Biochemistry. 1975;14(24):5294-5299.
- [Google Scholar]
- Horiguchi, H., Oguma, E., Kayama, F., 2011. Cadmium induces anemia through interdependent progress of hemolysis, body iron accumulation, and insufficient erythropoietin production in rats. Toxicol. Sci. 122, 198–210.
- Respiratory protein-generated reactive oxygen species as an antimicrobial strategy. Nat. Immunol.. 2007;8(10):1114-1122.
- [Google Scholar]
- Metoclopramide attenuates iminodipropionitrile-induced oxidative stress and neurobehavioral toxicity in rats. Pharmacol. Biochem. Behav.. 2004;79(3):555-561.
- [Google Scholar]
- Distribution pattern of eight heavy metals in the outer and inner tissues of ten commonly used vegetables. Int. J. Food Prop.. 2012;15(6):1212-1219.
- [Google Scholar]
- Soil contamination with cadmium, consequences and remediation using organic amendments. Sci. Total Environ.. 2017;601-602:1591-1605.
- [Google Scholar]
- Red blood cells express a functional endothelial nitric oxide synthase. Blood. 2006;107:2943-2951.
- [Google Scholar]
- Insight into the oxidative stress induced by lead and/or cadmium in blood, liver and kidneys. Food Chem. Toxicol.. 2015;78:130-140.
- [Google Scholar]
- The bioinorganic chemistry of cadmium in the context of its toxicity. Met Ions Life Sci.. 2013;11:1-29.
- [Google Scholar]
- Protective effects of oestradiol against cadmium-induced changes in blood parameters and oxidative damage in rats. Arh. Hig. Rada. Toksikol.. 2014;65:37-46.
- [Google Scholar]
- Biomarkers of oxidative stress in erythrocytes as a function of human age. World J. Methodol.. 2015;5(4):216.
- [CrossRef] [Google Scholar]
- Role of lipid mediators in the regulation of oxidative stress and inflammatory responses in dairy cattle. Res. Vet. Sci.. 2018;116:4-14.
- [Google Scholar]
- Occupational cadmium exposure-associated oxidative stress and erythrocyte fragility among jewelry workers in India. Am. J. Ind. Med.. 2014;57(9):1064-1072.
- [Google Scholar]
- Moore DM. Haematology of Camelid Species: Llamas and Camels. In: Schalm's Veterinary Haematology (Feldman, BF, JG Zinkl, NC Jain, Eds.). 5th ed. Lippincott Williams and Wilkins, Philadelphia, USA. 2000; pp. 1184-1190.
- Antioxidant levels in tissues of young and adult camels (Camelus dromedarius)Actividad antioxidante en tejidos de camello joven y adulto. J. Physiol. Biochem.. 2006;62(3):213-218.
- [Google Scholar]
- Effects of cadmium treatment in vitro on the antioxidant protection mechanism and activation of human blood platelets. Thromb. Res.. 1988;50:419-427.
- [Google Scholar]
- Cadmium levels in Europe: implications for human health. Environ. Geochem. Health. 2010;32(1):1-12.
- [Google Scholar]
- An update on oxidative stress-mediated organ pathophysiology. Food Chem. Toxicol.. 2013;62:584-600.
- [Google Scholar]
- Alterations of erythrocyte antioxidant mechanisms: antioxidant enzymes, lipid peroxidation and serum trace elements associated with anemia in bovine tropical theileriosis. Vet. Parasitol.. 2011;180(3-4):209-214.
- [Google Scholar]
- Biomarkers of oxidative stress in rat for assessing toxicological effects of heavy metal pollution in river water. Env. Sci. Poll. Res.. 2015;22(17):13453-13463.
- [Google Scholar]
- Dietary Fatty Acids Affect Red Blood Cell Membrane Composition and Red Blood Cell ATP Release in Dairy Cows. Int. J. Mol. Sci.. 2019;20(11):2769.
- [CrossRef] [Google Scholar]
- Erythrocyte plasma membrane redox system in human aging. Rejuvenation Res.. 2006;9(4):470-474.
- [Google Scholar]
- Clinical, hemato-biochemical alterations and oxidant-antioxidant biomarkers in Babesia-infected calves. Int. J. Vet. Sci. Med.. 2016;4:17-22.
- [Google Scholar]
- Hypotheses on the effect of cadmium on glutathione content of red blood corpuscles. Twin Res. Hum. Genet.. 2006;9(1):73-75.
- [Google Scholar]
- Control of oxidative reactions of hemoglobin in the design of blood substitutes: role of the ascorbate-glutathione antioxidant system. Artif. Organs. 2009;33(2):115-126.
- [Google Scholar]
- Significance of metabolic stress, lipid mobilization, and inflammation on transition cow disorders. Vet. Clin. North Am. Food Anim. Pract.. 2013;29(2):267-278.
- [Google Scholar]
- Al Deeb S (2002) Attenuation of iminodipropionitrile- induced behavioral syndrome by sodium salicylate in rats. Pharmacol. Biochem. Behav.. 2002;73:647-654.
- [Google Scholar]
- Reduction of erythrocyte catalase and superoxide dismutase activities in male inhabitants of a cadmium-polluted area in Jinzu river basin, Japan. Toxicol. Lett.. 2004;151(3):451-457.
- [Google Scholar]
- Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact.. 2006;160(1):1-40.
- [Google Scholar]
- Blood concentration of essential trace elements and heavy metals in workers exposed to lead and cadmium. Int. J. Occup. Med. Environ. Health. 2001;14:223-229.
- [Google Scholar]
- Induction of c-fos proto-oncogene in mesangial cells by cadmium. J. Biol. Chem.. 1998;273(1):73-79.
- [Google Scholar]
- Normal hematology of cattle, in Schalm’s Veterinary Hematology (6th edition,). Ames, Iowa, USA: Wiley-Blackwell; 2010. p. :829-835.
- Erythrocyte superoxide dismutase, glutathione peroxidase, and catalase activities and risk of coronary heart disease in generally healthy women: a prospective study. Am. J. Epidemiol.. 2014;180:901-908.
- [Google Scholar]







